Potassium Iodide-Functionalized Polyaniline Nanothin Film Chemiresistor for Ultrasensitive Ozone Gas Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensor Fabrication
2.2. Gas Sensing
3. Results and Discussion
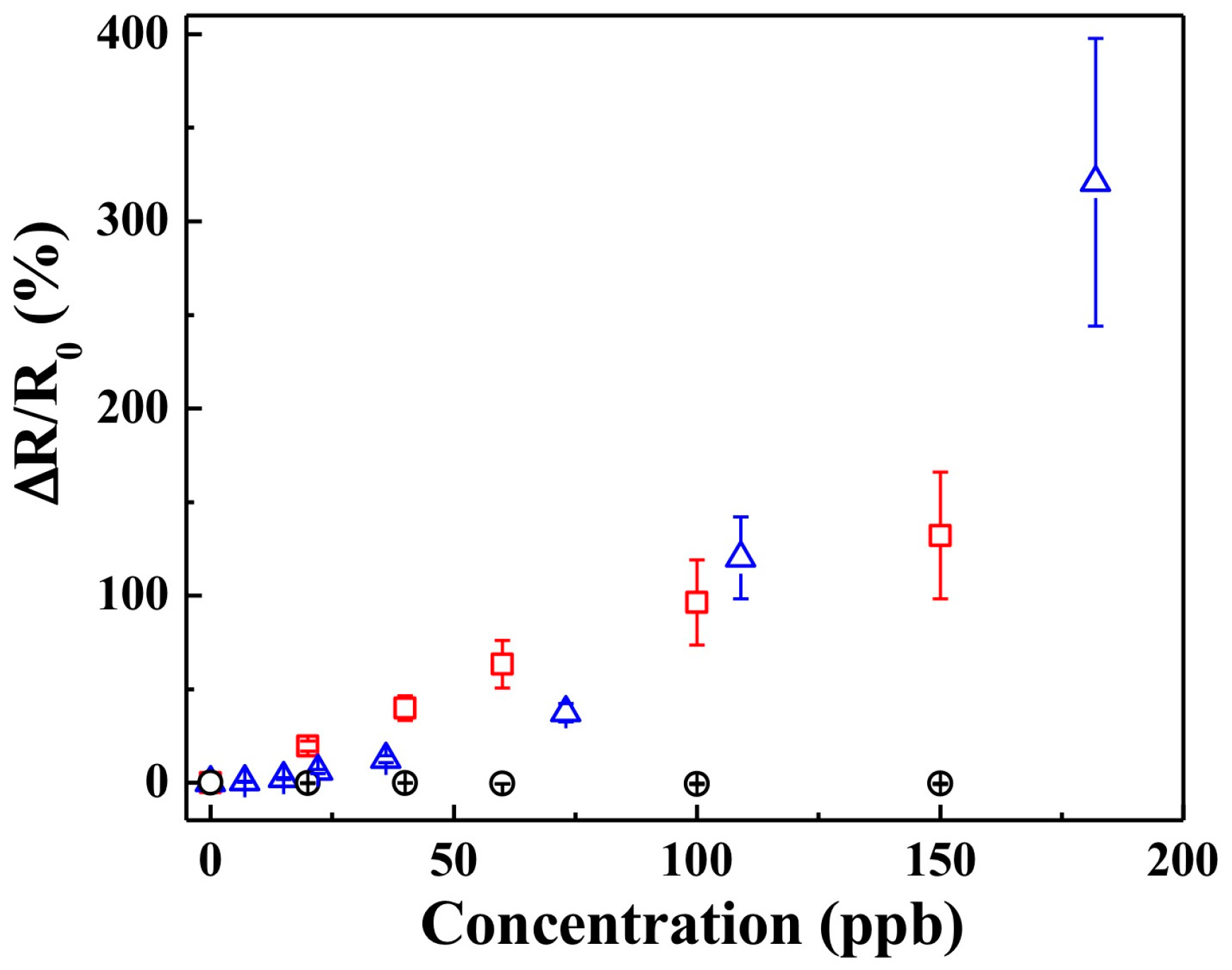
3.1. Sensing with Pristine PANI Nanothin Film
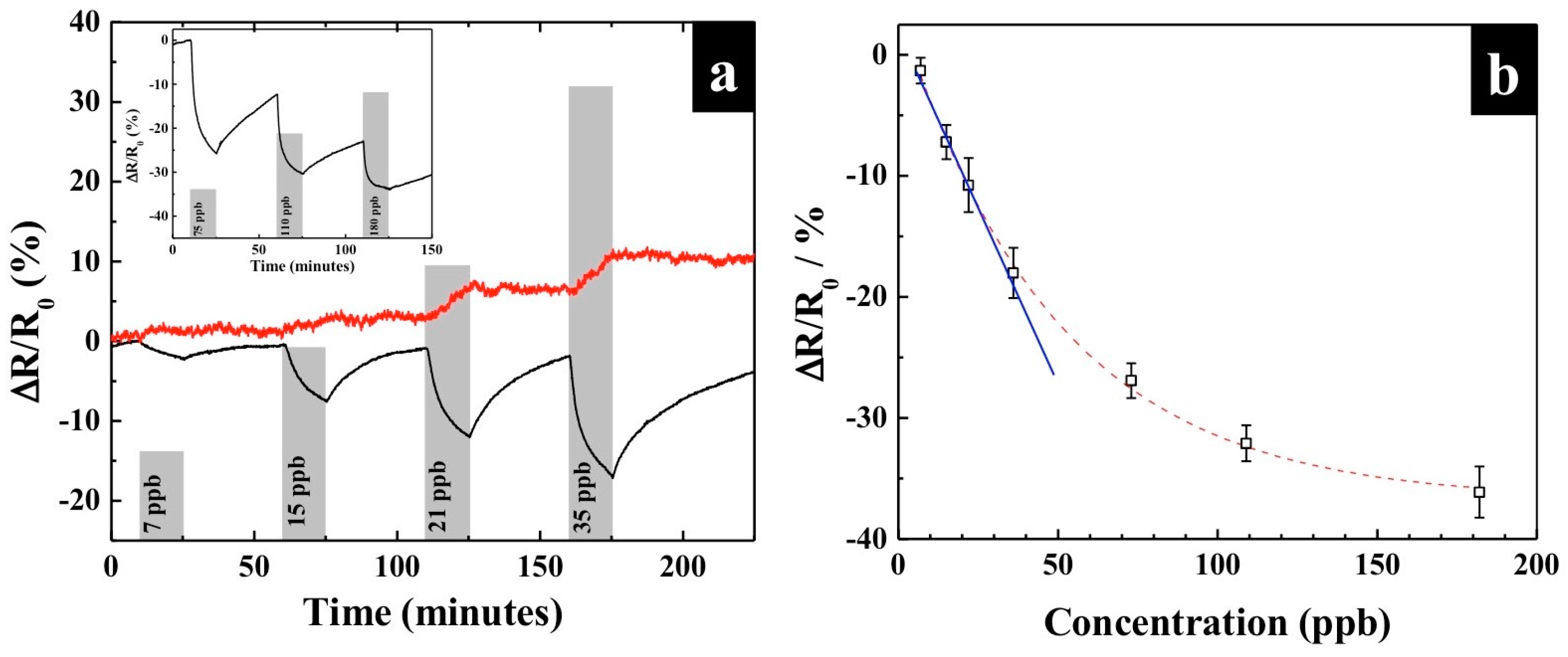
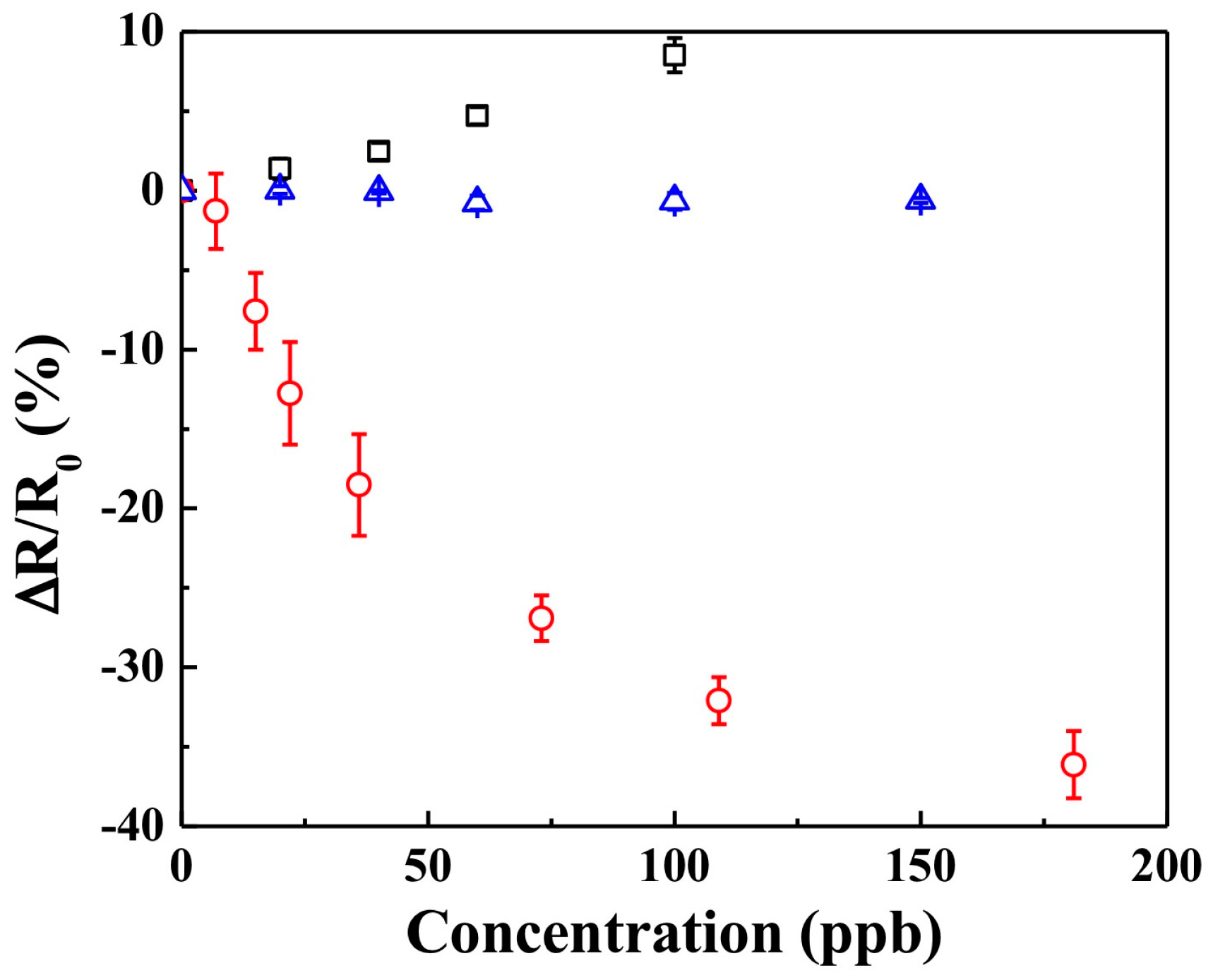
3.2. Sensing with KI-PANI Nanothin Film
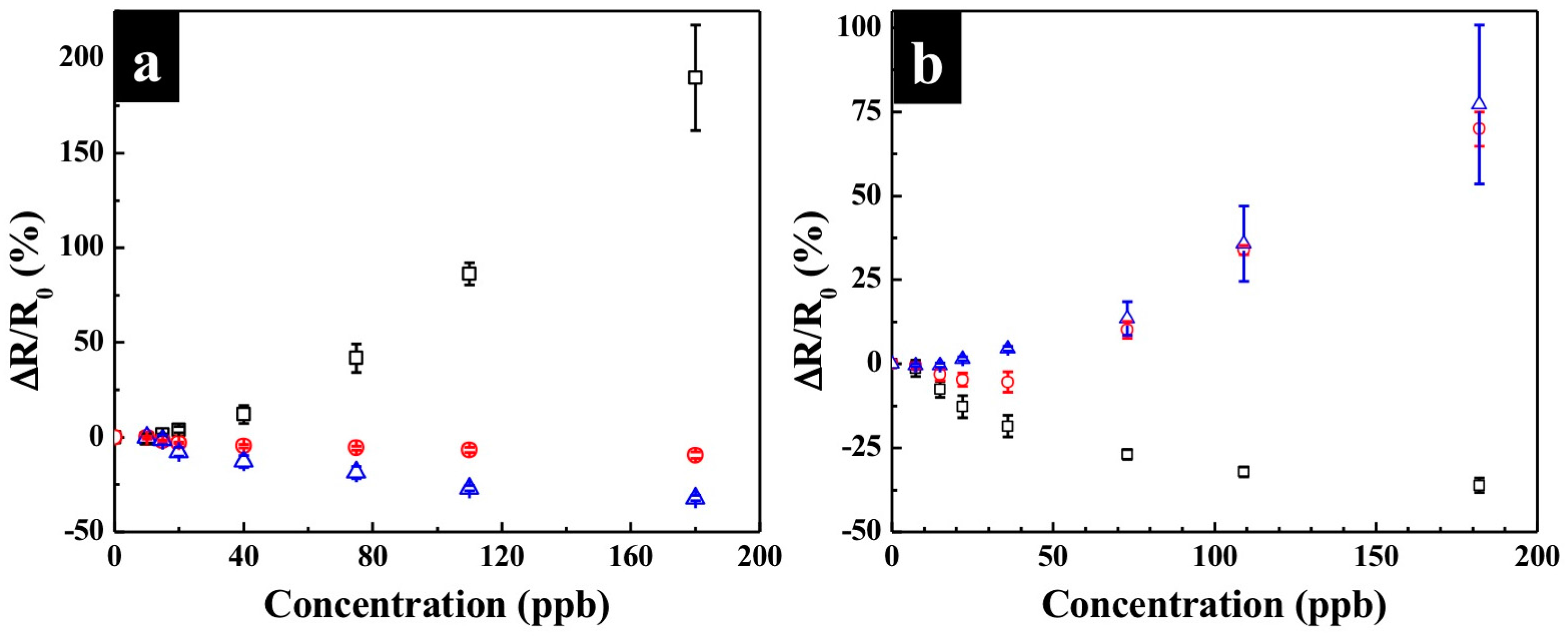
3.3. Effect of Humidity and Temperature
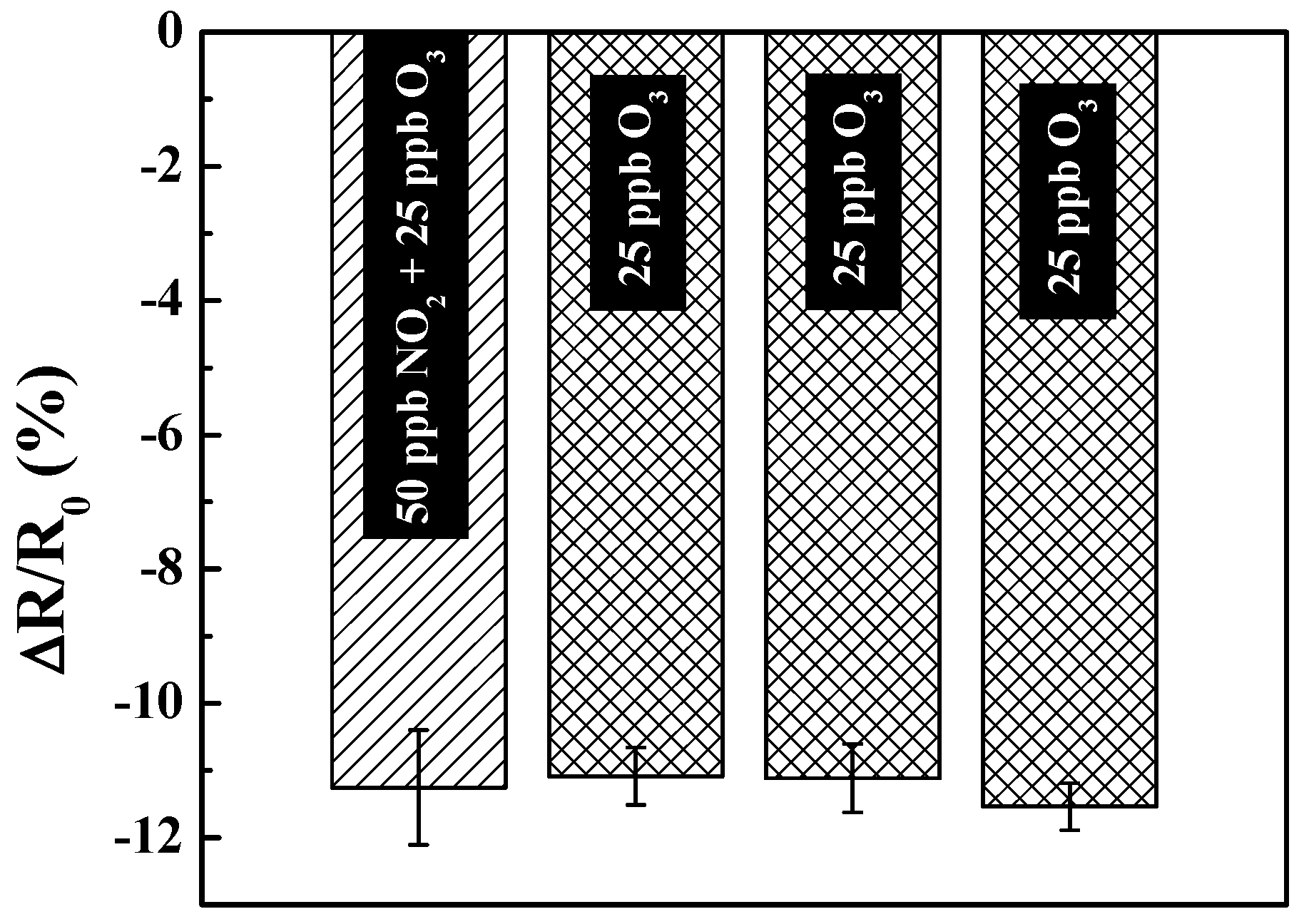
3.4. Cross-Reactivity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Byers, D.H.; Saltzman, B.E. Determination of Ozone in Air by Neutral and Alkaline Iodide Procedures. In Ozone Chemistry and Technology; American Chemical Society: Washington, DC, USA, 1959; pp. 93–101. [Google Scholar]
- Klausen, J.; Zellweger, C.; Buchmann, B.; Hofer, P. Uncertainty and bias of surface ozone measurements at selected Global Atmosphere Watch sites. J. Geophys. Res. 2003, 108, 4622. [Google Scholar] [CrossRef]
- Wang, R.; Tsow, F.; Zhang, X.; Peng, J.-H.; Forzani, E.S.; Chen, Y.; Crittenden, J.C.; Destaillats, H.; Tao, N. Real-Time Ozone Detection Based on a Microfabricated Quartz Crystal Tuning Fork Sensor. Sensors 2009, 9, 5655–5663. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, A.; Broder, B. Interference of some trace gases with ozone measurements by the KI method. Atmos. Environ. (1967) 1982, 16, 2187–2190. [Google Scholar] [CrossRef]
- Huang, H.; Dasgupta, P.K. Electrochemical sensing of gases based on liquid collection interfaces. Electroanalysis 1997, 9, 585–591. [Google Scholar] [CrossRef]
- Deng, Z.; Fang, X.; Li, D.; Zhou, S.; Tao, R.; Dong, W.; Wang, T.; Meng, G.; Zhu, X. Room temperature ozone sensing properties of p-type transparent oxide CuCrO2. J. Alloys Compd. 2009, 484, 619–621. [Google Scholar] [CrossRef]
- Viricelle, J.P.; Pauly, A.; Mazet, L.; Brunet, J.; Bouvet, M.; Varenne, C.; Pijolat, C. Selectivity improvement of semi-conducting gas sensors by selective filter for atmospheric pollutants detection. Mater. Sci. Eng. C 2006, 26, 186–195. [Google Scholar] [CrossRef]
- Park, Y.; Dong, K.-Y.; Lee, J.; Choi, J.; Bae, G.-N.; Ju, B.-K. Development of an ozone gas sensor using single-walled carbon nanotubes. Sens. Actuators B 2009, 140, 407–411. [Google Scholar] [CrossRef]
- Srinives, S.; Sarkar, T.; Hernandez, R.; Mulchandani, A. A miniature chemiresistor sensor for carbon dioxide. Anal. Chim. Acta 2015, 874, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Srinives, S.; Sarkar, T.; Mulchandani, A. Nanothin Polyaniline Film for Highly Sensitive Chemiresistive Gas Sensing. Electroanalysis 2013, 25, 1439–1445. [Google Scholar] [CrossRef]
- Srinives, S.; Sarkar, T.; Mulchandani, A. Primary amine-functionalized polyaniline nanothin film sensor for detecting formaldehyde. Sens. Actuators B 2014, 194, 255–259. [Google Scholar] [CrossRef]
- Cataldo, F. On the action of ozone on polyaniline. Polym. Degrad. Stab. 2002, 75, 93–98. [Google Scholar] [CrossRef]
- Xie, D.; Jiang, Y.; Pan, W.; Li, D.; Wu, Z.; Li, Y. Fabrication and characterization of polyaniline-based gas sensor by ultra-thin film technology. Sens. Actuators B 2002, 81, 158–164. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.; Bowmaker, G.A. Iodine vapour doped polyaniline. Polymer 2008, 49, 3070–3075. [Google Scholar] [CrossRef]
- Adhikari, S.; Banerji, P. Enhanced conductivity in iodine doped polyaniline thin film formed by thermal evaporation. Thin Solid Films 2010, 518, 5421–5425. [Google Scholar] [CrossRef]
- Stergiou, D.V.; Prodromidis, M.I.; Efstathiou, C.E. On the possibility of a pH-metric determination of ozone. Electrochem. Commun. 2010, 12, 262–265. [Google Scholar] [CrossRef]
- Brown, M.A.; Ashby, P.D.; Krisch, M.J.; Liu, Z.; Mun, B.S.; Green, R.G.; Giorgi, J.B.; Hemminger, J.C. Interfacial Dushman-like Chemistry in Hydrated KIO3 Layers Grown on KI. J. Phys. Chem. C 2010, 114, 14093–14100. [Google Scholar] [CrossRef]
- Brown, M.A.; Newberg, J.T.; Krisch, M.J.; Mun, B.S.; Hemminger, J.C. Reactive Uptake of Ozone on Solid Potassium Iodide. J. Phys. Chem. C 2008, 112, 5520–5525. [Google Scholar] [CrossRef]
- Sedlak, J.M.; Blurton, K.F. A new electrochemical analyser for nitric oxide and nitrogen dioxide. Talanta 1976, 23, 811–814. [Google Scholar] [CrossRef]
- Sedlak, J.M.; Blurton, K.F. The Electrochemical Reactions of Carbon Monoxide, Nitric Oxide, and Nitrogen Dioxide at Gold Electrodes. J. Electrochem. Soc. 1976, 123, 1476–1478. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinives, S.; Sarkar, T.; Hernandez, R.; Mulchandani, A. Potassium Iodide-Functionalized Polyaniline Nanothin Film Chemiresistor for Ultrasensitive Ozone Gas Sensing. Polymers 2017, 9, 80. https://doi.org/10.3390/polym9030080
Srinives S, Sarkar T, Hernandez R, Mulchandani A. Potassium Iodide-Functionalized Polyaniline Nanothin Film Chemiresistor for Ultrasensitive Ozone Gas Sensing. Polymers. 2017; 9(3):80. https://doi.org/10.3390/polym9030080
Chicago/Turabian StyleSrinives, Sira, Tapan Sarkar, Raul Hernandez, and Ashok Mulchandani. 2017. "Potassium Iodide-Functionalized Polyaniline Nanothin Film Chemiresistor for Ultrasensitive Ozone Gas Sensing" Polymers 9, no. 3: 80. https://doi.org/10.3390/polym9030080
APA StyleSrinives, S., Sarkar, T., Hernandez, R., & Mulchandani, A. (2017). Potassium Iodide-Functionalized Polyaniline Nanothin Film Chemiresistor for Ultrasensitive Ozone Gas Sensing. Polymers, 9(3), 80. https://doi.org/10.3390/polym9030080







