MgCl2-Supported Titanium Ziegler-Natta Catalyst Using Carbon Dioxide-Based Poly(propylene ether carbonate) Diols as Internal Electron Donor for 1-Butene Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts and Metal Complexes
2.3. 1-Butene Polymerization
2.4. Characterization
3. Results and Discussion
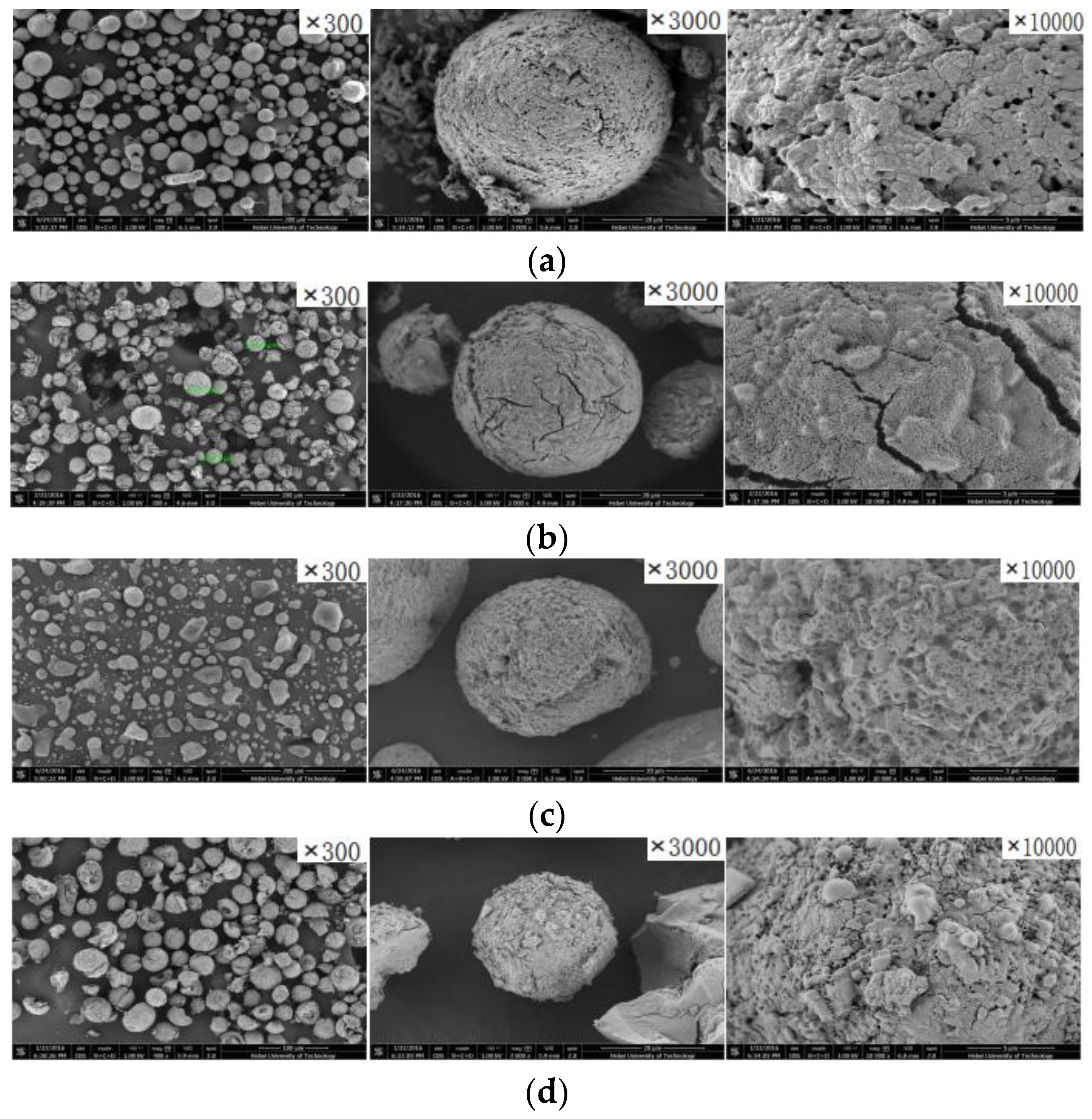
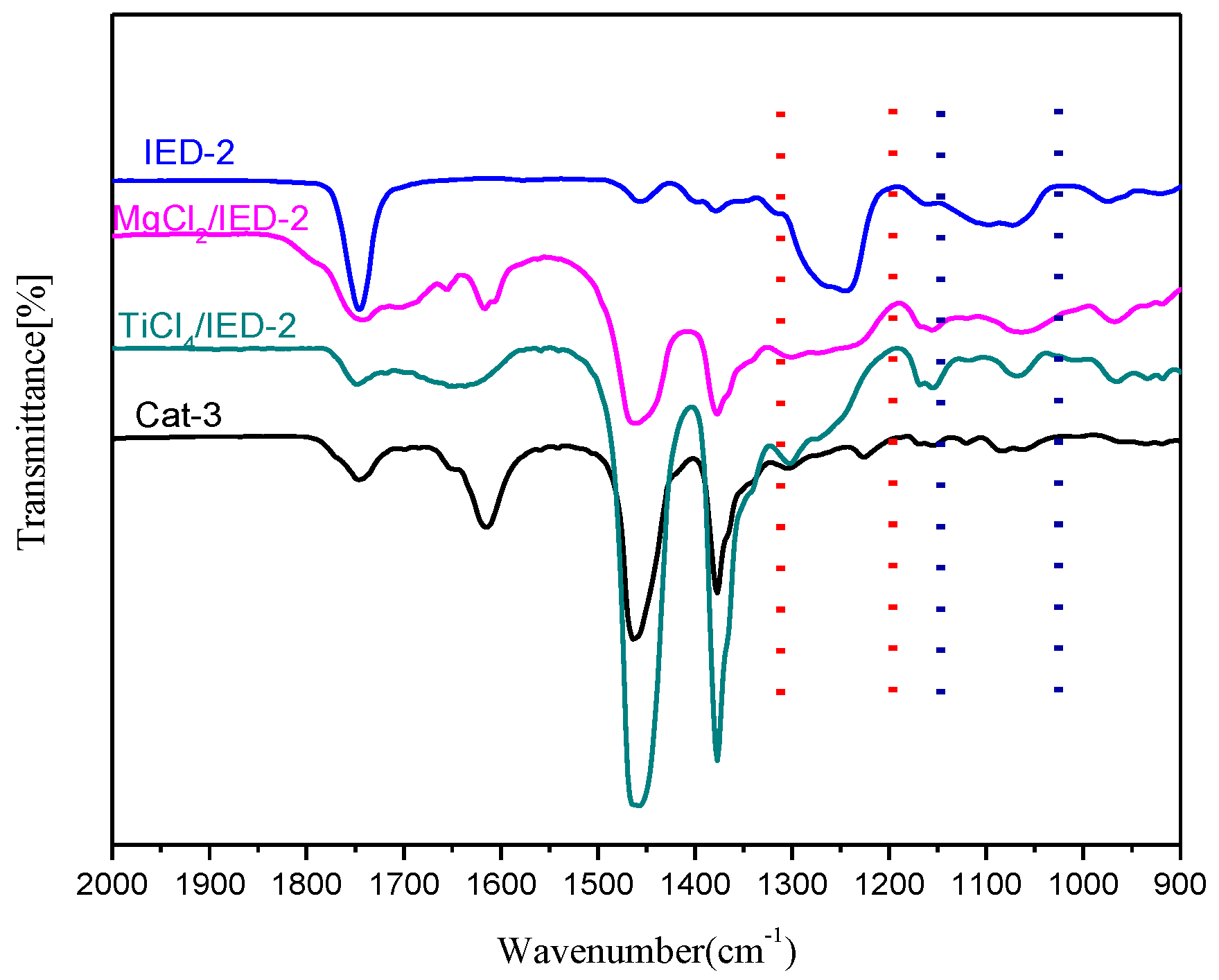
3.1. Components and Structures of the Support and Catalysts
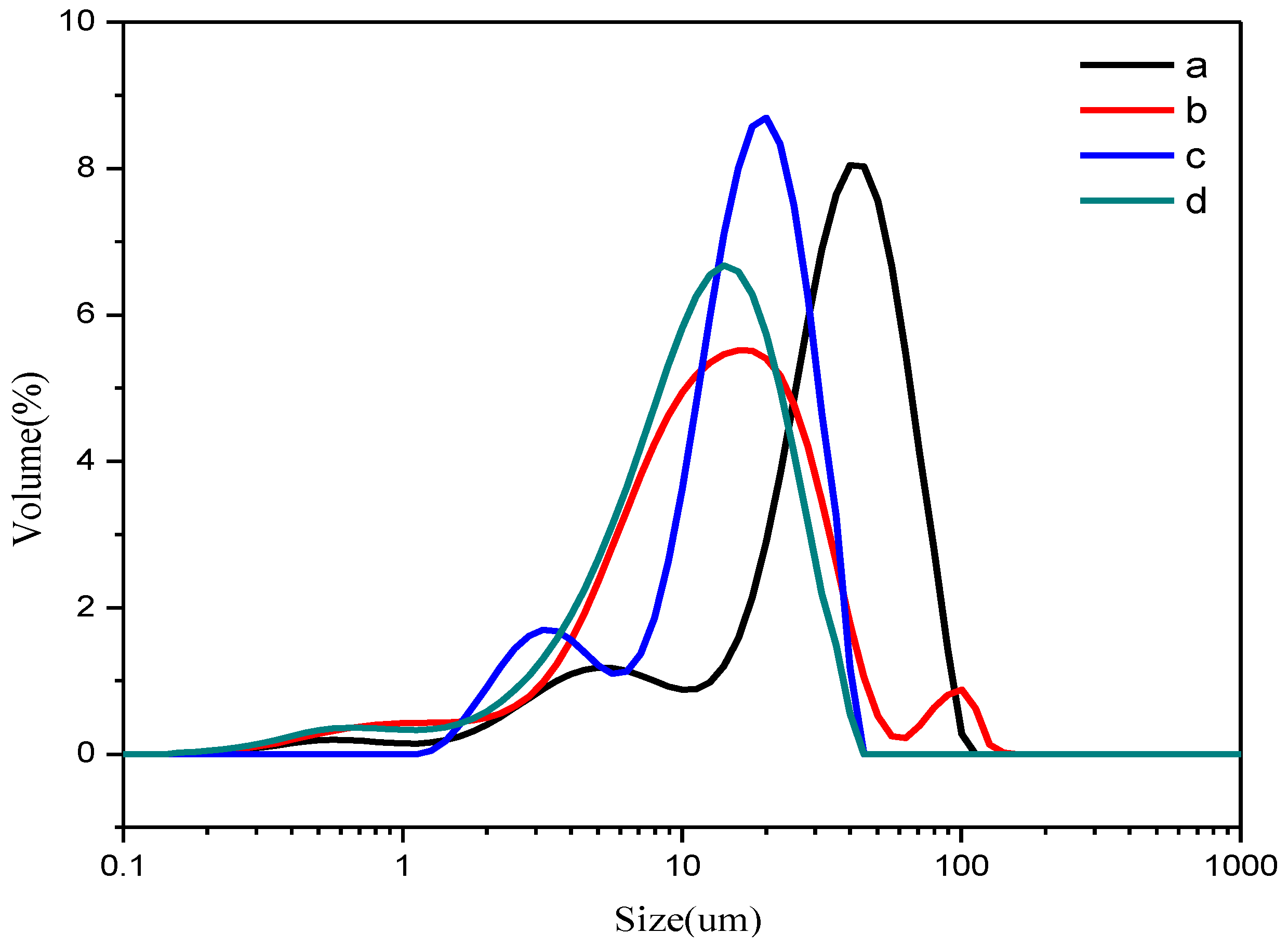
3.2. The Particle Size Distribution of the Support and Catalysts
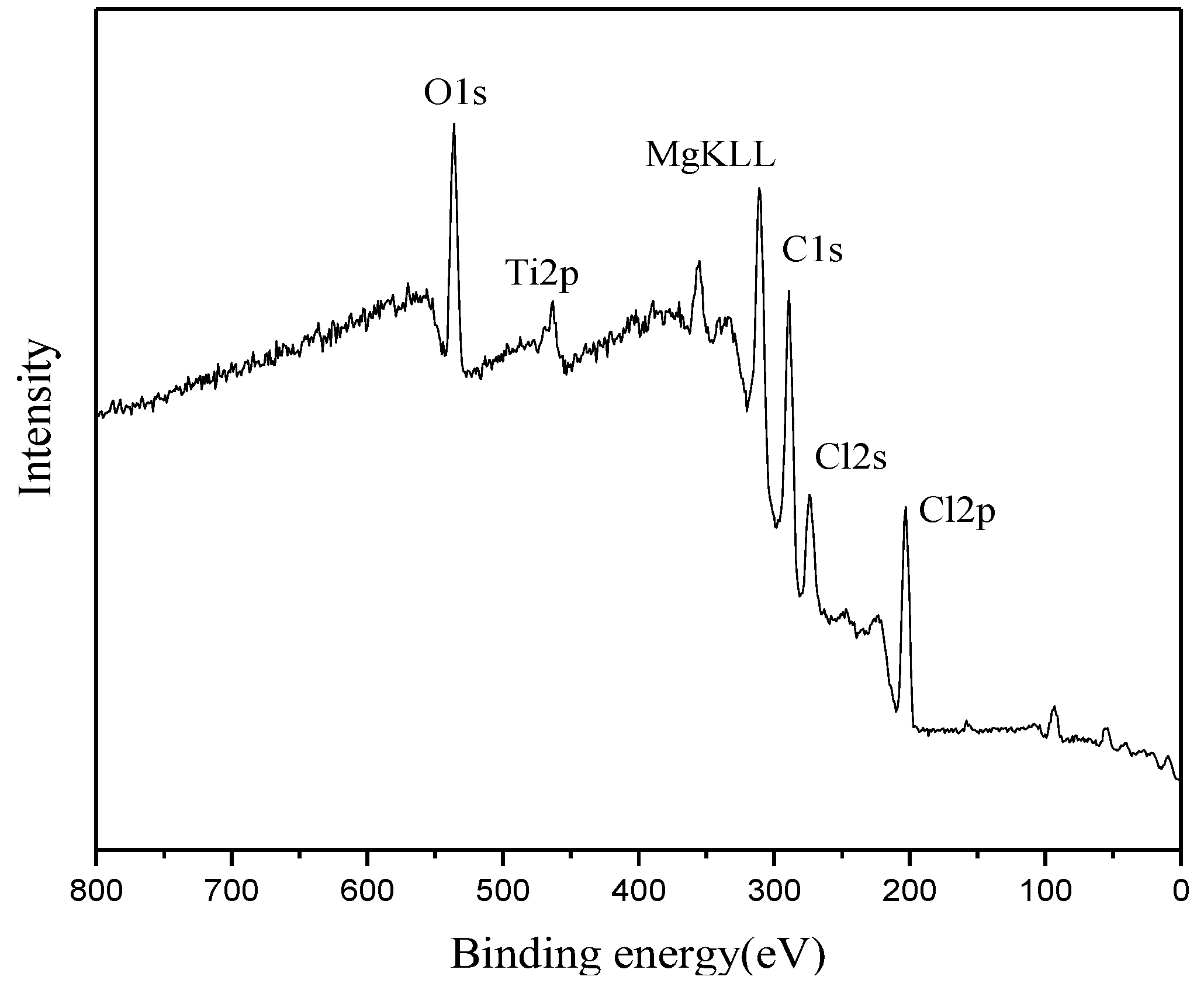
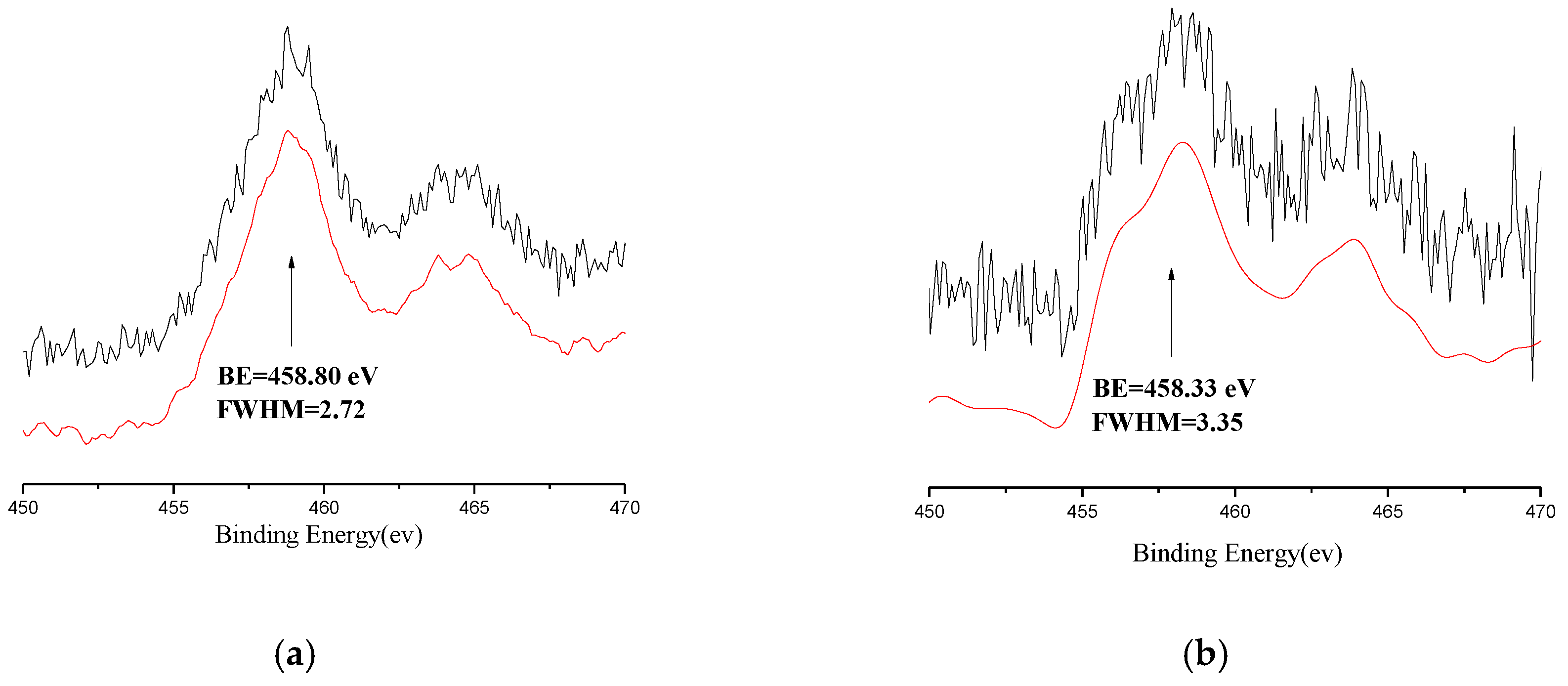
3.3. XPS and Fourier Transform Infrared Spectroscopy (FT-IR) Analyses of the Catalyst
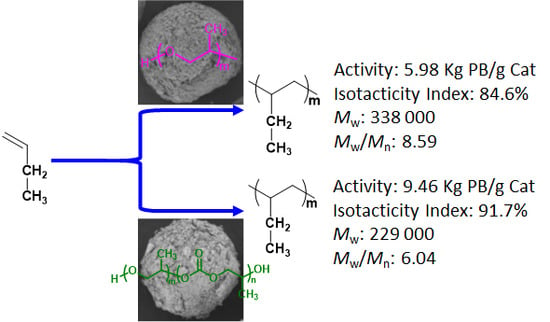
3.4. Effects of Different IEDs on Activity, Isotacticity Index, Molecular Weight and Molecular Weight Distribution of PB
3.5. Effect of Al/Ti, Al/Si Molar Ratios and the Amount of H2 on the Performance of the Cat-3 in 1-Butene Polymerization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luciani, L.; Seppälä, J.; Löfgren, B. Poly-1-butene: Its preparation, properties and challenges. Prog. Polym. Sci. 1988, 13, 37–62. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, C.G.; Zhao, Z.C.; Huang, B.C. Research progress in 1-butene copolymerization. China Synth. Rubber Ind. 2010, 33, 71–76. [Google Scholar]
- Li, P.Y.; Tu, S.T.; Xu, T.; Fu, Z.S.; Fan, Z.Q. The influence of combined external donor and combined cocatalyst on propylene polymerization with a MgCl2-supported Ziegler-Natta catalyst in the presence of hydrogen. J. Appl. Polym. Sci. 2015, 132, 41689. [Google Scholar] [CrossRef]
- Ratanasak, M.; Parasuk, V. Understanding the roles of novel electron donors in Ziegler-Natta catalyzed propylene polymerization. RSC. Adv. 2016, 6, 112776–112783. [Google Scholar] [CrossRef]
- Kang, J.; Cao, Y.; Li, H.L.; Li, J.P.; Chen, S.H.; Yang, F.; Xiang, M. Influence of the stereo-defect distribution on the crystallization behavior of Ziegler-Natta isotactic polypropylene. J. Polym. Res. 2012, 19, 37. [Google Scholar] [CrossRef]
- Xu, J.T.; Feng, L.X.; Yang, S.L. Formation mechanism of stereoblocks in polypropylene produced by supported Ziegler-Natta catalysts. Macromolecules 1997, 30, 2539–2541. [Google Scholar] [CrossRef]
- Credendino, R.; Liguori, D.; Morini, G.; Cavallo, L. Investigating phthalate and 1,3-diether coverage and dynamics on the (104) and (110) surfaces of MgCl2-supported iegler-Natta catalysts. J. Phys. Chem. C 2014, 118, 8050–8058. [Google Scholar] [CrossRef]
- Bazhenov, A.; Linnolahti, M.; Pakkanen, T.A.; Denifl, P.; Leinonen, T. Modeling the stabilization of surface defects by donors in Ziegler-Natta catalyst support. J. Phys. Chem. C 2014, 118, 4791–4796. [Google Scholar] [CrossRef]
- Potapov, A.G.; Politanskaya, L.V. The study of the adsorption of 1,3-diethers on the MgCl2 surface. J. Mol. Catal. A Chem. 2013, 159, 368–369. [Google Scholar] [CrossRef]
- Correa, A.; Piemontesi, F.; Morini, G.; Cavallo, L. Key elements in the structure and function relationship of the MgCl2/TiCl4/lewis base Ziegler-Natta catalytic system. Macromolecules 2007, 40, 9181–9189. [Google Scholar] [CrossRef]
- Song, B.G.; Ihm, S.K. The role of two different internal donors (Phthalate and 1,3-Diether) on the formation of surface structure in MgCl2-supported Ziegler-Natta catalysts and their catalytic performance of propylene polymerization. Appl. Polym. Sci. 2014, 131, 40536. [Google Scholar] [CrossRef]
- Pirinen, S.; Pakkanen, T.T. Polyethers as potential electron donors for Ziegler-Natta ethylene polymerization catalysts. J. Mol. Catal. A Chem. 2015, 398, 177–183. [Google Scholar] [CrossRef]
- Mignogna, A.; Balboni, D.; Cristofori, A.; Guidotti, S.; Morini, G.; Joachim, T.M.P. Catalyst Components for the Polymerization of Olefins. U.S. Patent 2,0140,142,262, 22 May 2014. [Google Scholar]
- Chen, L.F.; Gonzalez, K. Catalyst Composition with Alkoxyalkyl Ester Internal Electron Donor and Polymer from Same. U.S. Patent 860,414,6B2, 10 December 2013. [Google Scholar]
- Chen, B.; Zhang, Q.F.; Zhao, L.P.; Zhang, X.Q.; Zhang, H.X. Preparation and properties of isotactic polypropylene obtained from MgCl2-supported TiCl4 catalyst bearing bifunctional internal donor. Polym. Bull. 2013, 70, 2793–2800. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, Q.; Li, Q.; Zhang, L.Y.; Hu, Y.L. Polypropylene Catalyst Containing an External Electron Donor of Carbonate. CN. Patent 104,403,028, 11 March 2014. [Google Scholar]
- Von der Assen, N.; Bardow, A. Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: Insights from an industrial case study. Green Chem. 2014, 16, 3272–3280. [Google Scholar] [CrossRef]
- Ma, K.; Bai, Q.; Zhang, L.; Liu, B.Y. Synthesis of flame-retarding oligo (carbonate-ether) diols via double metal cyanide complex-catalyzed copolymerization of PO and CO2 using bisphenol A as a chain transfer agent. RSC Adv. 2016, 6, 48405–48410. [Google Scholar] [CrossRef]
- Gao, Y.G.; Qin, Y.S.; Zhao, X.J.; Wang, F.S.; Wang, X.H. Selective synthesis of oligo (carbonate-ether) diols from copolymerization of CO2 and propylene oxide under zinc-cobalt double metal cyanide complex. J. Polym. Res. 2012, 19, 9878. [Google Scholar] [CrossRef]
- Makwana, U.C.; Singala, K.J.; Patankar, R.B.; Singh, S.C.; Gupta, V.K. Propylene polymerization using supported Ziegler-Natta catalyst systems with mixed donors. J. Appl. Polym. Sci. 2012, 125, 896–901. [Google Scholar] [CrossRef]
- Wen, X.; Ji, J.M.; Yi, Q.F.; Niu, H.; Dong, J.Y. Magnesium chloride supported Ziegler-Natta catalysts containing succinate internal electron donors for the polymerization of propylene. J. Appl. Polym. Sci. 2010, 118, 1853–1858. [Google Scholar] [CrossRef]
- Salakhov, I.I.; Batyrshin, A.Z.; Sergeev, S.A.; Bukatov, G.D.; Barabanov, A.A.; Mats’ko, M.A.; Sakhabutdinov, A.G.; Zakharov, V.A. Effect of titanium-magnesium catalyst morphology on the properties of polypropylene upon propylene polymerization in a liquid monomer. Catal. Ind. 2016, 8, 213–216. [Google Scholar] [CrossRef]
- Kaur, S.; Bantu, B.; Singu, G.; kumar, N.; Kapur, G.S.; Basu, B. Particle Size Distribution Control through Internal Donor in Ziegler-Natta Catalyst. U.S. Patent 2,0160,009,833, 14 January 2016. [Google Scholar]
- Fregonese, D.; Glisenti, A.; Mortara, S.; Rizzi, G.A.; Tondello, E.; Bresadola, S. MgCl2/TiCl4/AlEt3 catalytic system for olefin polymerisation: A XPS study. J. Mol. Catal. A Chem. 2002, 178, 115–123. [Google Scholar] [CrossRef]
- Xiao, S.J.; Cai, S.M.; Chen, Z.P.; Liu, H.Q. The structural study of supported Ziegler-Natta catalysts for the polymerization of olefin. Stud. Surf. Sci. Catal. 1986, 25, 431–442. [Google Scholar]
- Mousty-Desbuquoit, C.; Riga, J.; Verbist, J.J. Solid state effects in the electronic structure of TiCl4 studied by XPS. J. Chem. Phys. 1983, 79, 26–32. [Google Scholar] [CrossRef]
- Mori, H.; Hasebe, K.; Terano, M. Variation in oxidation state of titanium species on MgCl2-supported Ziegler catalyst and its correlation with kinetic behavior for propylene polymerization. Polymer 1999, 40, 1389–1394. [Google Scholar] [CrossRef]
- Mori, H.; Hasebe, K.; Terano, M. XPS study of the interaction of titanium species with internal electron donors on MgCl2-supported Ziegler catalysts. J. Mol. Catal. A Chem. 1999, 140, 165–172. [Google Scholar] [CrossRef]
- Korányi, T.; Magni, I.E.; Somorjai, G.A. Surface science approach to the preparation and characterization of model Ziegler-Natta heterogeneous polymerization catalysts. Top. Catal. 1999, 7, 179–185. [Google Scholar] [CrossRef]
- Ren, H.G.; Yang, M.; Zhang, B.J.; Ren, X.F.; Liu, B.Y.; Wang, Y.J.; Kim, II. Isospecific polymerizations of 1-butene catalyzed by MgCl2/TiCl4/internal donor-AlR3/external donor system. Macromol. Res. 2012, 20, 985–989. [Google Scholar] [CrossRef]
- Stukalov, D.V.; Zakharov, V.A. Active site formation in MgCl2-supported Ziegler-Natta catalysts. A density functional theory study. J. Phys. Chem. C 2009, 113, 21376–21382. [Google Scholar] [CrossRef]
- Koivumäki, J.; Seppälä, J.V.; Kuutti, L. Effect of the cocatalyst on the copolymerization of ethylene and propylene with high activity Ziegler-Natta catalyst. Polym. Bull. 1992, 29, 185–191. [Google Scholar] [CrossRef]
- Dang, X.F.; Li, Q.; Li, H.Y.; Yang, Y.; Zhang, L.Y.; Hu, Y.L. Ziegler-Natta catalysts with novel internal electron donors for propylene polymerization. J. Polym. Res. 2014, 21, 619. [Google Scholar] [CrossRef]
- Zhang, H.X.; Lee, L.Y.; Park, J.R.; Lee, D.H.; Yoon, K.B. Control of molecular weight distribution for polypropylene obtained by a commercial Ziegler-Natta catalyst: Effect of a cocatalyst and hydrogen. J. Appl. Polym. Sci. 2011, 120, 101–108. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Hu, Y.L. Superactive and stereospecific catalysts. II. Kinetics of propylene polymerizations. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 2973–2989. [Google Scholar] [CrossRef]
- Chadwick, J.C.; van Kessel, G.M.M.; Sudmeijer, O. Regio-and stereospecificity in propene polymerization with MgCl2-supported Ziegler-Natta catalysts: Effects of hydrogen and the external donor. Macromol. Chem. Phys. 1995, 196, 1431–1437. [Google Scholar] [CrossRef]
- Chadwick, J.C.; Morini, G.; Balbontin, G.; Mingozzi, I.; Albizzati, E.; Sudmeijer, O. Propene polymerization with MgCl2-supported catalysts: Effects of using a diether as external donor. Macromol. Chem. Phys. 1997, 198, 1181–1188. [Google Scholar] [CrossRef]
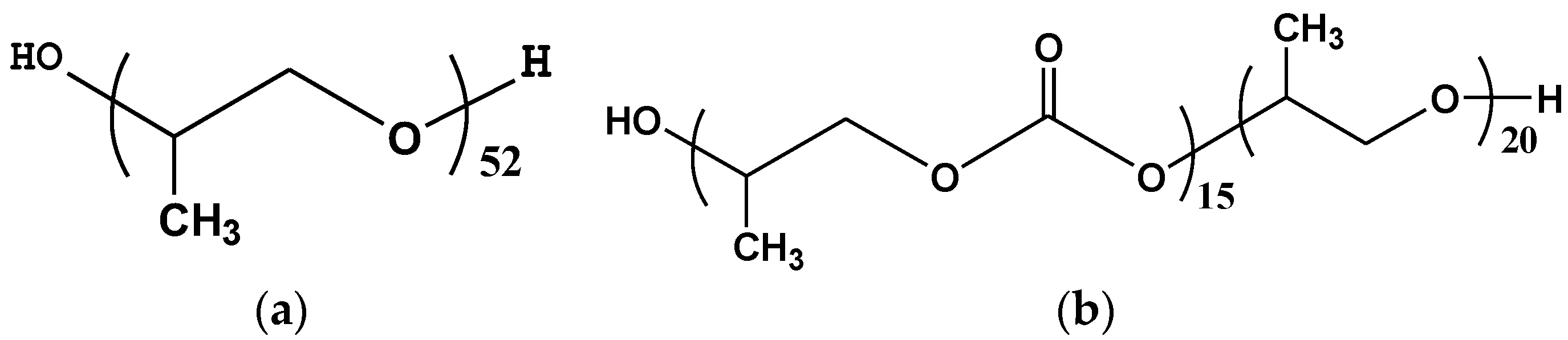
| Catalyst | Mg content (%) | Cl content (%) | Ti content (%) | Surface area (m2/g) | Pore volume (cm3/g) | Pore size (nm) |
|---|---|---|---|---|---|---|
| support | 10.40 | 33.43 | - | 20.80 | 0.12 | 19.43 |
| Cat-1 | 15.70 | 69.26 | 7.78 | 467.13 | 0.28 | 3.29 |
| Cat-2 | 14.01 | 63.40 | 10.78 | 362.30 | 0.33 | 3.98 |
| Cat-3 | 15.50 | 63.11 | 6.92 | 364.53 | 0.34 | 3.73 |
| Catalyst | Activity (Kg PB/g Ti) | I.I (%) | Mw b × 104 (g/mol) | MWD b |
|---|---|---|---|---|
| Cat-1 | 8.30 | 83.70 | 26.40 | 8.26 |
| Cat-2 | 5.98 | 84.60 | 33.75 | 8.59 |
| Cat-3 | 9.46 | 91.70 | 29.94 | 6.04 |
| Run | n (Al)/n (Ti) (mol.mol−1) | n (Al)/n (Si) (mol.mol−1) | H2 (mL) | Activity (Kg PB/gTi) | I.I (%) |
|---|---|---|---|---|---|
| 1 a | 100 | 0 | 0 | 7.23 | 73.2 |
| 2 a | 150 | 0 | 0 | 8.21 | 74.0 |
| 3 a | 200 | 0 | 0 | 9.75 | 76.2 |
| 4 a | 250 | 0 | 0 | 7.71 | 76.4 |
| 5 a | 300 | 0 | 0 | 6.59 | 79.5 |
| 6 a | 200 | 40 | 0 | 4.27 | 90.3 |
| 7 a | 200 | 35 | 0 | 4.74 | 89.9 |
| 8 a | 200 | 30 | 0 | 5.55 | 88.7 |
| 9 a | 200 | 20 | 0 | 5.27 | 90.2 |
| 10 a | 200 | 30 | 1 | 6.88 | 89.0 |
| 11 a | 200 | 30 | 3 | 9.44 | 89.9 |
| 12 a | 200 | 30 | 4 | 9.46 | 91.7 |
| 13 a | 200 | 30 | 5 | 5.65 | 92.1 |
| 14 b | 200 | 30 | 0 | 59.8 | 94.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Bai, Q.; Ma, K.; Yang, M.; Liu, B. MgCl2-Supported Titanium Ziegler-Natta Catalyst Using Carbon Dioxide-Based Poly(propylene ether carbonate) Diols as Internal Electron Donor for 1-Butene Polymerization. Polymers 2017, 9, 627. https://doi.org/10.3390/polym9110627
Cui X, Bai Q, Ma K, Yang M, Liu B. MgCl2-Supported Titanium Ziegler-Natta Catalyst Using Carbon Dioxide-Based Poly(propylene ether carbonate) Diols as Internal Electron Donor for 1-Butene Polymerization. Polymers. 2017; 9(11):627. https://doi.org/10.3390/polym9110627
Chicago/Turabian StyleCui, Xiaopeng, Qing Bai, Kai Ma, Min Yang, and Binyuan Liu. 2017. "MgCl2-Supported Titanium Ziegler-Natta Catalyst Using Carbon Dioxide-Based Poly(propylene ether carbonate) Diols as Internal Electron Donor for 1-Butene Polymerization" Polymers 9, no. 11: 627. https://doi.org/10.3390/polym9110627
APA StyleCui, X., Bai, Q., Ma, K., Yang, M., & Liu, B. (2017). MgCl2-Supported Titanium Ziegler-Natta Catalyst Using Carbon Dioxide-Based Poly(propylene ether carbonate) Diols as Internal Electron Donor for 1-Butene Polymerization. Polymers, 9(11), 627. https://doi.org/10.3390/polym9110627