Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids
Abstract
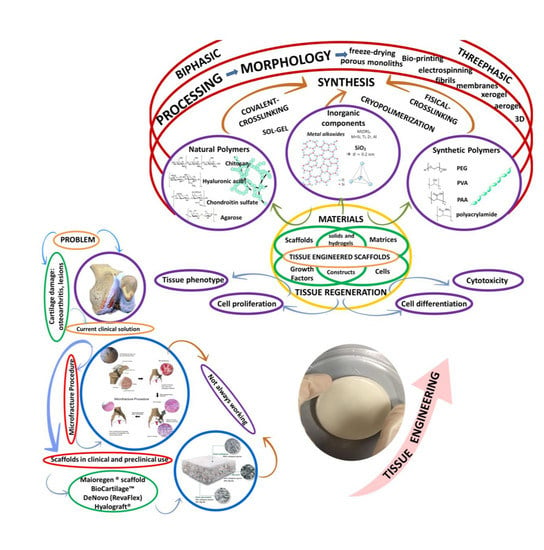
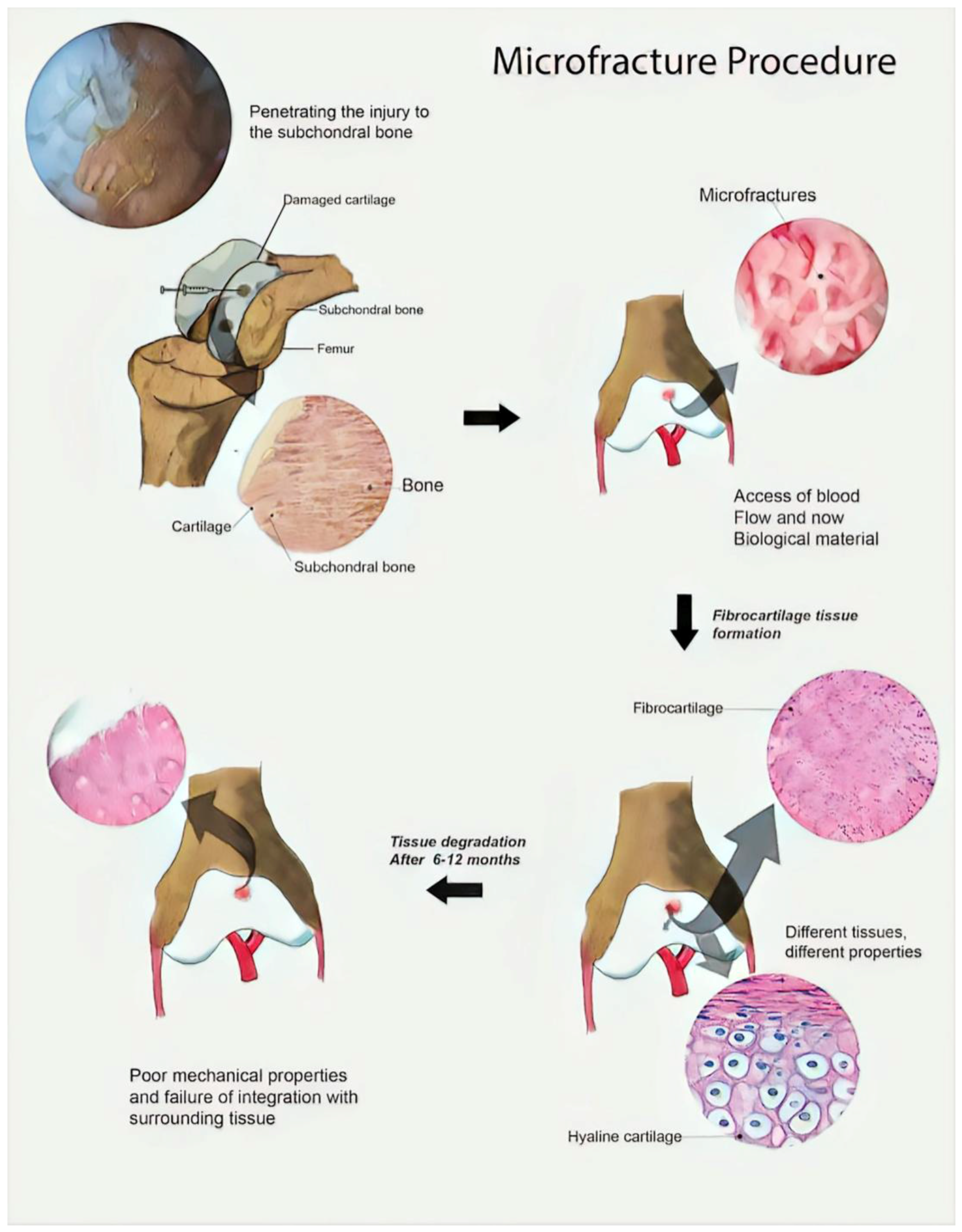
1. Introduction: Current Clinical Approaches and the Need for New Developments
2. Hydrogels in Cartilage Regeneration
2.1. Cell-Free Hydrogel Scaffolds
2.2. Cell-Seeded Hydrogel Scaffolds
3. Polysaccharides Versus Synthetic Hydrogels
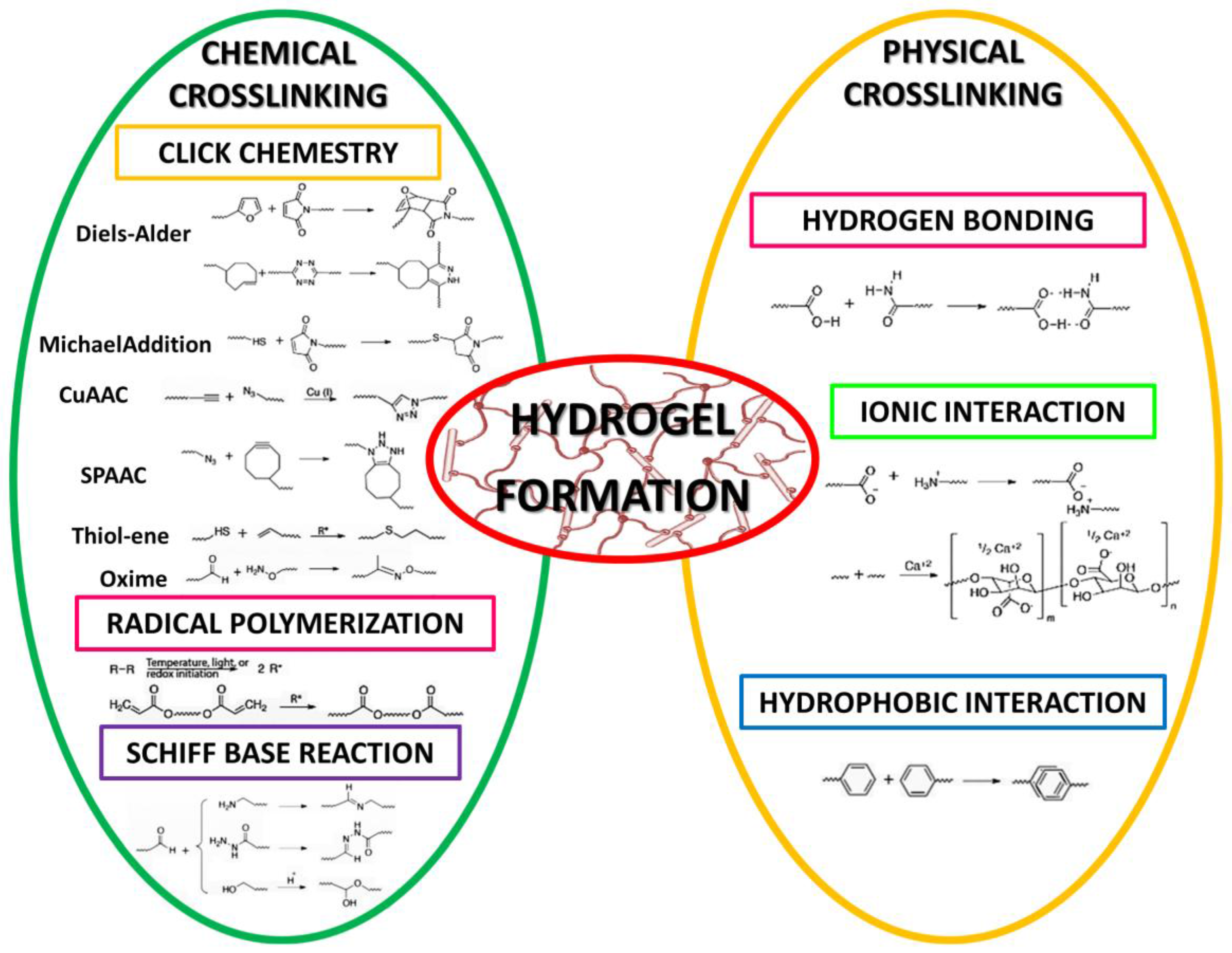
3.1. Manufacturing Techniques and Their Influence on Hydrogel Properties
3.2. Degradation Kinetics, Physical Properties (Applicability) and Biological Effects
3.3. Specificities of Polysaccharide-Based Hydrogels
3.4. Biological Response of Polysaccharide-Based Hydrogels
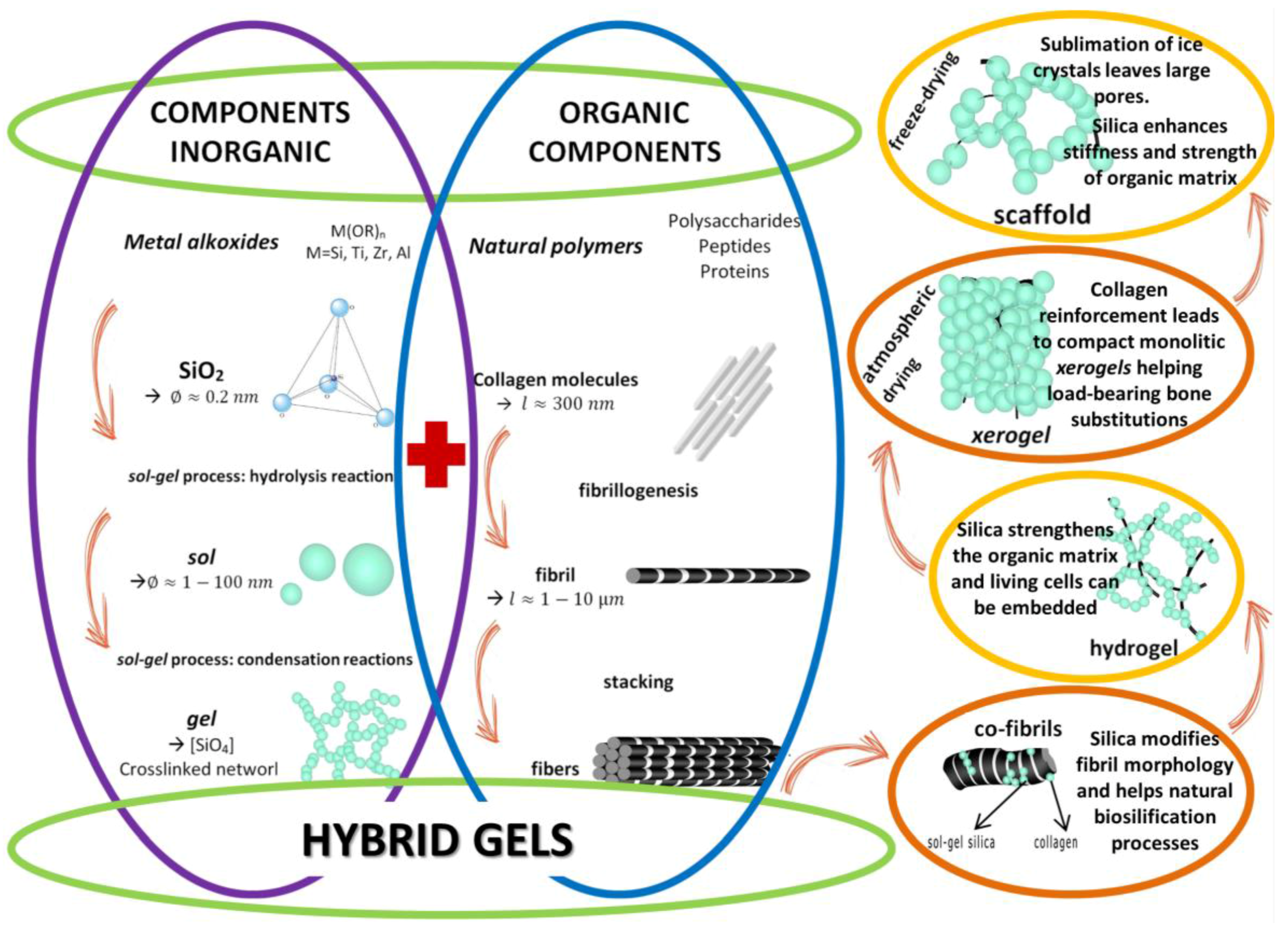
4. Future Trends: From Combination of Materials to Hybrid Hydrogels
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fickert, S.; Schattenberg, T.; Niks, M.; Weiss, C.; Their, S. Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: A case series. Arch. Orthop. Trauma Surg. 2014, 134, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Baynat, C.; Andro, C.; Vincent, J.P.; Schiele, P.; Buisson, P.; Dubrana, F.; Gunepin, F.X. Actifit® synthetic meniscal substitute: Experience with 18 patients in Brest, France. Orthop. Traumatol. Surg. Res. 2014, 100 (Suppl. 8), S385–S389. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, P.; Dhollander, A.; Almqvist, K.F.; Verdonk, R.; Victor, J. Treatment of osteochondral lesions in the knee using a cell-free scaffold. Bone Jt. J. 2015, 97B, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Case, J.M.; Scopp, J.M. Treatment of articular cartilage defects of the knee with microfracture and enhanced microfacture techniques. Sports Med. Arthrosc. 2016, 24, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Erggelet, C. Enhanced marrow stimulation techniques for cartilage repair. Oper. Tech. Orthop. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Gille, J.; Behrens, P.; Volpi, P.; de Girolamo, L.; Reiss, E.; Zoch, W.; Anders, S. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: Data of the AMIC registry. Arch. Orthop. Trauma Surg. 2013, 133, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Turajane, T.; Thitiset, T.; Honsawek, S.; Chaveewanakorn, U.; Aojanepong, J.; Papadopoulos, K.I. Assessment of chondrogenic differentiation potential of autologous activated peripheral blood stem cells on human early osteoarthritic cancellous tibial bone scaffold. Musculoskelet. Surg. 2014, 98, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Schexnailder, P.J.; Shmidt, G. Chapter 24: Nanocomposite polymer biomaterials for tissue repair of bone and cartilage: A material science perspective. In Nanomaterials Handbook; Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2011; pp. 1–20. [Google Scholar]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Xia, C.; Sherman, O.; Strauss, E.; Jazrawi, L.; Recht, M.P.; Regatte, R.R. High resolution morphologic imaging and T2 mapping of cartilage at 7 tesla: Comparison of cartilage repair patients and healthy controls. Magn. Reson. Mater. Phys. 2013, 26, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Chevrier, A.; Lascau-Coman, V.; Rivard, G.E.; Hoemann, C.D. Stereological analysis of subchondral angiogenesis induced by chitosan and coagulation factors in microdrilled articular cartilage defects. Osteoarthr. Cartil. 2013, 21, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Siclari, A.; Mascaro, G.; Gentili, C.; Kaps, C.; Cancedda, R.; Boux, E. Cartilage repair in the knee with subchondral drilling augmented with a platelet-rich plasma-immersed polymer-based implant. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Accardi, M.A.; McCullen, S.D.; Callanan, A.; Chung, S.; Cann, P.M.; Stevens, M.M.; Dini, D. Effects of fiber orientation on the frictional properties and damage of regenerative articular cartilage surfaces. Tissue Eng. Part A 2013, 19, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Lebourg, M.; Rochina, J.R.; Sousa, T.; Mano, J.; Ribelles, J.L. Different hyaluronic acid morphology modulates primary articular chondrocyte behavior in hyaluronic acid-coated polycaprolactone scaffolds. J. Biomed. Mater. Res. Part A 2013, 101A, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Levorson, E.J.; Hu, O.; Mountziaris, P.M.; Kasper, F.K.; Mikos, A.G. Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, Part 2: Construct devitalization and determination of chondroinductive capacity. Tissue Eng. Part C Methods 2014, 20, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Matsiko, A.; Dickson, G.R.; O’Brien, F.J.; Gleeson, J.P. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014, 10, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, A.J.; Beck, E.C.; Dennis, S.C.; Converse, G.L.; Hopkins, R.A.; Berkland, C.J.; Detamore, M.S. Decellularized cartilage may be a chondroinductive material for osteochondral tissue engineering. PLoS ONE 2015, 10, e0121966. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Tello, M.; Forriol, F.; Gastaldi, P.; Ruiz-Saurí, A.; Martín de Llano, J.J.; Novella-Maestre, E.; Antolinos-Turpín, C.M.; Gómez-Tejedor, J.A.; Gómez Ribelles, J.L.; Carda, C. Time evolution of in vivo articular cartilage repair induced by bone marrow stimulation and scaffold implantation in rabbits. Int. J. Artif. Organs 2015, 38, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Q.; Cheng, N.; Tao, X.; Zhang, Z.; Sun, X.; Zhang, Q. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater. Sci. Eng. C 2016, 61, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhang, X.; Pi, Y.; Yin, L.; Li, L.; Chen, H.; Zhou, C.; Ao, Y. Surface modification on polycaprolactone electrospun mesh and human decalcified bone scaffold with synovium-derived mesenchymal stem cells-affinity peptide for tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103A, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, C.L.; Chen, W.J.; Zhang, Q.; Wang, S.L. Dynamic compression combined with exogenous SOX-9 promotes chondrogenesis of adipose-derived mesenchymal stem cells in PLGA scaffold. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2671–2678. [Google Scholar] [PubMed]
- Lehmann, M.; Martin, F.; Mannigel, K.; Kaltschmidt, K.; Sack, U.; Anderer, U. Three-dimensional scaffold-free fusion culture: The way to enhanced chondrogenesis of in vitro propagated human articular chondrocytes. Eur. J. Histochem. 2013, 57, e31. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Sun, L.; Cairns, D.M.; Rainbow, R.S.; Preda, R.C.; Kaplan, D.L.; Zeng, L. The influence of scaffold material on chondrocytes under inflammatory conditions. Acta Biomater. 2013, 9, 6563–6575. [Google Scholar] [CrossRef] [PubMed]
- Pretzel, D.; Linss, S.; Ahrem, H.; Endres, M.; Kaps, C.; Klemm, D.; Kinne, R.W. A novel in vitro bovine cartilage punch model for assessing the regeneration of focal cartilage defects with biocompatible bacterial nanocellulose. Arthritis Res. Ther. 2013, 15, R59. [Google Scholar] [CrossRef] [PubMed]
- Chomchalao, P.; Pongcharoen, S.; Sutheerawattananonda, M.; Tiyaboonchai, W. Fibroin and fibroin blended three-dimensional scaffolds for rat chondrocyte culture. Biomed. Eng. Online 2013, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Lee, J.Y.; Joo, S.Y.; Lee, Y.S.; Jeong, C. Transplantation of a scaffold-free cartilage tissue analogue for the treatment of physeal cartilage injury of the proximal tibia in rabbits. Yonsei Med. J. 2016, 57, 441–448. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, N.A.; Kobayashi, S.; Ranka, M.P.; Zaleski, K.L.; Yaremchuk, M.J.; Bonassar, L.J.; Randolph, M.A. Adhesion and integration of tissue engineered cartilage to porous polyethylene for composite ear reconstruction. J. Biomed. Mater. Res. Part B 2015, 103B, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, I.; Lips, K.S.; Sommer, U.; Schappat, I.; Martin, A.P.; Szalay, G.; Schnettler, R. Allogenous bone with collagen for repair of deep osteochondral defects. J. Surg. Res. 2013, 185, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.J.; Lam, C.F.; Lin, C.C.; Chen, W.L.; Li, C.F.; Lin, Y.T.; Yeh, M.L. Transplantation of autologous endothelial progenitor cells in porous PLGA scaffolds create a microenvironment for the regeneration of hyaline cartilage in rabbits. Osteoarthr. Cartil. 2013, 21, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lee, A.; Choi, K.; Kim, K.; Youn, I.; Trippel, S.B.; Panitch, A. Biomimetic aggrecan reduces cartilage extracellular matrix from degradation and lowers catabolic activity in ex vivo and in vivo models. Macromol. Biosci. 2013, 13, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Qiao, Z.; Jiang, W.; Li, H.; Wei, J.; Zhou, G.; Dai, K. Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials 2013, 34, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Waldorff, E.I.; Roessler, B.J.; Zachos, T.A.; Miller, B.S.; McHugh, J.; Goldstein, S.A. Preclinical evaluation of a novel implant for treatment of a full-thickness distal femoral focal cartilage defect. J. Arthroplast. 2013, 28, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Weder, C.; Foster, E.J. Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials 2016, 74, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Wanivenhaus, F.; Ng, K.W.; Doty, S.; Warren, R.F.; Maher, S.A. Matrix generation within a macroporous non-degradable implant for osteochondral defects is not enhanced with partial enzymatic digestion of the surrounding tissue: Evaluation in an in vivo rabbit model. J. Mater. Sci. Mater. Med. 2013, 24, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Irion, V.H.; Flanigan, D.C. New and emerging techniques in cartilage repair: Other scaffold-based cartilage treatment options. Oper. Tech. Sports Med. 2013, 21, 125–137. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, D.Y.; Cho, Y.H.; Chang, J.W.; Choi, J.W.; Park, J.K.; Min, B.H.; Shin, Y.S.; Kim, C.H. Cultured chondrocyte and porcine cartilagederived substance (PCS) construct as a possible dorsal augmentation material in rhinoplasty: A preliminary animal study. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Perdisa, F.; Di Matteo, B.; Di Martino, A.; Iacono, F.; Zaffagnini, S.; Balboni, F.; Vaccari, V.; Marcacci, M. Osteochondral scaffold reconstruction for complex knee lesions: A comparative evaluation. Knee 2013, 20, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Di Martino, A.; Busacca, M.; Moio, A.; Perdisa, F.; Marcacci, M. Clinical results and mri evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am. J. Sports Med. 2014, 42, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Perdisa, F.; Di Martino, A.; Busacca, M.; Balboni, F.; Sessa, A.; Marcacci, M. A one-step treatment for chondral and osteochondral knee defects: Clinical results of a biomimetic scaffold implantation at 2 years of follow-up. J. Mater. Sci. Mater. Med. 2014, 25, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Caminal, M.; Moll, X.; Codina, D.; Rabanal, R.M.; Morist, A.; Barrachina, J.; Garcia, F.; Pla, A.; Vives, J. Transitory improvement of articular cartilage characteristics after implantation of polylactide: Polyglycolic acid (PLGA) scaffolds seeded with autologous mesenchymal stromal cells in a sheep model of critical-sized chondral defect. Biotechnol. Lett. 2014, 36, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Delcogliano, M.; de Caro, F.; Scaravella, E.; Ziveri, G.; De Biase, C.F.; Marotta, D.; Marenghi, P.; Delcogliano, A. Use of innovative biomimetic scaffold in the treatment for large osteochondral lesions of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- McCormick, F.; Cole, B.J.; Nwachukwu, B.; Harris, J.D.; Adkisson, H.D.; Farr, J. Treatment of focal cartilage defects with a juvenile allogeneic 3-dimensional articular cartilage graft. Oper. Techniq. Sports Med. 2013, 21, 95–99. [Google Scholar] [CrossRef]
- Chiang, H.; Liao, C.J.; Hsieh, C.H.; Shen, C.Y.; Huang, Y.Y.; Jiang, C.C. Clinical feasibility of a novel biphasic osteochondral compositefor matrix-associated autologous chondrocyte implantation. Osteoarthr. Cartil. 2013, 21, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Venieri, G.; Perdisa, F.; Marcacci, M. Tibial plateau lesions. Surface reconstruction with a biomimetic osteochondral scaffold: Results at 2 years of follow-up. Inj. Int. J. Care Inj. 2014, 45S, S121–S125. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.A.; Mall, N.A.; Fortier, L.A.; Roller, B.L.; Cole, B.J. BioCartilage: Background and operative technique. Oper. Tech. Sports Med. 2013, 21, 116–124. [Google Scholar] [CrossRef]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.E.; et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci. Transl. Med. 2013, 5, 167ra6. [Google Scholar] [CrossRef] [PubMed]
- Quarch, V.M.; Enderle, E.; Lotz, J.; Frosch, K.H. Fate of large donor site defects in osteochondral transfer procedures in the knee joint with and without TruFit plugs. Arch. Orthop. Trauma Surg. 2014, 134, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Bartha, L.; Hamann, D.; Pieper, J.; Péters, F.; Riesle, J.; Vajda, A.; Novak, P.K.; Hangody, L.R.; Vasarhelyi, G.; Bodó, L.; et al. A clinical feasibility study to evaluate the safety and efficacy of PEOT/PBT implants for human donor site filling during mosaicplasty. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Porcellini, G.; Merolla, G.; Campi, F.; Pellegrini, A.; Bodanki, C.S.; Paladini, P. Arthroscopic treatment of early glenohumeral arthritis. J. Orthop. Traumatol. 2013, 14, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Krase, A.; Abedian, R.; Steck, E.; Hurschler, C.; Richter, W. BMP activation and WNT-signalling affect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthr. Cartil. 2014, 22, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Panadero, J.A.; Vikingsson, L.V.; Gomez Ribelles, J.L.; Lanceros-Mendez, S.; Sencadas, V. In vitro mechanical fatigue behavior of poly-ε-caprolactone macroporous scaffolds for cartilage tissue engineering: Influence of pore filling by a poly(vinyl alcohol) gel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Motavalli, M.; Whitney, G.A.; Dennis, J.E.; Mansour, J.M. Investigating a continuous shear strain function for depth-dependent properties of native and tissue engineering cartilage using pixel-size data. J. Mech. Behav. Biomed. Mater. 2013, 28, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.A.; Moroni, L.; Riesle, J.; de Wijn, J.R.; van Blitterswijk, C.A. The effect of scaffold-cell entrapment capacity and physico-chemical properties on cartilage regeneration. Biomaterials 2013, 34, 4259–4265. [Google Scholar] [CrossRef] [PubMed]
- Griebel, A.J.; Khoshgoftar, M.; Novak, T.; van Donkelaar, C.C.; Neu, C.P. Direct noninvasive measurement and numerical modeling of depth-dependent strains in layered agarose constructs. J. Biomech. 2014, 47, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, K.J.; Park, S.Y.; Huh, J.E.; Kim, H.J.; Yu, W.R. 3D braid scaffolds for regeneration of articular cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 37–46. [Google Scholar] [CrossRef] [PubMed]
- MacBarb, R.F.; Chen, A.L.; Hu, J.C.; Athanasiou, K.A. Engineering functional anisotropy in fibrocartilage neotissues. Biomaterials 2013, 34, 9980–9989. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Pramanik, S.P.; Ataollahi, F.; Khalil, A.A.; Kamarul, T.; Pingguan-Murphy, B. A comparison study of different physical treatments on cartilage matrix derived porous scaffolds for tissue engineering applications. Sci. Technol. Adv. Mater. 2014, 15, 065001. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; McNary, S.M.; Athanasiou, K.A.; Reddi, A.H. Surface zone articular chondrocytes modulate the bulk and surface mechanical properties of the tissue-engineered cartilage. Tissue Eng. Part A 2014, 20, 3332–3341. [Google Scholar] [CrossRef] [PubMed]
- Causin, P.; Sacco, R.; Verri, M. A multiscale approach in the computational modeling of the biophysical environment in artificial cartilage tissue regeneration. Biomech. Model. Mechanobiol. 2013, 12, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Da, H.; Jia, S.J.; Meng, G.L.; Cheng, J.H.; Zhou, W.; Xiong, Z.; Mu, Y.J.; Liu, J. The impact of compact layer in biphasic scaffold on osteochondral tissue engineering. PLoS ONE 2013, 8, e54838. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Moriguchi, Y.; Ando, W.; Nansai, R.; Fujie, H.; Hart, D.A.; Gobbi, A.; Kita, K.; Horibe, S.; Shino, K.; et al. Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial mesenchymal stem cells and a hydroxyapatite-based artificial bone. Tissue Eng. Part A 2014, 20, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, Y.; Li, L.P. Influences of the depth-dependent material inhomogeneity of articular cartilage on the fluid pressurization in the human knee. Med. Eng. Phys. 2013, 35, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.M.; Ricken, T.; Holzapfel, G.A. Modeling sample/patient-specific structural and diffusional responses of cartilage using DT-MRI. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.P.; Guilak, F.; Jay, G.D.; Zauscher, S. Interaction of lubricin with type II collagen surfaces: Adsorption, friction, and normal forces. J. Biomech. 2014, 47, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.W.; Olszewska, A.; Osterberg, M.; Zhu, H.; Horn, R. A cartilage-inspired lubrication system. Soft Matter 2014, 10, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, K.C.; de Albuquerque Haach, L.C.; dos Santos, A.R., Jr.; de Almeida Rollo, J.M.D.; Bezerra de Menezes Reiff, R.; Minarelli Gaspar, A.M.; de Moraes Purquerio, B.; Fortulan, C.A. Cartilage reconstruction using self-anchoring implant with functional gradient. Mater. Res. 2014, 17, 638–649. [Google Scholar] [CrossRef]
- Utzschneider, S.; Lorber, V.; Dedic, M.; Paulus, A.C.; Schröder, C.; Gottschalk, O.; Schmitt-Sody, M.; Jansson, V. Biological activity and migration of wear particles in the knee joint: An in vivo comparison of six different polyethylene materials. J. Mater. Sci. Mater. Med. 2014, 25, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, S.; Meyer, C.; Ponomarev, I.; Barnewitz, D.; Resinger, C.; Klepal, W.; Albrecht, C.; Marlovits, S. Equine articular chondrocytes on MACT scaffolds for cartilage defect treatment. Anat. Histol. Embryol. 2013, 42, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Vahdati, A.; Wagner, D.R. Implant size and mechanical properties influence the failure of the adhesive bond between cartilage implants and native tissue in a finite element analysis. J. Biomech. 2013, 46, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Bulman, S.E.; Coleman, C.M.; Murphy, J.M.; Medcalf, N.; Ryan, A.E.; Barry, F. Pullulan: A new cytoadhesive for cell-mediated cartilage repair. Stem Cell Res. Ther. 2015, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Vikingsson, L.; Gómez-Tejedor, J.A.; Gallego Ferrer, G.; Gómez Ribelles, J.L. An experimental fatigue study of a porous scaffold for the regeneration of articular cartilage. J. Biomech. 2015, 48, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Delighkaris, K.; Tadele, T.S.; Olthuis, W.; van den Berg, A. Hydrogel-based devices for biomedical applications. Sens. Actuators B Chem. 2010, 147, 765–774. [Google Scholar] [CrossRef]
- Kim, I.L.; Mauck, R.L.; Burdick, J.A. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011, 32, 8771–8782. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.A.N.; Michelacci, Y.M.; Baccarin, R.Y.A.; Cogliati, B.; Silva, L.S.L.C. Evaluation of chitosan-GP hydrogel biocompatibility in osteochondral defects: An experimental approach. BMC Vet. Res. 2014, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Kazusa, H.; Nakasa, T.; Shibuya, H.; Ohkawa, S.; Kamei, G.; Adachi, N.; Deie, M.; Nakajima, N.; Hyon, S.H.; Ochi, M. Strong adhesiveness of a new biodegradable hydrogel glue, LYDEX, for use on articular cartilage. J. Appl. Biomater. Funct. Mater. 2013, 11, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Mesallati, T.; Buckley, C.T.; Kelly, D.J. Engineering articular cartilage-like grafts by self-assembly of infrapatellar fat pad-derived stem cells. Biotechnol. Bioeng. 2014, 111, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Lidgren, L.; Kumar, A. In vitro neo-cartilage formation on a three-dimensional composite polymeric cryogel matrix. Macromol. Biosci. 2013, 13, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Foss, C.; Merzari, E.; Migliaresi, C.; Motta, A. Silk fibroin/hyaluronic acid 3D matrices for cartilage tissue engineering. Biomacromolecules 2013, 14, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Boere, K.W.; Visser, J.; Seyednejad, H.; Rahimian, S.; Gawlitta, D.; van Steenbergen, M.J.; Dhert, W.J.; Hennink, W.E.; Vermonden, T.; Malda, J. Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 2014, 10, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.P.; Horejs, C.M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.; Stevens, M.M.; Paresh, A.; Parmar, L.W.C.; et al. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Omobono, M.A.; Zhao, X.; Furlong, M.A.; Kwon, C.H.; Gill, T.J.; Randolph, M.A.; Redmond, R.W. Enhancing the stiffness of collagen hydrogels for delivery of encapsulated chondrocytes to articular lesions for cartilage regeneration. J. Biomed. Mater. Res. Part A 2015, 103A, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Liao, I.C.; Moutos, F.T.; Estes, B.T.; Zhao, X.; Guilak, F. Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage. Adv. Funct. Mater. 2013, 23, 5833–5839. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, E.; Lobo, A.O.; Ferretti, M.; Cohen, M.; Marciano, F.R.; Corat, E.J.; Trava-Airoldi, V.J. An evaluation of chondrocyte morphology and gene expression on superhydrophilic vertically-aligned multi-walled carbon nanotube films. Mater. Sci. Eng. C 2013, 33, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. An anisotropic hydrogel with electrostatic repulsion between cofacially aligned nanosheets. Nature 2015, 517, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.; Bidini, A.B.; Lamponi, S.; Magnani, A. States of water, surface and rheological characterisation of a new biohydrogel as articular cartilage substitute. Polym. Adv. Technol. 2013, 24, 824–833. [Google Scholar] [CrossRef]
- Drira, Z.; Yadavalli, V.K. Nanomechanical measurements of polyethyleneglycol hydrogels using atomic force microscopy. J. Mech. Behav. Biomed. Mater. 2013, 18, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ronken, S.; Wirz, D.; Daniels, A.U.; Kurokawa, T.; Gong, J.P.; Arnold, M.P. Double-network acrylamide hydrogel compositions adapted to achieve cartilage-like dynamic stiffness. Biomech. Model. Mechanobiol. 2013, 12, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Liu, Y.; Kelly, D.J. Combining freshly isolated chondroprogenitor cells from the infrapatellar fat pad with a growth factor delivery hydrogel as a putative single stage therapy for articular cartilage repair. Tissue Eng. Part A 2014, 20, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Mesallati, T.; Buckley, C.T.; Kelly, D.J. A comparison of self-assembly and hydrogel encapsulation as a means to engineer functional cartilaginous grafts using culture expanded chondrocytes. Tissue Eng. Part C 2014, 20, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Rajagopal, K.; Vaikkath, D.; Nair, P.D.; Madhuri, V. Enhanced encapsulation of chondrocytes within a chitosan/ hyaluronic acid hydrogel: A new technique. Biotechnol. Lett. 2014, 36, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.W.; Park, Y.B.; Chung, J.Y.; Park, Y.G. Cartilage repair using composites of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel in a minipig model. Stem Cells Transl. Med. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Chen, X.; Zhang, Q.; Yu, F.; Li, Y.; Yao, Y. Redifferentiation of dedifferentiated chondrocytes in a novel three-dimensional microcavitary hydrogel. J. Biomed. Mater. Res. Part A 2015, 103A, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sakai, N.; Yamaguchi, T.; Yarimitsu, S.; Nakashima, K.; Sawae, Y.; Suzuki, A. Evaluation of a superior lubrication mechanism with biphasic hydrogels for artificial cartilage. Tribol. Int. 2015, 89, 19–26. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, D.; Cui, X.; Wang, Q. Research on swing friction lubrication mechanisms and the fluid load support characteristics of PVA–HA composite hydrogel. Tribol. Int. 2015, 90, 412–419. [Google Scholar] [CrossRef]
- Baykal, D.; Underwood, R.J.; Mansmann, K.; Marcolongo, M.; Kurtz, S.M. Evaluation of friction properties of hydrogels based on a biphasic cartilage model. J. Mech. Behav. Biomed. Mater. 2013, 28, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, J.; Bai, D.; Wang, H.; Zheng, X.; Guo, W.; Tian, W. Hemiarthroplasty of the shoulder joint using a custom-designed high-density nano-hydroxyapatite/polyamide prosthesiswith a polyvinyl alcohol hydrogel humeral head surface in rabbits. Artif. Organs 2014, 38, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-W.; Chen, W.-C.; Liu, H.-W.; Chen, C.-H. Application of synovial fluid mesenchymal stem cells: Platelet-rich plasma hydrogel for focal cartilage defect. J. Exp. Clin. Med. 2014, 6, 118–124. [Google Scholar] [CrossRef]
- Sridhar, B.V.; Brock, J.L.; Silver, J.S.; Leight, J.L.; Randolph, M.A.; Anseth, K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv. Healthc. Mater. 2015, 4, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, P.; Lai, J.H.; Dhulipala, L.; Behn, A.W.; Goodman, S.B.; Smith, R.L.; Maloney, W.J.; Yang, F.; Bhutani, N. Comparative potential of juvenile and adult human articular chondrocytes for cartilage tissue formation in three-dimensional biomimetic hydrogels. Tissue Eng. Part A 2015, 21, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; He, W.; Yan, Y.; Zhang, H.; Zhang, G.; Tian, D.; Gao, H. The application of polysaccharide biocomposites to repair cartilage defects. Int. J. Polym. Sci. 2014, 2014, 654597. [Google Scholar] [CrossRef]
- Zeng, L.; Yao, Y.; Wang, D.A.; Chen, X. Effect of microcavitary alginate hydrogel with different pore sizes on chondrocyte culture for cartilage tissue engineering. Mater. Sci. Eng. C 2014, 34, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rackwitz, L.; Djouad, F.; Janjanin, S.; Nöth, U.; Tuan, R.S. Functional cartilage repair capacity of de-differentiated, chondrocyteand mesenchymal stem cell-laden hydrogels in vitro. Osteoarthr. Cartil. 2014, 22, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Ponnurangam, S.; O’Connell, G.D.; Chernyshova, I.V.; Wood, K.; Hung, C.T.; Somasundaran, P. Beneficial effects of cerium oxide nanoparticles in development of chondrocyte-seeded hydrogel constructs and cellular response to interleukin insults. Tissue Eng. Part A 2014, 20, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Dashtdar, H.; Murali, M.R.; Abbas, A.A.; Suhaeb, A.M.; Selvaratnam, L.; Tay, L.X.; Kamarul, T. PVA-chitosan composite hydrogel versus alginate beads as a potential mesenchymal stem cell carrier for the treatment of focal cartilage defects. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Kim, S.; Fan, J.; Kowalski, T.; Petrigliano, F.; Evseenko, D.; Lee, M. Covalently conjugated transforming growth factor-β1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomater. Sci. 2015, 3, 742. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Seol, D.; Magnetta, M.J.; Ramakrishnan, P.S.; Kurriger, G.L.; Choe, H.; Jang, K.; Martin, J.A.; Lim, T.H. Biocompatibility and preclinical feasibility tests of a temperature-sensitive hydrogel for the purpose of surgical wound pain control and cartilage repair. J. Biomed. Mater. Res. Part B 2013, 101B, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Sandker, M.; Müller, B.; Meyboom, R.; van Midwoud, P.; Bruin, P.; Redout, E.M.; Versluijs-Helder, M.; van der Lest, C.H.; Buwalda, S.J.; et al. Release behavior and intra-articular biocompatibility of celecoxib-loaded acetyl-capped PCLA-PEG-PCLA thermogels. Biomaterials 2014, 35, 7919–7928. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Walton, R.S.; Brand, D.D.; Czernuszka, J.T. Characterisation of freeze-dried type ii collagen and chondroitin sulfate scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, K.Y. Cartilage regeneration using biodegradable oxidized alginate/hyaluronate hydrogels. J. Biomed. Mater. Res. Part A 2014, 102A, 4519–4525. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.J.; Madihally, S.V. Anisotropic temperature sensitive chitosan-based injectable hydrogels mimicking cartilage matrix. J. Biomed. Mater. Res. Part B 2015, 103B, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, E.J.; Mesallati, T.; Vinardell, T.; Kelly, D.J. Engineering cartilage or endochondral bone: A comparison of different naturally derived hydrogels. Acta Biomater. 2015, 13, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kaderli, S.; Viguier, E.; Watrelot-Virieux, D.; Roger, T.; Gurny, R.; Scapozza, L.; Möller, M.; Boulocher, C.; Jordan, O. Efficacy study of two novel hyaluronic acid-based formulations for viscosupplementation therapy in an early osteoarthrosic rabbit model. Eur. J. Pharm. Biopharm. 2015, 96, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.D.; Gao, S.; Kurisawa, M.; Ying, J.Y. Cartilage synthesis in hyaluronic acid–tyramine constructs. J. Mater. Chem. B 2015, 3, 1942. [Google Scholar] [CrossRef]
- Kim, J.; Lin, B.; Kim, S.; Choi, B.; Evseenko, D.; Lee, M. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J. Biol. Eng. 2015, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, Z.; Radosavljević, A.; Kačarević-Popović, Z.; Stojkovska, J.; Perić-Grujić, A.; Ristić, M.; Matić, I.Z.; Juranić, Z.D.; Obradovic, B.; Mišković-Stanković, V. Bioreactor validation and biocompatibility of Ag/poly(n-vinyl-2-pyrrolidone) hydrogel nanocomposites. Colloids Surf. B Biointerfaces 2013, 105, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Dua, R.; Centeno, J.; Ramaswamy, S. Augmentation of engineered cartilage to bone integration using hydroxyapatite. J. Biomed. Mater. Res. Part B 2014, 102 B, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shen, Q.; Pan, C.; Wang, J. Compressive mechanical characteristics of multi-layered gradient hydroxyapatite reinforced poly (vinyl alcohol) gel biomaterial. J. Mater. Sci. Technol. 2013, 29, 551–556. [Google Scholar] [CrossRef]
- Yodmuang, S.; McNamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.A.; Chao, P.H.; Hung, C.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Parkes, M.; Myant, C.; Dini, D.; Cann, P. Tribology-optimised silk protein hydrogels for articular cartilage repair. Tribol. Int. 2015, 89, 9–18. [Google Scholar] [CrossRef]
- Su, R.S.; Kim, Y.; Liu, J.C. Resilin: Protein-based elastomeric biomaterials. Acta Biomater. 2014, 10, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Chen, C.H.; Hsiao, C.Y.; Chen, J.P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gwon, H.J.; Lim, Y.M.; Nho, Y.C.; Kim, S.Y. Hyaluronic acid/chondroitin sulfate-based hydrogel prepared by gamma irradiation technique. Carbohydr. Polym. 2014, 102, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Tang, Z.; Cao, W.; Lin, H.; Fan, Y.; Guo, L.; Zhang, X. Tough and elastic hydrogel of hyaluronic acid and chondroitin sulfate as potential cell scaffold materials. Int. J. Biol. Macromol. 2015, 74, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, B.; Hu, J.; Lee, M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013, 9, 4779–4789. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tan, L.; Cen, L.; Zhang, Z. An injectable scaffold based on crosslinked hyaluronic acid gel for tissue regeneration. RSC Adv. 2016, 6, 16838. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. Injectable in situ forming hydrogels based on natural and synthetic polymers for potential application in cartilage repair. RSC Adv. 2015, 5, 19715. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Matsushita, T.; Tabata, Y.; Saito, T.; Matsumoto, T.; Nagai, K.; Kuroda, R.; Kurosaka, M. Intra-articular administration of gelatin hydrogels incorporating rapamycinemicelles reduces the development of experimental osteoarthritis in a murine model. Biomaterials 2014, 35, 9904–9911. [Google Scholar] [CrossRef] [PubMed]
- Nims, R.J.; Cigan, A.D.; Albro, M.B.; Hung, C.T.; Ateshian, G.A. Synthesis rates and binding kinetics of matrix products in engineered cartilage constructs using chondrocyte-seeded agarose gels. J. Biomech. 2014, 47, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xiong, D.S.; Peng, Y.; Wang, N. Effects of polymerization degree on recovery behavior of PVA/PVP hydrogels as potential articular cartilage prosthesis after fatigue test. eXPRESS Polym. Lett. 2016, 10, 125–138. [Google Scholar] [CrossRef]
- Bichara, D.A.; Bodugoz-Sentruk, H.; Ling, D.; Malchau, E.; Bragdon, C.R.; Muratoglu, O.K. Osteochondral defect repair using a polyvinyl alcohol-polyacrylic acid (PVA-PAAc) hydrogel. Biomed. Mater. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; van Ee, R.J.; Timmer, K.; Craenmehr, E.G.M.; Huang, J.H.; Öner, F.C.; Dhert, W.J.A.; Kragten, A.H.M.; Willems, N.; Grinwis, G.C.M.; et al. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015, 23, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Choi, W.H.; Song, B.R.; Jin, H.; Lee, S.J.; Lee, S.H.; Lee, J.; Kim, Y.J.; Park, S.R.; Park, S.-H.; et al. Fabrication of an osteochondral graft with using a solid freeform fabrication system. Tissue Eng. Regen. Med. 2015, 12, 239–248. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.H.; Jung, Y. In situ chondrogenic differentiation of bone marrow stromal cells in bioactive self-assembled peptide gels. J. Biosci. Bioeng. 2015, 120, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Song, M.; Lee, C.H.; Kim, J.A.; Ha, C.W. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J. Orthop. Res. 2015, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Kawazoe, N.; Chen, G. Cellular uptake of single-walled carbon nanotubes in 3D extracellular matrix-mimetic composite collagen hydrogels. J. Nanosci. Nanotechnol. 2014, 14, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Aberle, T.; Franke, K.; Rist, E.; Benz, K.; Schlosshauer, B. Cell-type specific four-component hydrogel. PLoS ONE 2014, 9, e86740. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Song, M.; Ha, C.W.; Kim, J.A.; Lee, C.H.; Park, Y.B. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res. Ther. 2014, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Salamon, A.; van Vlierberghe, S.; van Nieuwenhove, I.; Baudisch, F.; Graulus, G.-J.; Benecke, V.; Alberti, K.; Neumann, H.-G.; Rychly, J.; Martins, J.C.; et al. Gelatin-based hydrogels promote chondrogenic differentiation of human adipose tissue-derived mesenchymal stem cells in vitro. Materials 2014, 7, 1342–1359. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.B.; Henning, E.A.; Söegaard, N.B.; Dodge, G.R.; Steinberg, D.R.; Mauck, R.L. Maximizing cartilage formation and integration via a trajectory-based tissue engineering approach. Biomaterials 2014, 35, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, L.; Li, W.; Zhu, Y.; Jiao, Z.; Lim, M.; Fang, M.; Shi, F.; Wang, L.; Liu, T. Three-dimensional dynamic fabrication of engineered cartilage based on chitosan/gelatin hybrid hydrogel scaffold in a spinner flaskwith a special designed steel frame. Mater. Sci. Eng. C 2015, 55, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Möller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. A 2014, 20, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.; Müller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yoshioka, K.; Urano, K.; Tanaka, Y.; Hatanaka, T.; Nii, A. Biocompatibility of cross-linked hyaluronate (Gel-200) for the treatment of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Mazaki, T.; Shiozaki, Y.; Yamane, K.; Yoshida, A.; Nakamura, M.; Yoshida, Y.; Zhou, D.; Kitajima, T.; Tanaka, M.; Ito, Y.; et al. A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci. Rep. 2014, 4, 4457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, Z.; Liu, K.; Wan, Y.Q.; Li, X.D.; Luo, X.W.; Bai, Y.G.; Yang, Z.L.; Feng, G. Repair of articular cartilage defects in rabbits through tissue-engineered cartilage constructed with chitosan hydrogel and chondrocytes. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2015, 16, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Kyomoto, M.; Moro, T.; Yamane, S.; Hashimoto, M.; Takatori, Y.; Ishihara, K. Effect of UV-irradiation intensity on graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on orthopedic bearing substrate. Mater. Res. Part A 2014, 102A, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, V.M.; Lima, L.L.; Belangero, W.D.; Zavaglia, C.A.; Bavaresco, V.P.; Gomes, J.R. Tribological characterization of polyvinyl alcohol hydrogel as substitute of articular cartilage. Wear 2013, 301, 218–225. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, D. Microstructure and friction properties of PVA/PVP hydrogels for articular cartilage repair as function of polymerization degree and polymer concentration. Wear 2013, 35, 280–285. [Google Scholar] [CrossRef]
- Chen, T.; Hilton, M.J.; Brown, E.B.; Zuscik, M.J.; Awad, H.A. Engineering superficial zone features in tissue engineered cartilage. Biotechnol. Bioeng. 2013, 110, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.M.; Ovaert, T.C. Low friction hydrogel for articular cartilage repair: Evaluation of mechanical and tribological properties in comparison with natural cartilage tissue. Mater. Sci. Eng. C 2013, 33, 4377–4383. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Liao, L.; Zhang, C.; Liu, L. A tough double network hydrogel for cartilage tissue engineering. J. Mater. Chem. B 2013, 1, 4251–4258. [Google Scholar] [CrossRef]
- Blum, M.M.; Ovaert, T.C. Investigation of friction and surface degradation of innovative boundary lubricant functionalized hydrogel material for use as artificial articular cartilage. Wear 2013, 301, 201–209. [Google Scholar] [CrossRef]
- Giavaresi, G.; Bondioli, E.; Melandri, D.; Giardino, R.; Tschon, M.; Torricelli, P.; Cenacchi, G.; Rotini, R.; Castagna, A.; Veronesi, F.; et al. Response of human chondrocytes and mesenchymal stromal cells to a decellularized human dermis. BMC Musculoskelet. Disord. 2013, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Dalzoppo, D.; Lora, S.; Sartore, L.; Folin, M.; Parnigotto, P.P.; Grandi, C. Tailored PVA/ECM scaffolds for cartilage regeneration. Biomed. Res. Int. 2014, 2014, 762189. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, K.; Yang, S.S.; Park, S.H.; Song, B.R.; Yun, H.W.; Jeong, S.I.; Kim, Y.J.; Min, B.H.; Kim, M.S. Preparation of extracellular matrix developed using porcine articular cartilage and in vitro feasibility study of porcine articular cartilage as an anti-adhesive film. Materials 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.; He, X.; Xu, Y.; Yang, Z.; Chen, L.; Zhou, S.; Yang, Y.; Zhou, Z.; Sheng, W.; et al. Chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells in type I collagen-hydrogel for cartilage engineering. Inj. Int. J. Care Inj. 2013, 44, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gonzalez, S.; Shah, S.; Kyupelyan, L.; Petrigliano, F.A.; McAllister, D.R.; Adams, J.S.; Karperien, M.; Tuan, T.L.; Benya, P.D.; et al. Extracellular matrix domain formation as an indicator of chondrocyte dedifferentiation and hypertrophy. Tissue Eng. Part C 2014, 20, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Lu, S.; Lee, E.J.; Trachtenberg, J.E.; Meretoja, V.V.; Dahlin, R.L.; van den Beucken, J.J.; Tabata, Y.; Wong, M.E.; Jansen, J.A.; et al. Osteochondral defect repair using bilayered hydrogels encapsulating both chondrogenically and osteogenically pre-differentiated mesenchymal stem cells in a rabbit model. Osteoarthr. Cartil. 2014, 22, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-T.; Sheu, M.-T.; Lin, Y.-F.; Lan, J.; Chin, Y.-P.; Hsieh, M.-S.; Cheng, C.-W.; Chen, C.-H. Injectable hyaluronic-acid-doxycycline hydrogel therapy in experimental rabbit osteoarthritis. Vet. Res. 2013, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.H.; Ren, X.; Afizah, M.H.; Chian, K.S.; Mikos, A.G. Oligo[poly(ethylene glycol)fumarate] hydrogel enhances osteochondral repair in porcine femoral condyle defects. Clin. Orthop. Relat. Res. 2013, 471, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Rainbow, R.S.; Sun, L.; Hui, C.K.; Cairns, D.M.; Preda, R.C.; Kaplan, D.L.; Zeng, L. Scaffold structure and fabrication method affect proinflammatory milieu in three-dimensional-cultured chondrocytes. J. Biomed. Mater. Res. Part A 2015, 103A, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.A.; McCullen, S.D.; Callanan, A.; Autefage, H.; Accardi, M.A.; Dini, D.; Stevens, M.M. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater. 2014, 10, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Perrier-Groult, E.; Pasdeloup, M.; Malbouyres, M.; Galéra, P.; Mallein-Gerin, F. Control of collagen production in mouse chondrocytes by using a combination of bone morphogenetic protein-2 and small interfering RNA targeting Col1a1 for hydrogel-based tissue-engineered cartilage. Tissue Eng. Part C 2013, 19, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, B.V.; Doyle, N.R.; Randolph, M.A.; Anseth, K.S. Covalently tethered TGF-β1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production. J. Biomed. Mater. Res. Part A 2014, 102A, 4464–4472. [Google Scholar] [CrossRef] [PubMed]
- Simson, J.A.; Strehin, I.A.; Lu, Q.; Uy, M.O.; Elisseeff, J.H. An adhesive bone marrow scaffold and bone morphogenetic-2 protein carrier for cartilage tissue engineering. Biomacromolecules 2013, 14, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Ayerst, B.I.; Day, A.J.; Nurcombe, V.; Cool, S.M.; Merry, C.L. New strategies for cartilage regeneration exploiting selected glycosaminoglycans to enhance cell fate determination. Biochem. Soc. Trans. 2014, 42, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Kelly, D.J. A comparison of fibrin, agarose and gellan gum hydrogels as carriers of stem cells and growth factor delivery microspheres for cartilage regeneration. Biomed. Mater. 2013, 8, 035004. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.D.; Hurtig, M.B.; Pilliar, R.M.; Stanford, W.L.; Kandel, R.A. Engineering of hyaline cartilage with a calcified zone using bone marrow stromal cells. Osteoarthr. Cartil. 2015, 23, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Kaderli, S.; Boulocher, C.; Pillet, E.; Watrelot-Virieux, D.; Rougemont, A.L.; Roger, T.; Viguier, E.; Gurny, R.; Scapozza, L.; Jordan, O. A novel biocompatible hyaluronic acid–chitosan hybrid hydrogel for osteoarthrosis therapy. Int. J. Pharm. 2015, 483, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Medved, F.; Gonser, P.; Lotter, O.; Albrecht, D.; Amr, A.; Schaller, H.E. Severe posttraumatic radiocarpal cartilage damage: First report of autologous chondrocyte implantation. Arch. Orthop. Trauma Surg. 2013, 133, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Niemietz, T.; Zass, G.; Hagmann, S.; Diederichs, S.; Gotterbarm, T.; Richter, W. Xenogeneic transplantation of articular chondrocytes into full-thickness articular cartilage defects in minipigs: Fate of cells and the role of macrophages. Cell Tissue Res. 2014, 358, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Ochi, M.; Deie, M.; Nakamae, A.; Kamei, G.; Uchio, Y.; Iwasa, J. Implantation of tissue-engineered cartilage-like tissue for the treatment for full-thickness cartilage defects of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Vikingsson, L.; Gallego Ferrer, G.; Gómez-Tejedor, J.A.; Gómez Ribelles, J.L. An “in vitro” experimental model to predict the mechanical behavior of macroporous scaffolds implanted in articular cartilage. J. Mech. Behav. Biomed. Mater. 2014, 32, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Yusong, P.; Qianqian, S.; Chengling, P.; Jing, W. Prediction of mechanical properties of multilayer gradient hydroxyapatite reinforced poly(vinyl alcohol) gel biomaterial. J. Biomed. Mater. Res. Part B 2013, 101B, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xiong, D. Stress-relaxation models of nano-HA/PVA gel biocomposites. Mech. Time-Depend. Mater. 2013, 17, 195–204. [Google Scholar] [CrossRef]
- Du, G.; Gao, G.; Hou, R.; Cheng, Y.; Chen, T.; Fu, J. Tough and fatigue resistant biomimetic hydrogels of interlaced self-assembled conjugated polymer belts with a polyelectrolyte network. Chem. Mater. 2014, 26, 3522–3529. [Google Scholar] [CrossRef]
- Cao, Yi.; Xiong, D.; Niu, Y.; Mei, Y.; Yin, Z.; Gui, J. Compressive properties and creep resistance of a novel, porous, semidegradable poly(vinyl alcohol)/poly(lactic-co-glycolic acid) scaffold for articular cartilage repair. J. Appl. Polym. Sci. 2014, 131, 40311. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Alvarez, V.A. Mechanical properties of polyvinyl alcohol/hydroxyapatite cryogel as potential artificial cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, D.; Dai, Z.; Wang, S.; Ge, S. Research on the interstitial fluid load support characteristics and start-up friction mechanisms of PVA-HA-silk composite hydrogel. J. Bionic Eng. 2014, 11, 378–388. [Google Scholar] [CrossRef]
- Mansour, J.M.; Gu, D.W.; Chung, C.Y.; Heebner, J.; Althans, J.; Abdalian, S.; Schluchter, M.D.; Liu, Y.; Welter, J.F. Towards the feasibility of using ultrasound to determine mechanical properties of tissues in a bioreactor. Ann. Biomed. Eng. 2014, 40, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Yun, A.; Lee, S.-H.; Kim, J. A phase-field model for articular cartilage regeneration in degradable scaffolds. Bull. Math. Biol. 2013, 75, 2389–2409. [Google Scholar] [CrossRef] [PubMed]
- Sandker, M.J.; Petit, A.; Redout, E.M.; Siebelt, M.; Müller, B.; Bruin, P.; Meyboom, R.; Vermonden, T.; Hennink, W.E.; Weinans, H. In situ forming acyl-capped PCLA-PEG-PCLA triblock copolymer based hydrogels. Biomaterials 2013, 34, 8002–8011. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Kitamura, N.; Kurokawa, T.; Yokota, M.; Kondo, E.; Gong, J.P.; Yasuda, K. Intra-articular administration of hyaluronic acid increases the volume of the hyaline cartilage regenerated in a large osteochondral defect by implantation of a double-network gel. J. Mater. Sci. Mater. Med. 2014, 25, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Haaparanta, A.M.; Järvinen, E.; Cengiz, I.F.; Ellä, V.; Kokkonen, H.T.; Kiviranta, I.; Kellomäki, M. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Cheng, A.W.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. Part A 2014, 2014, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zuo, Y.; Qu, D.; Liu, Y.; Chen, T.; Jiang, N.; Li, H.; Li, J. Osteogenesis and chondrogenesis of biomimetic integrated porous PVA/gel/V-n-HA/pa6 scaffolds and BMSCs construct in repair of articular osteochondral defect. J. Biomed. Mater. Res. Part A 2015, 103A, 3226–3236. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Tovar, G.E.; Borchers, K. Biopolymer-based hydrogels for cartilage tissue engineering. Bioinspir. Biomim. Nanobiomater. 2016, 5, 51–66. [Google Scholar] [CrossRef]
- Tuan, R.S.; Chen, A.F.; Klatt, B.A. Cartilage regeneration. J. Am. Acad Orthop. Surg. 2013, 21, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Zhou, F.; Gao, C. Biological hydrogel synthesized from hyaluronic acid, gelatin and chondroitin sulfate by click chemistry. Acta Biomater. 2011, 7, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Mirahmadi, F.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A.; Bonakdar, S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Moreira Teixeira, L.S.; Patterson, J.; Luyten, F.P. Skeletal tissue regeneration: Where can hydrogels play a role? Int. Orthop. 2014, 38, 1861–1876. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Pot, M.W.; Faraj, K.A.; Adawy, A.; van Enckevort, W.J.; van Moerkerk, H.T.; Vlieg, E.; Daamen, W.F.; van Kuppevelt, T.H. Versatile wedge-based system for the construction of unidirectional collagen scaffolds by directional freezing: Practical and theoretical considerations. Appl. Mater. Interfaces 2015, 7, 8495–8505. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Kawazoe, N.; Chen, G. Cell response to single-walled carbon nanotubes in hybrid porous collagen sponges. Colloids Surf. B Biointerfaces 2015, 126, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.J.; Park, J.Y.; Jeong, W.; Kim, T.H.; Kim, S.Y.; Cho, D.W. Development of hybrid scaffolds using ceramic and hydrogel for articular cartilage tissue regeneration. J. Biomed. Mater. Res. Part A 2015, 103A, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 015001. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Portalatín, N.; Kilmer, C.E.; Panitch, A.; Liu, J.C. Characterization of collagen type I and II blended hydrogels for articular cartilage tissue engineering. Biomacromolecules 2016, 17, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Mintz, B.R.; Cooper, J.A., Jr. Hybrid hyaluronic acid hydrogel/poly(ε-caprolactone) scaffold provides mechanically favorable platform for cartilage tissue engineering studies. J. Biomed. Mater. Res. Part A 2014, 102A, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xue, B.; Li, Y.; Qin, M.; Wu, J.; Lu, K.; Wu, J.; Cao, Y.; Jiang, Q.; Wang, W. Polymer-supramolecular polymer double-network hydrogel. Adv. Funct. Mater. 2016, 26, 9044–9052. [Google Scholar] [CrossRef]
- Formica, F.A.; Öztürk, E.; Hess, S.C.; Stark, W.J.; Maniura-Weber, K.; Rottmar, M.; Zenobi-Wong, M. A bioinspired ultraporous nanofiber-hydrogel mimic of the cartilage extracellular matrix. Adv. Funct. Mater. 2016, 5, 3129–3138. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.L.; Jeong, S.Y.; Im, G.I. Hyaluronic acid hydrogel functionalized with self-assembled micelles of amphiphilic PEGylated kartogenin for the treatment of osteoarthritis. Tissue Eng. Part A 2017, 23, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, X.; Hu, X.; Dai, L.; Zhu, J.; Man, Z.; Chen, H.; Zhou, C.; Ao, Y. Directing chondrogenic differentiation of mesenchymal stem cells with a solid-supported chitosan thermogel for cartilage tissue engineering. Biomed. Mater. 2014, 9, 035008. [Google Scholar] [CrossRef] [PubMed]
- Buchtová, N.; Réthoré, G.; Boyer, C.; Guicheux, J.; Rambaud, F.; Vallé, K.; Belleville, P.; Sanchez, C.; Chauvet, O.; Weiss, P.; et al. Nanocomposite hydrogels for cartilage tissue engineering: Mesoporous silica nanofibers interlinked with siloxane derived polysaccharide. J. Mater. Sci. Mater. Med. 2013, 24, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lai, J.H.; Yang, F. Effects of hydrogel stiffness and extracellular compositions on modulating regeneration by mixed populations of stem cells and chondrocytes in vivo. Tissue Eng. Part A 2016, 22, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C., Jr.; Ribiero-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Hauser, A.W.; Hayward, R.C. Shape-morphing materials from stimuli-responsive hydrogel hybrids. Acc. Chem. Res. 2017, 50, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.E.; Vaquette, C.; Theodoropoulos, C.; Klein, T.J.; Hutmacher, D.W. Multiphasic construct studied in an ectopic osteochondral defect model. J. R. Soc. Interface 2014, 11, 20140184. [Google Scholar] [CrossRef] [PubMed]
- Childs, A.; Hemraz, U.D.; Castro, N.J.; Fenniri, H.; Zhang, L.G. Novel biologically-inspired rosette nanotube PLLA scaffolds for improving human mesenchymal stem cell chondrogenic differentiation. Biomed. Mater. 2013, 8, 065003. [Google Scholar] [CrossRef] [PubMed]
- Mačiulaitis, J.; Deveikytė, M.; Rekštytė, S.; Bratchikov, M.; Darinskas, A.; Šimbelytė, A.; Daunoras, G.; Laurinavičienė, A.; Laurinavičius, A.; Gudas, R.; et al. Preclinical study of SZ20180 material 3D microstructured scaffolds for cartilage tissue engineering made by femtosecond direct laser writing lithography. Biofabrication 2015, 7, 015015. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Coradin, T.; Desimone, M.F. Bio-inspired silica-collagen materials: Applications and perspectives in the medical field. Biomater. Sci. 2013, 1, 688–702. [Google Scholar] [CrossRef]
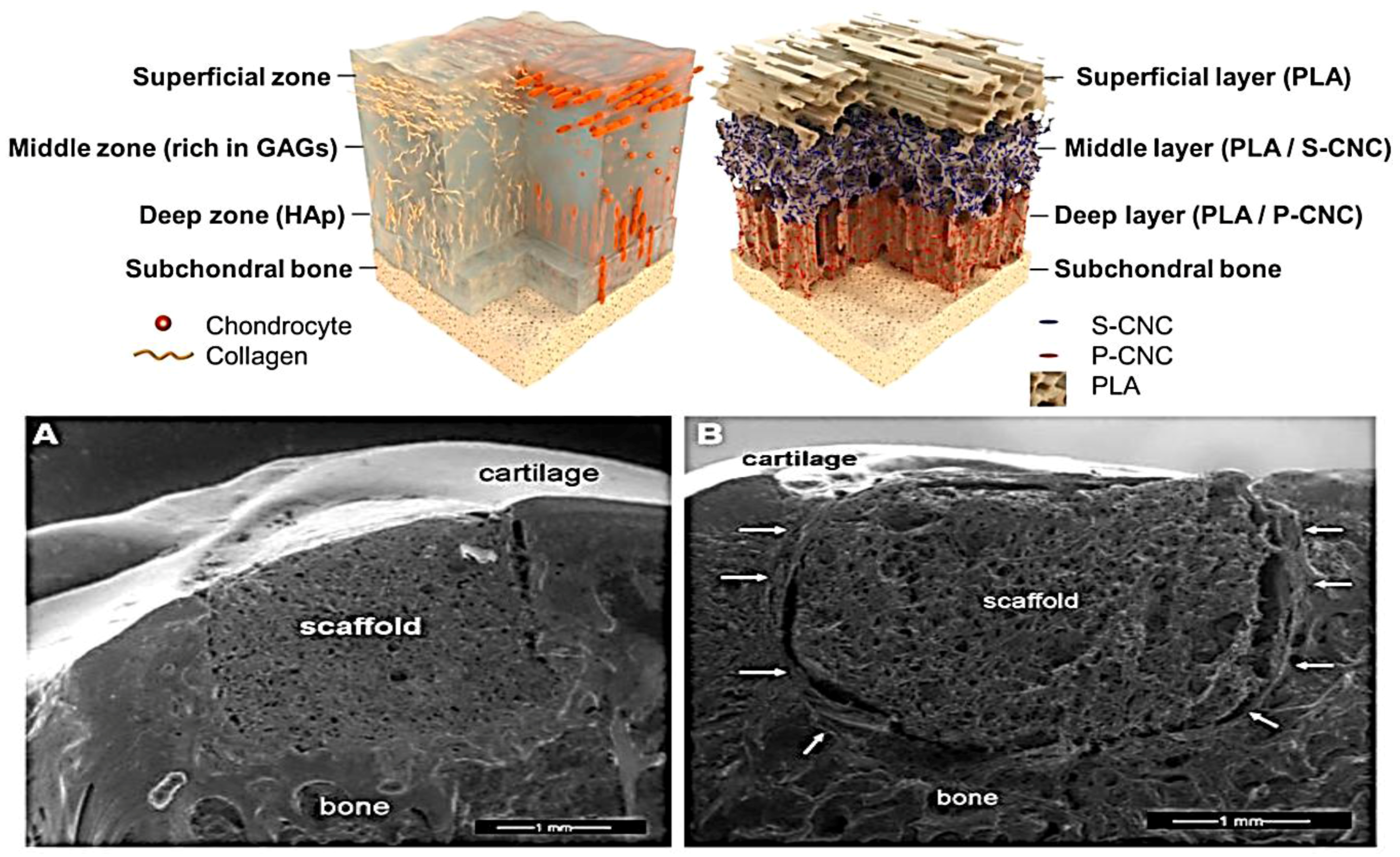
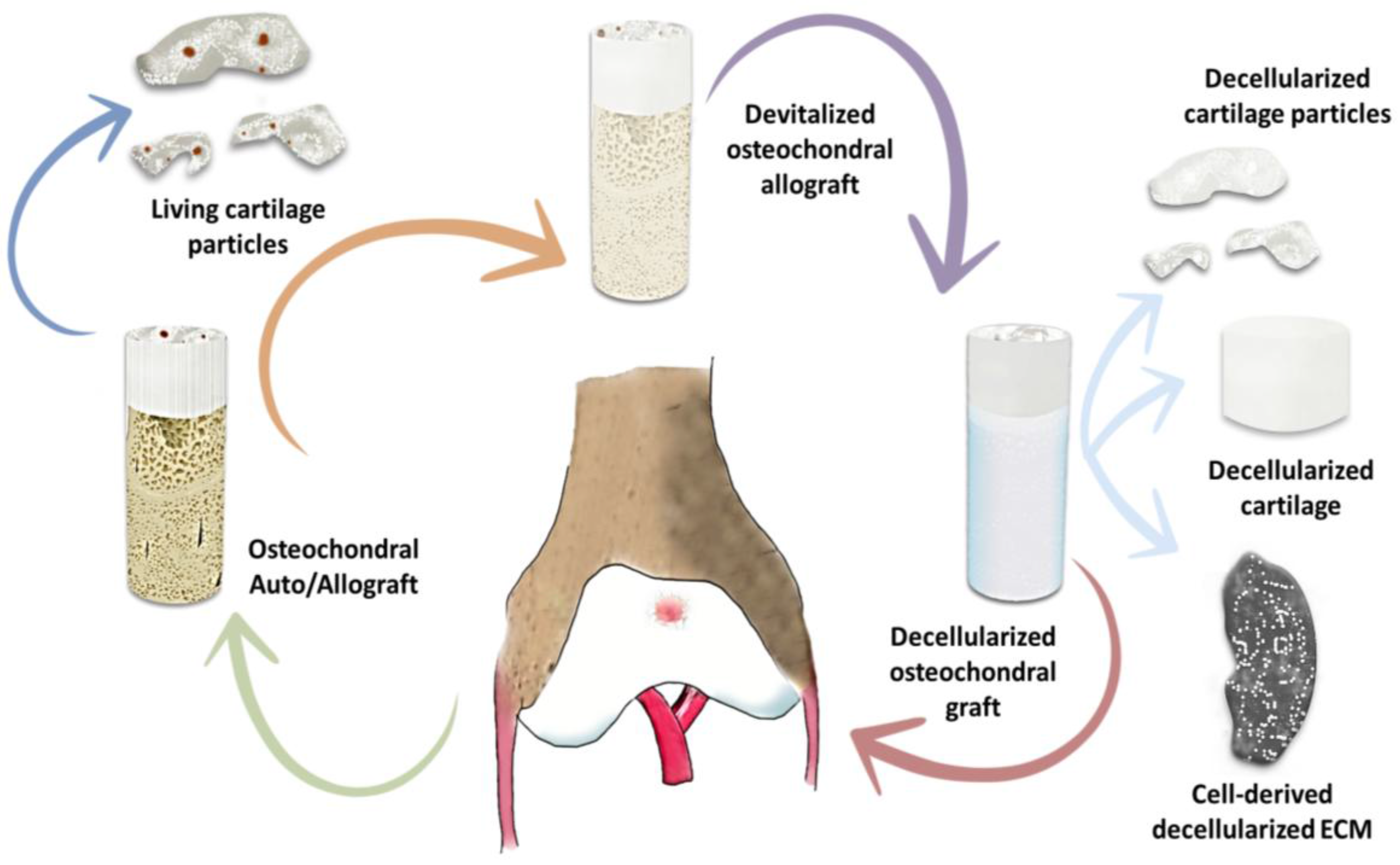
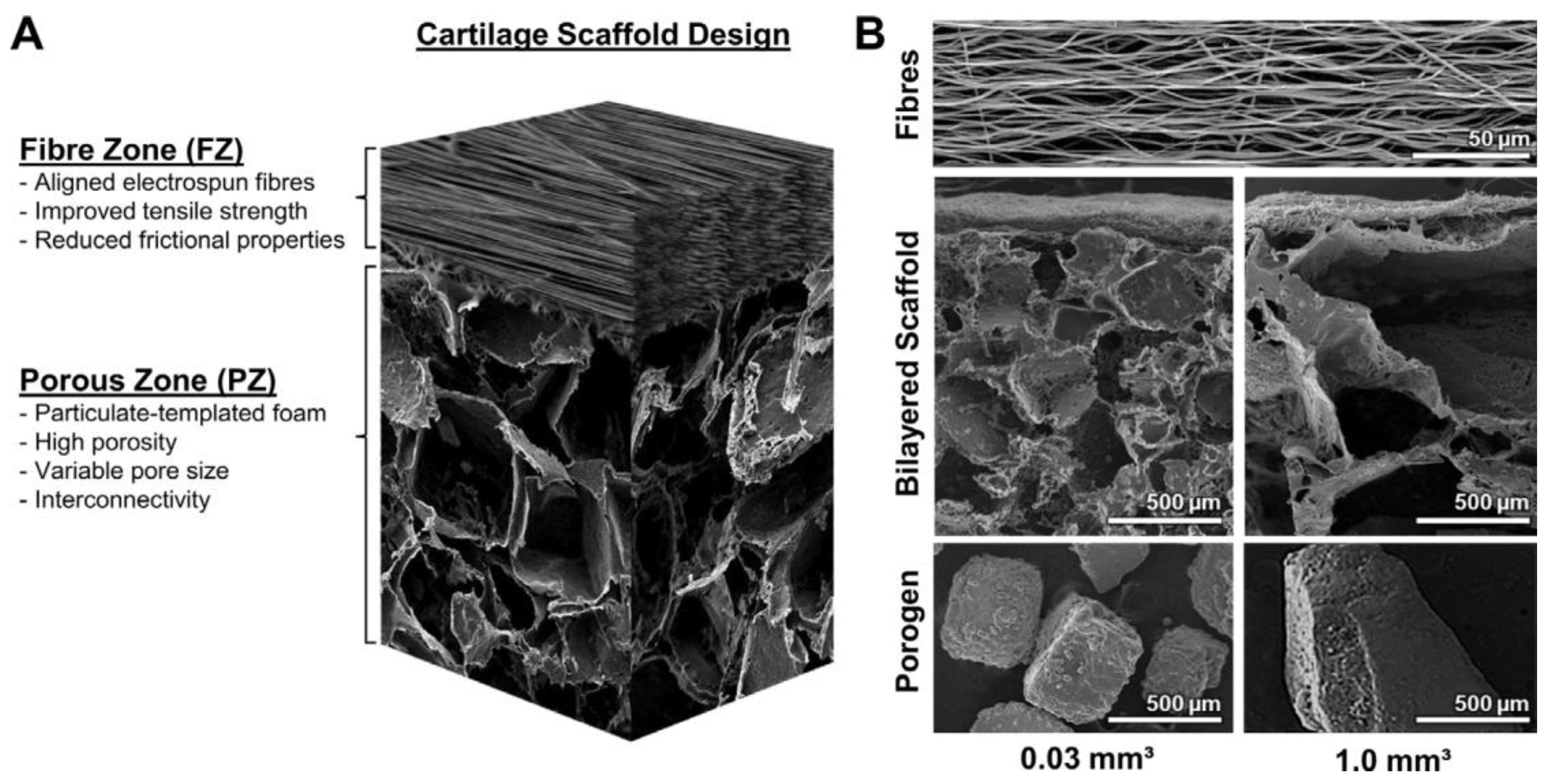
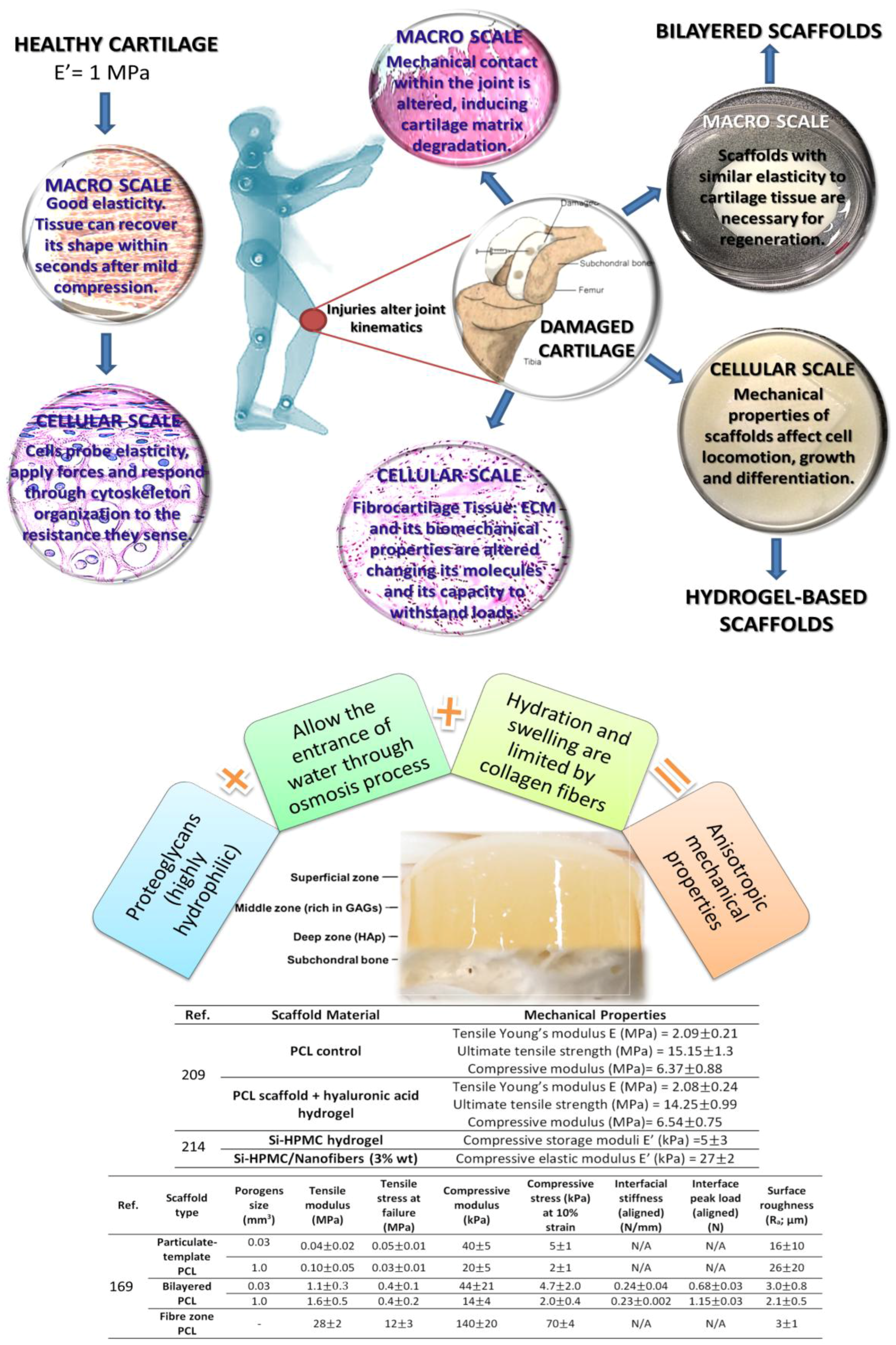
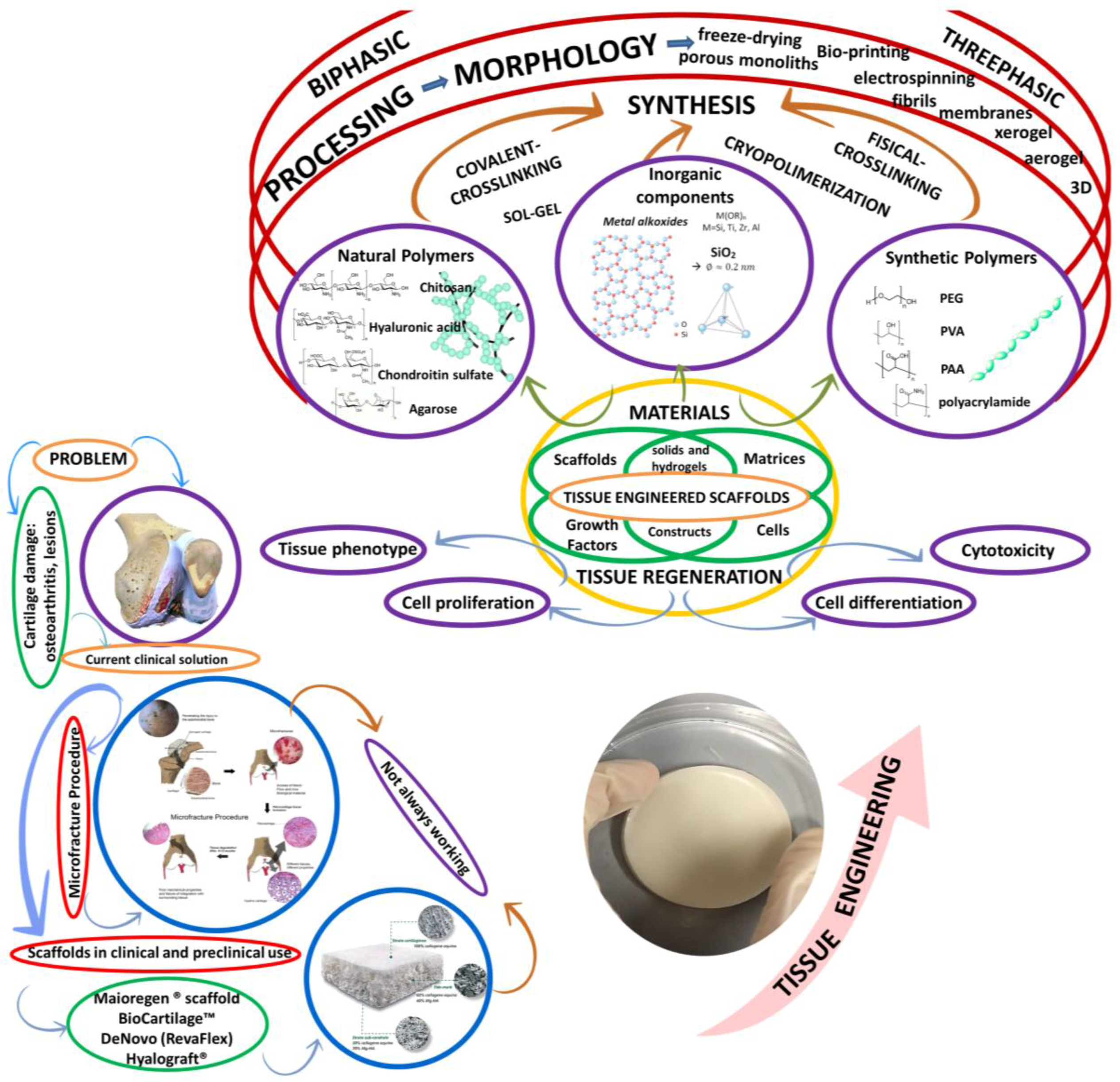
| Application | Material | Problem-result | Ref. |
|---|---|---|---|
| Nose (dorsal augmentation material in rhinoplasty) | Tissue-engineered chondrocyte PCS (Porcine Cartilage-derived Substance) scaffold construct. | Preliminary animal study: Excellent biocompatibility, neocartilage formation starts. However, it was not confirmed that the constructs contributed to the formation of neocartilage. | [36] |
| Knee (subchondral bone) | Osteochondral biomimetic nanostructured scaffold Maioregen® | Better results in healing complex lesions in comparison with the implantation of a purely chondral scaffold. | [37] |
| Cell-free biphasic scaffold: collagen-hydroxyapatite osteochondral scaffold | Statistically significant improvement in clinical scores. At 5 years, between 60.9% and 78.3% of the cases showed complete filling of the cartilage, complete integration of the graft, intact repaired tissue surface and homogeneous repaired tissue. | [38] | |
| Nanostructured biomimetic three-phasic collagen-hydroxiapatite construct | The implantations to treat chondral and osteochondral knee defects were effective in terms of clinical outcome, although MRI detected abnormal findings. | [39] | |
| Knee (chondral defects) | Autologous ovine MNC Cell-seeded and cell-free dl-poly-lactide–co–glycolide (PLGA) scaffolds | The engineered tissue had not local or systemic adverse effects. However, only a poor integration of the tissue engineering product into adjacent tissue was reached and the formed ECM was not mature enough for long-lasting weight-loading resistance. | [40] |
| Type I collagen-hydroxyapatite (Maioregen®) nanostructural biomimetic osteochondral scaffold | The use of the Maioregen® scaffold is a good procedure for the treatment of large osteochondral defects; however, the lesion site seems to influence the results. Patient affected in the medial femoral condyle showed better results. | [41] | |
| DeNovo (RevaFlex) engineered tissue graft | Preliminary evidence suggests that DeNovo ET implant is capable of spontaneous matrix formation with no immune response, improving function and recreating hyaline-like cartilage. | [42] | |
| Knee (femoral condyles) | Biphasic cylindrical osteochondral composite construct of dl-poly-lactide–co–glycolide (PLGA). Its lower body is impregnated with β-tricalcium phosphate (TCP) | The regenerated osteochondral tissue was evaluated as a tissue of acceptable quality. Regenerated cartilage was defined as being hyaline when the ground substance was homogeneous without fibrous texture. | [43] |
| Tibial plateau (osteochondral scaffold) | Osteochondral biomimetic collagen-hydroxiapatite scaffold (Maioregen®, Fin-ceramica, Faenza, Italy) | MRI abnormalities. Clinical outcome with stable results up to a mid-term follow-up. | [44] |
| Microfractured defect (for filling microfractures) | BioCartilageTM, product containing dehydrated, micronized allogeneic cartilage, implanted with the addition of platelet rich plasma | No human clinical outcomes data available. Data regarding results are limited to expert opinion. | [45] |
| Chondroitin sulfate adhesive-Poly(ethylene glycol) diacrylate (PEGDA) hydrogel system combined with standard microfracture surgery | Significant increase in tissue fillers with defects in a short-term follow-up. | [46] | |
| Knee (for donor site filling) | Artificial TruFit cylinders made of fully synthetic material called PolyGraft®-Material: 50% copolymer (PDLG), composed of 85% poly(d,l-lactide) and 15% glycolide; 40% calcium sulfphae, 10% PGA fibers | No clinical improvement could be found. The regeneration of the filled defects took more than 2 years, even though TruFit Plugs are supposed to stimulate cartilage and bone cell migration from the surrounding tissue to the synthetic cylinders. | [47] |
| Porous poly(ethylene oxide)terephthalate/butylene terephthalate) (PEOT/PBT) implants | Treated defects did not cause postoperative bleeding. Well integration. Surface stiffness was minimally improved compared to controls. Considerable biodegradation after 9 months. Congruent fibrocartilaginous surface repair with interspersed fibrous tissue formation in implanted sites. Donor site: fibrocartilaginous surface repair. | [48] | |
| Shoulder | Engineered hyaluronic acid membrane, Hyalograft® | Using the hyaluronic membrane had no effect on the final outcome. No difference was observed between the fibrocartilage tissue formed after implementing microfractures and the fibrocartilage tissue grown on the hyaluronic acid membrane scaffold. | [49] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. https://doi.org/10.3390/polym9120671
Sánchez-Téllez DA, Téllez-Jurado L, Rodríguez-Lorenzo LM. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers. 2017; 9(12):671. https://doi.org/10.3390/polym9120671
Chicago/Turabian StyleSánchez-Téllez, Daniela Anahí, Lucía Téllez-Jurado, and Luís María Rodríguez-Lorenzo. 2017. "Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids" Polymers 9, no. 12: 671. https://doi.org/10.3390/polym9120671
APA StyleSánchez-Téllez, D. A., Téllez-Jurado, L., & Rodríguez-Lorenzo, L. M. (2017). Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers, 9(12), 671. https://doi.org/10.3390/polym9120671