Combining ATRP and FRP Gels: Soft Gluing of Polymeric Materials for the Fabrication of Stackable Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Swelling Measurements
2.2.1. Stackable Gel Synthesis: Solid/Liquid Sequential Procedure
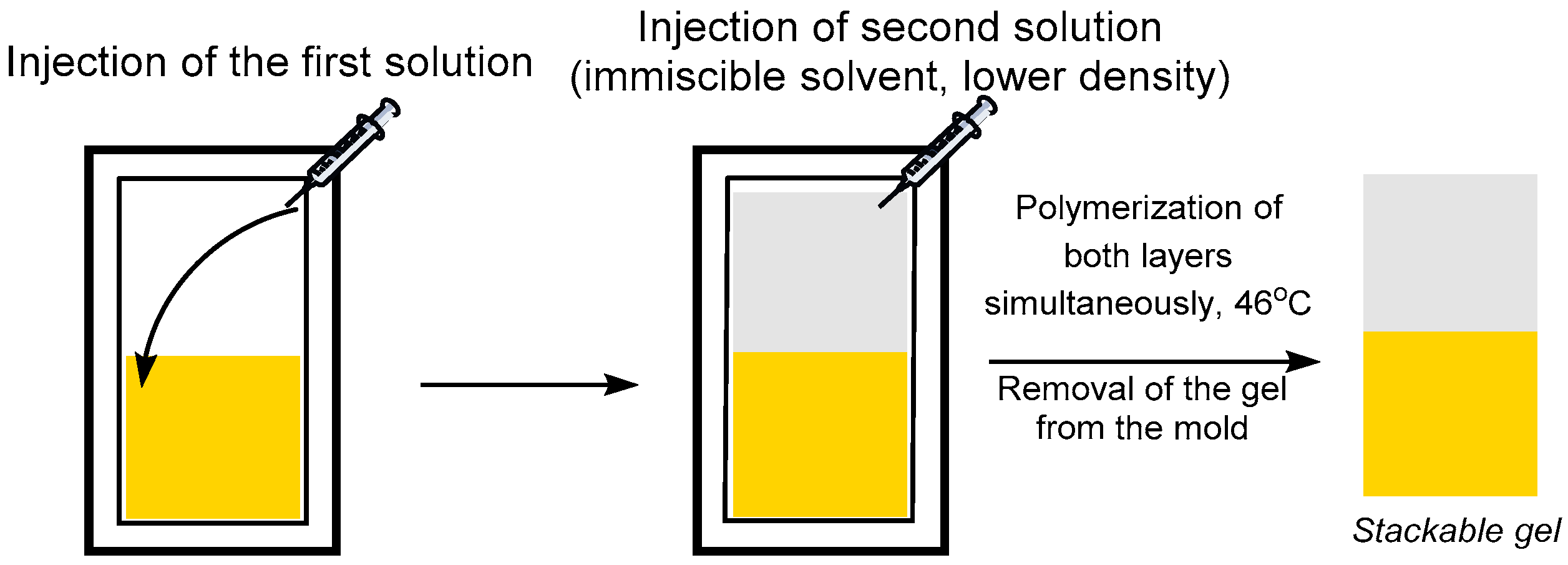
2.2.2. Stackable Gel Synthesis: One Pot Liquid/Liquid Procedure
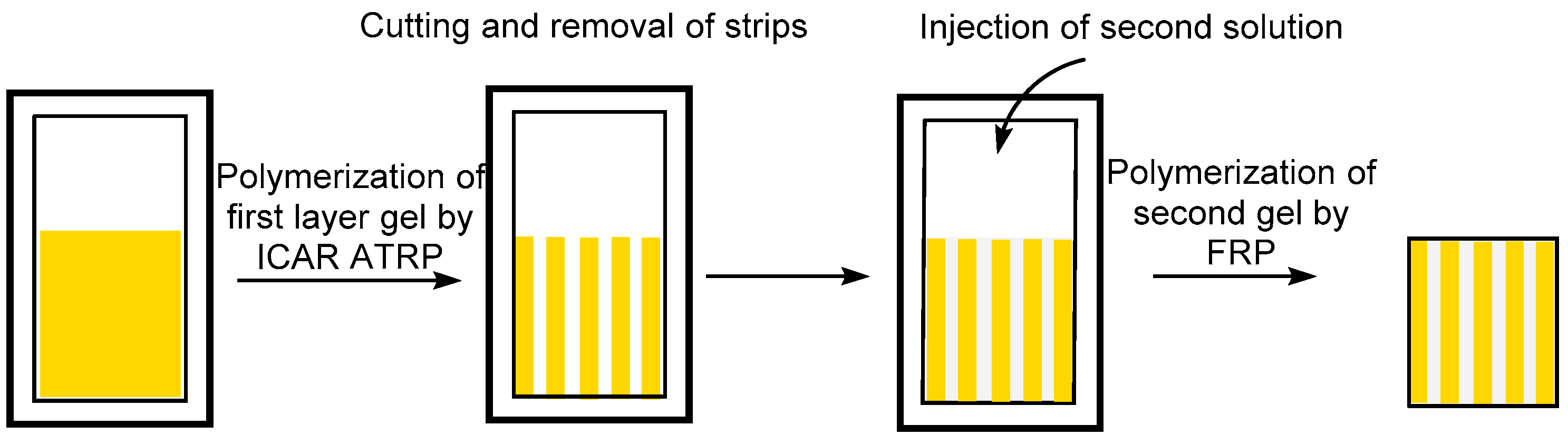
2.3. Synthesis of n-Layer Stackable Gel Combining ATRP/FRP by Patching Method
2.3.1. Composition of the Pre-Gel Solutions
2.3.2. Synthesis of PBMA Layer by FRP (BF)
2.3.3. Synthesis of PDMAEMA Layer by FRP (DF)
2.3.4. Synthesis of PEO2MA Layer by ATRP (PA)
3. Results and Discussion
3.1. Stackable Gels
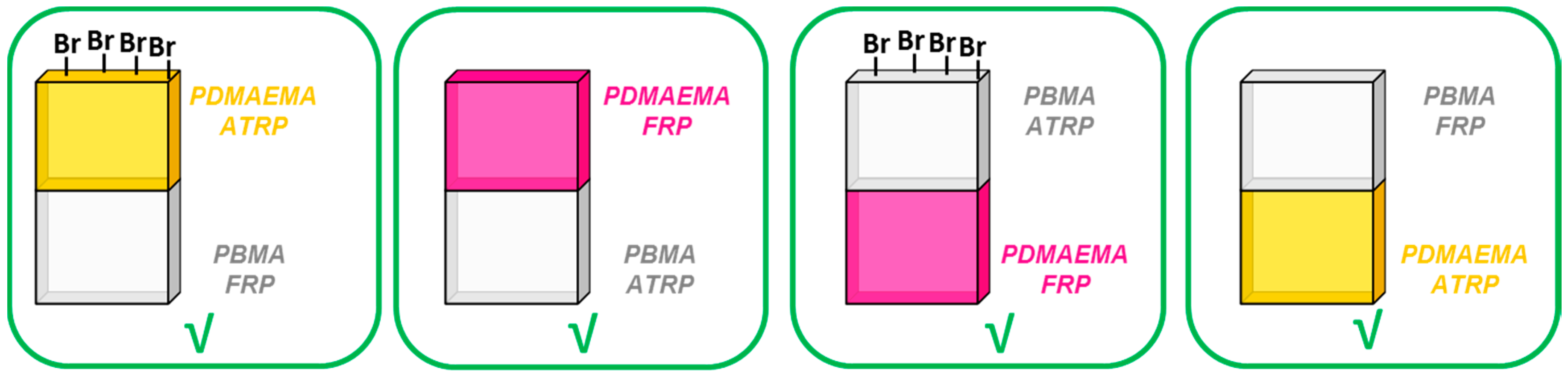
3.2. Formation of Amphiphilic Stackable Gels Combining ATRP and FRP by Solid/Liquid Sequential Polymerization
3.3. Formation of Amphiphilic Stackable Gels by Combining ATRP and FRP in a Liquid/Liquid One Pot Polymerization
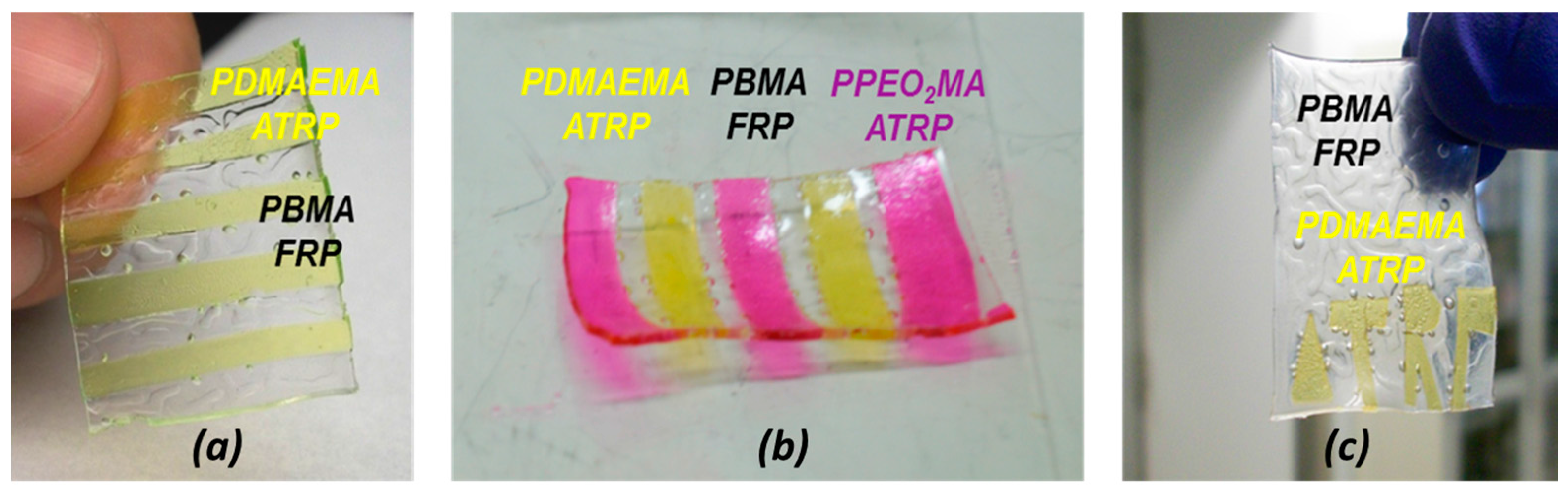
3.4. FRP Gels as Soft Glues: Synthesis of Multi-Layer Gels by Patching Method
- 10-layer stackable gel incorporating PDMAEMA and PEO2MA layers by ATRP and PBMA layers made by FRP (PA|BF|DA|BF|PA|BF|DA|BF|BF|PA|BF) (Figure 5b).
- “ATRP” letters formed by DA in a BF matrix (Figure 5c).
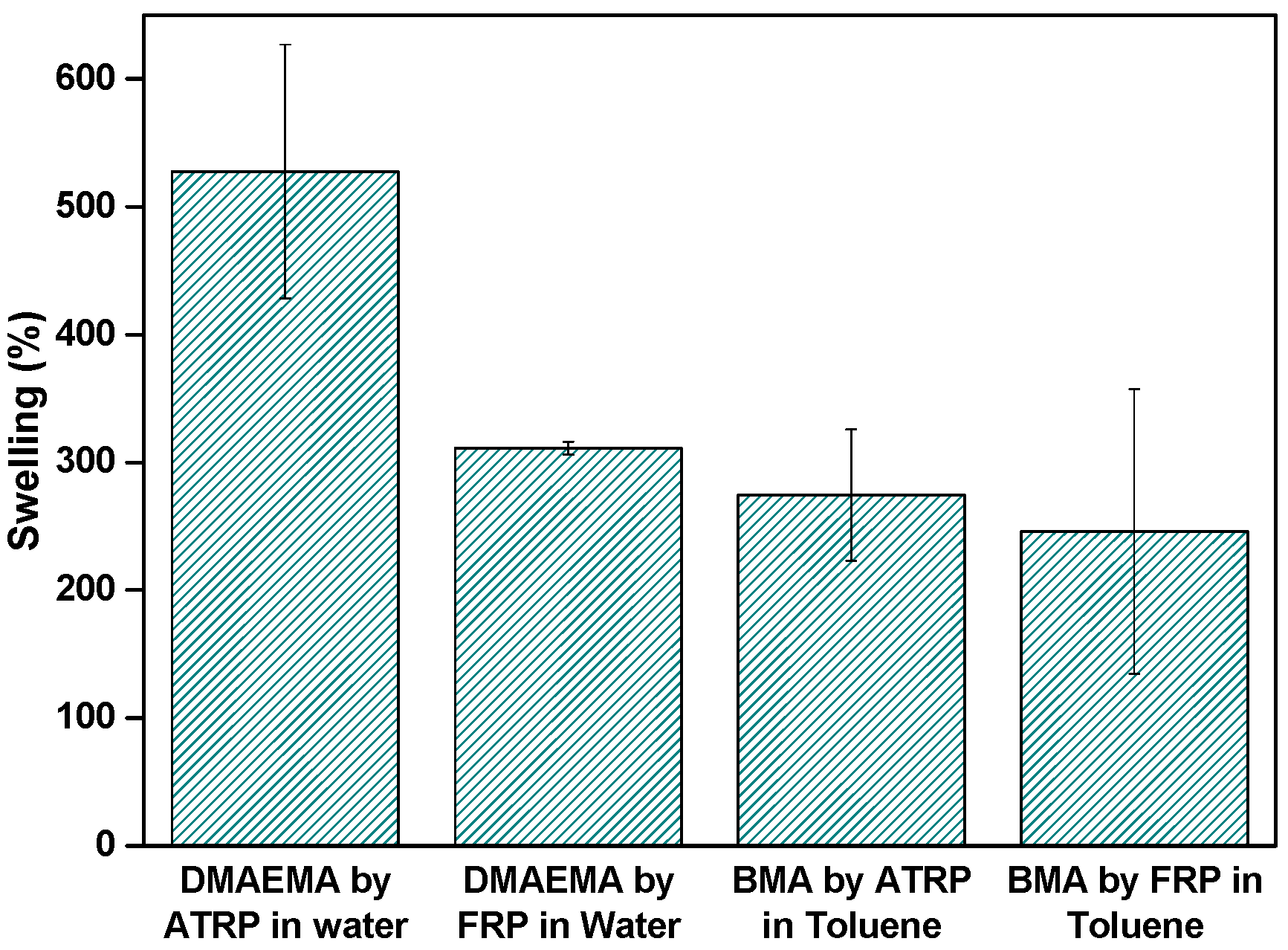
3.5. Influence of the Polymerization Technique: Swelling of the PBMA and PDMAEMA Gels
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent hydrogels that are robust and highly stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, Y.-T.; Liu, X.-Y.; Shi, F.-K.; Zhang, L.-Q.; Zhu, M.-F.; Xie, X.-M. Dually cross-linked single network poly(acrylic acid) hydrogels with superior mechanical properties and water absorbency. Soft Matter 2016, 12, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Alhadrami, H.A.; Hussain, M.A.; Aldhahri, M.; Al Nowaiser, F.; Al-Hazmi, F.; Oklu, R.; Khademhosseini, A. Hydrogels 2.0: Improved properties with nanomaterial composites for biomedical applications. Biomed. Mater. 2015, 11, 014104. [Google Scholar] [CrossRef] [PubMed]
- Vintiloiu, A.; Leroux, J.-C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Sagiri, S.S.; Behera, B.; Rafanan, R.R.; Bhattacharya, C.; Pal, K.; Banerjee, I.; Rousseau, D. Organogels as matrices for controlled drug delivery: A review on the current state. Soft Mater. 2014, 12, 47–72. [Google Scholar] [CrossRef]
- Banik, S.J.; Fernandes, N.J.; Thomas, P.C.; Raghavan, S.R. A new approach for creating polymer hydrogels with regions of distinct chemical, mechanical, and optical properties. Macromolecules 2012, 45, 5712–5717. [Google Scholar] [CrossRef]
- Rose, S.; Prevoteau, A.; Elziere, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Ma, Q.; Yu, H.; Zhang, Y.; Yan, Z.; Liu, F.; Liu, C.; Jiang, H.; Chen, Y. Macroscopic organohydrogel hybrid from rapid adhesion between dynamic covalent hydrogel and organogel. ACS Macro Lett. 2015, 4, 467–471. [Google Scholar] [CrossRef]
- Chiang, M.-Y.; Hsu, Y.-W.; Hsieh, H.-Y.; Chen, S.-Y.; Fan, S.-K. Constructing 3D heterogeneous hydrogels from electrically manipulated prepolymer droplets and crosslinked microgels. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Matyjaszewski, K. Controlled living radical polymerization—Atom-transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J.H. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” atom transfer radical polymerization: From process design to preparation of well-defined environmentally friendly polymeric materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Seiffert, S. Nanostructural heterogeneity in polymer networks and gels. Polym. Chem. 2015, 6, 5515–5528. [Google Scholar] [CrossRef]
- Kurochkin, S.A.; Grachev, V.P. Reversible deactivation radical polymerization of polyfunctional monomers. Polym. Sci. Ser. C 2015, 57, 20–31. [Google Scholar] [CrossRef]
- Gao, H.; Miasnikova, A.; Matyjaszewski, K. Effect of cross-linker reactivity on experimental gel points during atrcp of monomer and cross-linker. Macromolecules 2008, 41, 7843–7849. [Google Scholar] [CrossRef]
- Ide, N.; Fukuda, T. Nitroxide-controlled free-radical copolymerization of vinyl and divinyl monomers. 2. Gelation. Macromolecules 1999, 32, 95–99. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, S.; Zhang, H.; Ding, Y.; Zhu, S. Comparison of reaction kinetics and gelation behaviors in atom transfer, reversible addition–fragmentation chain transfer and conventional free radical copolymerization of oligo(ethylene glycol) methyl ether methacrylate and oligo(ethylene glycol) dimethacrylate. Polymer 2009, 50, 3488–3494. [Google Scholar] [CrossRef]
- Gao, H.; Min, K.; Matyjaszewski, K. Determination of gel point during atom transfer radical copolymerization with cross-linker. Macromolecules 2007, 40, 7763–7770. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Polanowski, P.; Jeszka, J.K.; Krysiak, K.; Matyjaszewski, K. Influence of intramolecular crosslinking on gelation in living copolymerization of monomer and divinyl cross-linker. Monte Carlo simulation studies. Polymer 2015, 79, 171–178. [Google Scholar] [CrossRef]
- Polanowski, P.; Jeszka, J.K.; Li, W.; Matyjaszewski, K. Effect of dilution on branching and gelation in living copolymerization of monomer and divinyl cross-linker: Modeling using dynamic lattice liquid model (DLL) and flory-stockmayer (FS) model. Polymer 2011, 52, 5092–5101. [Google Scholar] [CrossRef]
- Polanowski, P.; Jeszka, J.K.; Matyjaszewski, K. Modeling of branching and gelation in living copolymerization of monomer and divinyl cross-linker using dynamic lattice liquid model (DLL) and flory-stockmayer model. Polymer 2010, 51, 6084–6092. [Google Scholar] [CrossRef]
- Pastorczak, M.; Okrasa, L.; Yoon, J.A.; Kowalewski, T.; Matyjaszewski, K. Kinetics of the temperature-induced volume phase transition in poly(2-(2-methoxyethoxy)ethyl methacrylate) hydrogels of various topologies. Polymer 2017, 110, 25–35. [Google Scholar] [CrossRef]
- Yoon, J.A.; Bencherif, S.A.; Aksak, B.; Kim, E.K.; Kowalewski, T.; Oh, J.K.; Matyjaszewski, K. Thermoresponsive hydrogel scaffolds with tailored hydrophilic pores. Chem. Asian J. 2011, 6, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.A.; Oh, J.K.; Li, W.; Kowalewski, T.; Matyjaszewski, K. Atrp: A versatile tool toward uniformly crosslinked hydrogels with controlled architecture and multifunctionality. In Hydrogel Micro and Nanoparticles; Lyon, L.A., Serpe, M.J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 169–186. [Google Scholar]
- Gao, H.; Polanowski, P.; Matyjaszewski, K. Gelation in living copolymerization of monomer and divinyl cross-linker: Comparison of atrp experiments with monte carlo simulations. Macromolecules 2009, 42, 5925–5932. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Guo, X.; Zhang, H. Ambient temperature synthesis of narrow or monodisperse, highly cross-linked, and “living” polymer microspheres by atom transfer radical precipitation polymerization. RSC Adv. 2012, 2, 5651–5662. [Google Scholar] [CrossRef]
- Krys, P.; Matyjaszewski, K. Kinetics of atom transfer radical polymerization. Eur. Polym. J. 2017, 89, 482–523. [Google Scholar] [CrossRef]
- Yong, X.; Simakova, A.; Averick, S.; Gutierrez, J.; Kuksenok, O.; Balazs, A.C.; Matyjaszewski, K. Stackable, covalently fused gels: Repair and composite formation. Macromolecules 2015, 48, 1169–1178. [Google Scholar] [CrossRef]
- Beziau, A.; Singh, A.; de Menezes, R.N.L.; Ding, H.; Simakova, A.; Kuksenok, O.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. Miktoarm star copolymers as interfacial connectors for stackable amphiphilic gels. Polymer 2016, 101, 406–414. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.; Braunecker, W.A.; Tsarevsky, N.V. Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 15309–15314. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Magenau, A.J.D.; Averick, S.E.; Simakova, A.; He, H.; Matyjaszewski, K. Icar atrp with ppm cu catalyst in water. Macromolecules 2012, 45, 4461–4468. [Google Scholar] [CrossRef]
- Tyeklar, Z.; Jacobson, R.R.; Wei, N.; Murthy, N.N.; Zubieta, J.; Karlin, K.D. Reversible reaction of dioxygen (and carbon monoxide) with a copper(I) complex. X-ray structures of relevant mononuclear Cu(I) precursor adducts and the trans-(.mu.-1,2-peroxo)dicopper(II) product. J. Am. Chem. Soc. 1993, 115, 2677–2689. [Google Scholar] [CrossRef]
- Xia, J.; Matyjaszewski, K. Controlled/“living” radical polymerization. Atom transfer radical polymerization catalyzed by Copper(I) and picolylamine complexes. Macromolecules 1999, 32, 2434–2437. [Google Scholar] [CrossRef]
- Lee, S.B.; Russell, A.J.; Matyjaszewski, K. Atrp synthesis of amphiphilic random, gradient, and block copolymers of 2-(dimethylamino)ethyl methacrylate and n-butyl methacrylate in aqueous media. Biomacromolecules 2003, 4, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Singh, A.; Beziau, A.; Kowalewski, T.; Matyjaszewski, K.; Balazs, A.C. Modeling the formation of layered, amphiphilic gels. Polymer 2017, 111, 214–221. [Google Scholar] [CrossRef]
- Yoon, J.A.; Gayathri, C.; Gil, R.R.; Kowalewski, T.; Matyjaszewski, K. Comparison of the thermoresponsive deswelling kinetics of poly(2-(2-methoxyethoxy)ethyl methacrylate) hydrogels prepared by ATRP and FRP. Macromolecules 2010, 43, 4791–4797. [Google Scholar] [CrossRef]

| Abb. | Gel | Method | Solvent | Rad. In. | [M]:[I]:[X]:[CuBr2]:[TPMA]:[Rad.In.] 1 |
|---|---|---|---|---|---|
| DA | PDMAEMA | ICAR ATRP | Water | VA-44 | 75:1:5:0.1:0.8:0.1 |
| BA | PBMA | ICAR ATRP | Toluene | V-70 | 75:1:5:0.1:0.8:0.9 |
| DF | PDMAEMA | FRP | Water | VA-44 | 75:0:5:0:0:0.1 |
| BF | PBMA | FRP | Toluene | V-70 | 75:0:5:0:0:0.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beziau, A.; De Menezes, R.N.L.; Biswas, S.; Singh, A.; Cuthbert, J.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. Combining ATRP and FRP Gels: Soft Gluing of Polymeric Materials for the Fabrication of Stackable Gels. Polymers 2017, 9, 186. https://doi.org/10.3390/polym9060186
Beziau A, De Menezes RNL, Biswas S, Singh A, Cuthbert J, Balazs AC, Kowalewski T, Matyjaszewski K. Combining ATRP and FRP Gels: Soft Gluing of Polymeric Materials for the Fabrication of Stackable Gels. Polymers. 2017; 9(6):186. https://doi.org/10.3390/polym9060186
Chicago/Turabian StyleBeziau, Antoine, Rafael N. L. De Menezes, Santidan Biswas, Awaneesh Singh, Julia Cuthbert, Anna C. Balazs, Tomasz Kowalewski, and Krzysztof Matyjaszewski. 2017. "Combining ATRP and FRP Gels: Soft Gluing of Polymeric Materials for the Fabrication of Stackable Gels" Polymers 9, no. 6: 186. https://doi.org/10.3390/polym9060186
APA StyleBeziau, A., De Menezes, R. N. L., Biswas, S., Singh, A., Cuthbert, J., Balazs, A. C., Kowalewski, T., & Matyjaszewski, K. (2017). Combining ATRP and FRP Gels: Soft Gluing of Polymeric Materials for the Fabrication of Stackable Gels. Polymers, 9(6), 186. https://doi.org/10.3390/polym9060186








