Shape Memory Polymers Containing Higher Acrylate Content Display Increased Endothelial Cell Attachment
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Polymerization
2.3. Dynamic Mechanical Analysis
2.4. Contact Angle
2.5. Atomic Force Microscopy (AFM)
2.6. Cell Culture
2.7. Light Microscopy
2.8. Live/Dead Assay
2.9. Cell Metabolism
2.10. Statistical Analysis
3. Results
3.1. Dynamic Mechanical Analysis
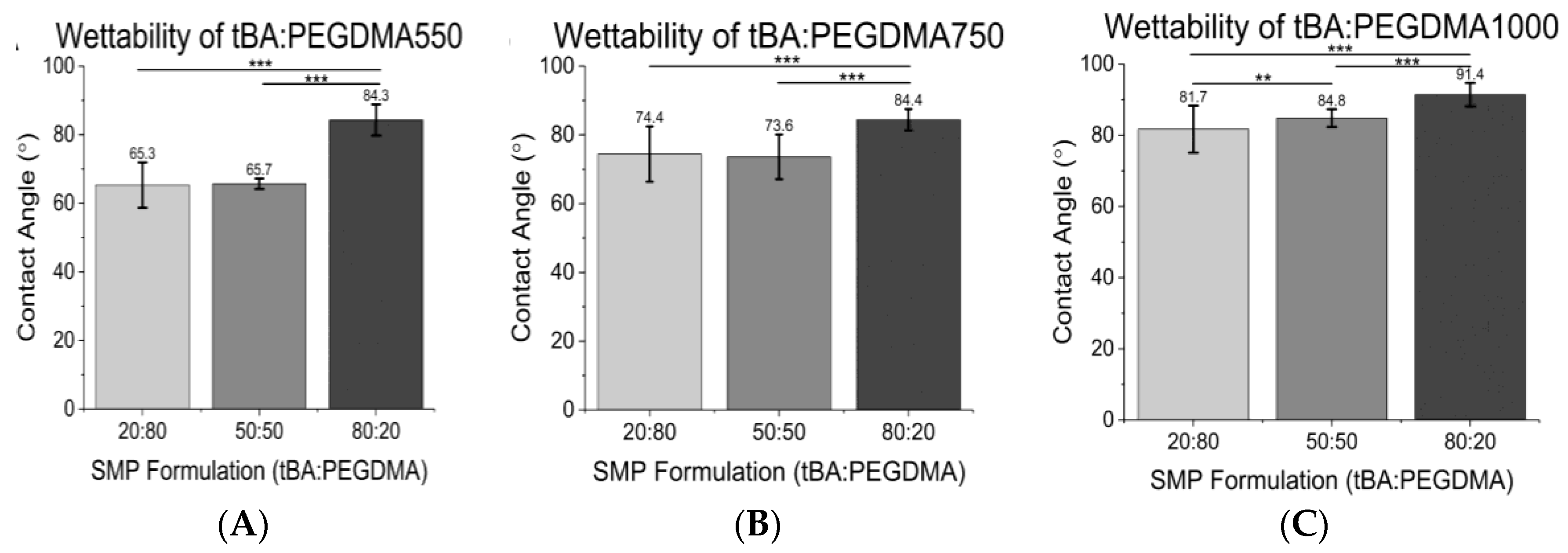
3.2. Contact Angle
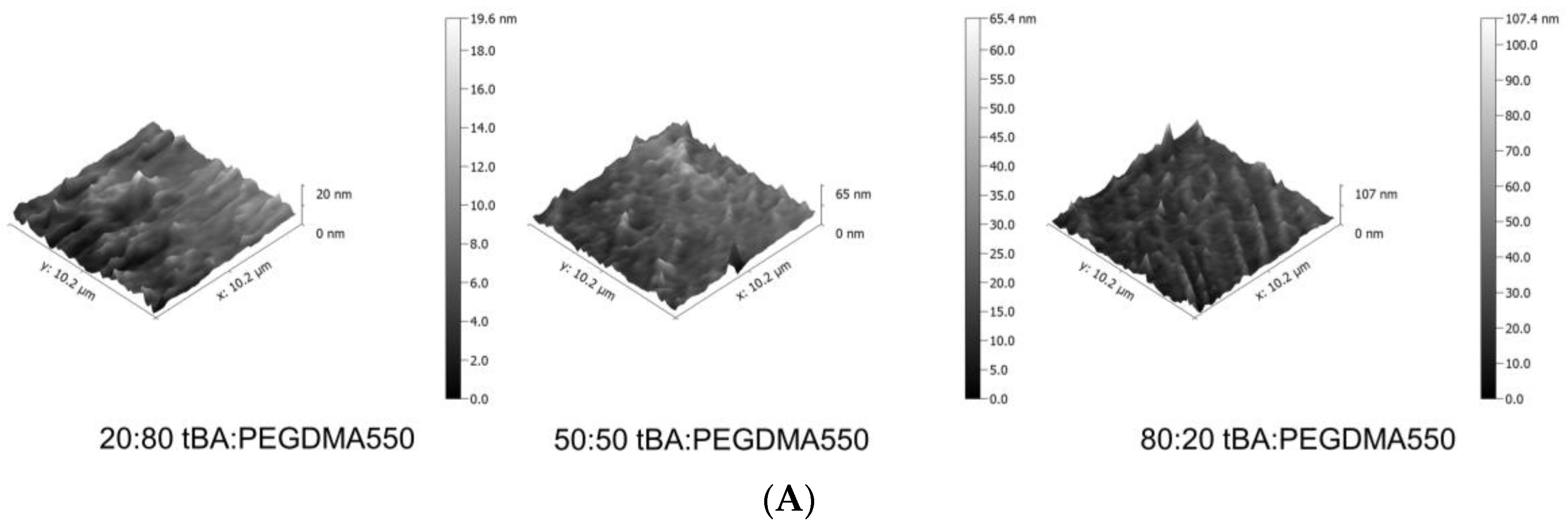
3.3. Atomic Force Microscopy (AFM)
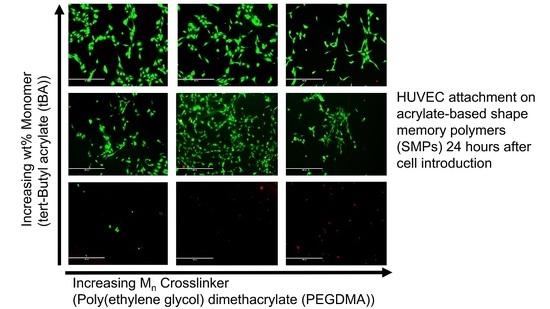
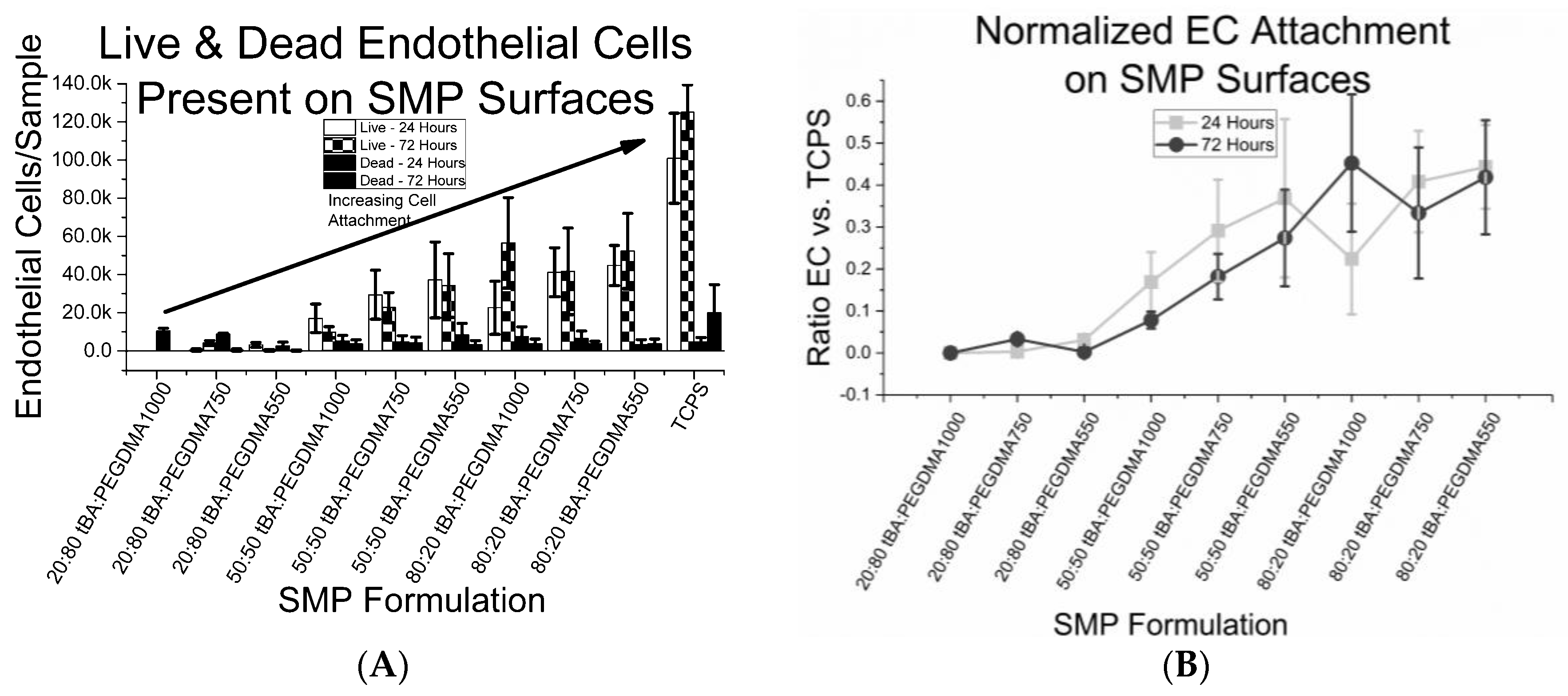
3.4. Cell Viability
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez, A.W.; Chaikof, E.L. Microfabrication and nanotechnology in stent design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.C.; Thapa, A.; Haberstroh, K.M.; Webster, T.J. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials 2004, 25, 53–61. [Google Scholar] [CrossRef]
- Chandy, T.; Das, G.S.; Wilson, R.F.; Rao, G.H.R. Use of plasma glow for surface-engineering biomolecules to enhance bloodcompatibility of Dacron and PTFE vascular prosthesis. Biomaterials 2000, 21, 699–712. [Google Scholar] [CrossRef]
- Reape, T.J.; Groot, P.H.E. Chemokines and atherosclerosis. Atherosclerosis 1999, 147, 213–225. [Google Scholar] [CrossRef]
- Morice, M.C.; Serruys, P.W.; Kappetein, A.P.; Feldman, T.E.; Stahle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Choi, J.W.; Ruzyllo, W.; et al. Five-Year Outcomes in Patients with Left Main Disease Treated with Either Percutaneous Coronary Intervention or Coronary Artery Bypass Grafting in the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery Trial. Circulation 2014, 129, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Luscher, T.F.; Steffel, J.; Eberli, F.R.; Joner, M.; Nakazawa, G.; Tanner, F.C.; Viramani, R. Drug-Eluting Stent and Coronary Thrombosis: Biological Mechanisms and Clinical Implications. Circulation 2007, 115, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Nguyen, K.T.; Brilakis, E.S.; Yang, J.; Fuh, E.; Banerjee, S. Enhanced Endothelialization of a New Stent Polymer Through Surface Enhancement and Incorporation of Growth Factor-Delivering Microparticles. J. Cardiovasc. Transl. Res. 2012, 5, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Grabow, N.; Martin, D.P.; Schmitz, K.-P.; Sternberg, K. Absorbable polymer stent technologies for vascular regeneration. J. Chem. Technol. Biotechnol. 2010, 85, 744–751. [Google Scholar] [CrossRef]
- Boura, C.; Menu, P.; Payan, E.; Picart, C.; Voegel, J.C.; Muller, S.; Stoltz, J.F. Endothelial cells grown on thin polyelectrolyte mutlilayered films: An evaluation of a new versatile surface modification. Biomaterials 2003, 24, 3521–3530. [Google Scholar] [CrossRef]
- McFarland, C.D.; Mayer, S.; Scotchford, C.; Dalton, B.A.; Steele, J.G.; Downes, S. Attachment of cultured human bone cells to novel polymers. J. Biomed. Mater. Res. 1999, 44, 1–11. [Google Scholar] [CrossRef]
- Pislaru, S.V.; Harbuzariu, A.; Agarwal, G.; Latg, T.W.; Sandhu, N.; Aa, C.M.; Kalra, M.; Simari, R.D.; Sandhu, G.S. Magnetic Forces Enable Rapid Endothelialization of Synthetic Vascular Grafts. Circulation 2006, 114, I-314–I-318. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Holmes, D.R.J. Drug-Eluting Coronary-Artery Stents. N. Eng. J. Med. 2013, 368, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Wise, S.G.; Yin, Y.; Wu, B.; James, B.; Zreiqat, H.; Mckenzie, D.R.; Bao, S.; Weiss, A.S.; Ng, M.K.C.; et al. In vivo biocompatibility of a plasma-activated, coronary stent coating. Biomaterials 2012, 33, 7984–7992. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Coltart, J. Miracle stents’—A future without restenosis. McGill J. Med. MJM 2007, 10, 105–111. [Google Scholar] [PubMed]
- Wise, S.G.; Waterhouse, A.; Michael, P.; Ng, M.K.C. Extracellular Matrix Molecules Facilitating Vascular Biointegration. J. Funct. Biomater. 2012, 3, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, V.; McSweene, P.A.; Shi, Q.; Bruno, B.; Ishida, A.; Nash, R.; Storb, R.F.; Sauvage, L.R.; Hammond, W.P.; Wu, M.H.-D. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34+ bone marrow cells: Presented to the Western Vascular Society, Whistler, BC, Canada, September 11, 1998. Blood 2000, 95, 581–585. [Google Scholar] [PubMed]
- Lee, J.H.; Park, J.W.; Lee, H.B. Cell adhesion and growth on polymer surfaces with hydroxyl groups prepared by water vapour plasma treatment. Biomaterials 1991, 12, 443–448. [Google Scholar] [CrossRef]
- McMillan, R.; Meeks, B.; Bensebaa, F.; Deslandes, Y.; Sheardown, H. Cell adhesion peptide modification of gold-coated polyurethanes for vascular endothelial cell adhesion. J. Biomed. Mater. Res. 2001, 54, 272–283. [Google Scholar] [CrossRef]
- Balcells, M.; Edelman, E.R. Effect of pre-adsorbed proteins on attachment, proliferation, and function of endothelial cells. J. Cell. Physiol. 2002, 191, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-I.; Lu, S.-K.; Tian, T.-Y.; Hong, R.-C.; Lee, W.-H.; Tsai, C.-H. Comparison of endothelial cells grown on different stent materials. J. Biomed. Mater. Res. A 2006, 76A, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Herring, M.; Gardner, A.; Glover, J. A single-staged technique for seeding vascular grafts with autogenous endothelium. Surgery 1978, 84, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Sprague, E.A.; Tio, F.; Ahmed, S.H.; Granada, J.F.; Bailey, S.R. Impact of Parallel Micro-Engineered Stent Grooves on Endothelial Cell Migration, Proliferation, and Function an In Vivo Correlation Study of the Healing Response in the Coronary Swine Model. Circ. Cardiovasc. Interv. 2012, 5, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Nichol, J.W.; Bae, H.; Tekin, H.; Bischoff, J.; Khademhosseini, A. Hydrogel surfaces to promote attachment and spreading of endothelial progenitor cells. J. Tissue Eng. Regen. Med. 2013, 7, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Yang, P.; Leng, Y.X.; Wang, J.; Sun, H.; Wu, X.; Zhao, A.S. Improving Blood Compatibility of Cardiovascular Devices by Surface Modification. Key Eng. Mater. 2007, 342–343, 801–804. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Shandas, R.; Lanning, C.; Rech, B.; Eckstein, A.; Gall, K. Unconstrained recovery characterization of shape-memory polymer networks for cardiovascular applications. Biomaterials 2007, 28, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Kelch, S. Shape-Memory Polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- El Feninat, F.; Laroche, G.; Fiset, M.; Mantovani, D. Shape Memory Materials for Biomedical Applications. Adv. Eng. Mater. 2002, 4, 91–104. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Shandas, R.; Safranski, D.; Ortega, A.M.; Sassaman, K.; Gall, K. Strong, Tailored, Biocompatible Shape-Memory Polymer Networks. Adv. Funct. Mater. 2008, 18, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Behl, M.; Hiebl, B.; Wischke, C. Shape-memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Devices 2010, 7, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, T. Review on the Functional Determinants and Durability of Shape Memory Polymers. Polymers 2010, 2, 120–158. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Ding, Z.; Zhao, Y.; Wang, C.C.; Purnawali, H.; Tang, C. Stimulus-responsive shape memory materials: A review. Mater Des. 2012, 33, 577–640. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49–50, 79–120. [Google Scholar] [CrossRef]
- Xie, T. Recent advances in polymer shape memory. Polymer 2011, 52, 4985–5000. [Google Scholar] [CrossRef]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a Key Functional Requirement of Next-Generation Medical Devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, T.; Ecker, M.; Schildhauer, M.; Maskos, M. Switchable information carriers based on shape memory polymer. J. Mater Chem. 2012, 22, 7757–7766. [Google Scholar] [CrossRef]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 964–1726. [Google Scholar] [CrossRef]
- Mirtschin, N.; Pretsch, T. Programming of One- and Two-Step Stress Recovery in a Poly (ester urethane). Polymers 2017, 9, 98. [Google Scholar] [CrossRef]
- Behl, M.; Kratz, K.; Noechel, U.; Sauter, T.; Lendlein, A. Temperature-memory polymer actuators. Proc. Natl. Acad. Sci. USA 2013, 110, 12555–12559. [Google Scholar] [CrossRef] [PubMed]
- Bothe, M.; Pretsch, T. Bidirectional actuation of a thermoplastic polyurethane elastomer. J. Mater. Chem. A 2013, 1, 14491–14497. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Lu, H.B.; Wang, C.C.; Zhang, J.L. Shape memory technology for active assembly/disassembly: Fundamentals, techniques and example applications. Assem. Autom. 2014, 34, 78–93. [Google Scholar] [CrossRef]
- Bar-Cohen, Y.; Zhang, Q. Electroactive polymer actuators and sensors. MRS Bull. 2008, 33, 173–181. [Google Scholar] [CrossRef]
- Xiao, X.; Kong, D.; Qiu, X.; Zhang, W.; Liu, Y.; Zhang, S.; Zhang, F.; Hu, Y.; Leng, J. Shape memory polymers with high and low temperature resistant properties. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Du, H.Y.; Liu, L.W.; Leng, J.S. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 023001. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Lyons, M.B.; Rech, B.; Gall, K.; Shandas, R. Cytotoxicity and thermomechanical behavior of biomedical shape-memory polymer networks post-sterilization. Biomed. Mater. 2008, 3, 15010. [Google Scholar] [CrossRef] [PubMed]
- Gall, K.; Yakacki, C.M.; Liu, Y.; Shandas, R.; Willett, N.; Anseth, K.S. Thermomechanics of the shape memory effect in polymers for biomedical applications. J. Biomed. Mater. Res. A 2005, 73, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zimkowski, M.M.; Rentschler, M.E.; Schoen, J.A.; Mandava, N.; Shandas, R. Biocompatibility and tissue integration of a novel shape memory surgical mesh for ventral hernia: In vivo animal studies. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Zimkowski, M.M.; Rentschler, M.E.; Schoen, J.; Rech, B.A.; Mandava, N.; Shandas, R. Integrating a novel shape memory polymer into surgical meshes decreases placement time in laparoscopic surgery: An in vitro and acute in vivo study. J. Biomed. Mater. Res. A 2013, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.L.; Frank, B.; Suo, Y.; Garoff, S. Physics of contact angle measurement. Colloids Surf. Physicochem. Eng. Asp. 1999, 156, 177–189. [Google Scholar] [CrossRef]
- Westra, K.L.; Thomson, D.J. Effect of tip shape on surface roughness measurements from atomic force microscopy images of thin films. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1995, 13, 344–349. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Yakacki, C.M.; Willis, S.; Luders, C.; Gall, K. Deformation Limits in Shape-Memory Polymers. Adv. Eng. Mater. 2008, 10, 112–119. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Yakacki, C.M.; Brahmbhatt, P.D.; Chambers, M.L. Modeling the Relaxation Mechanisms of Amorphous Shape Memory Polymers. Adv. Mater. 2010, 22, 3411–3423. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and Mechanical Properties Significantly Influence Cell Attachment, Proliferation, and Migration within Collagen Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2010, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Tirrell, M.; Kokkoli, E.; Biesalski, M. The role of surface science in bioengineered materials. Surf. Sci. 2002, 500, 61–83. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Lakhera, N.; Yakacki, C.M.; Nguyen, T.D.; Frick, C.P. Partially constrained recovery of (meth)acrylate shape-memory polymer networks. J. Appl. Polym. Sci. 2012, 126, 72–82. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, S.; Benavene, J.; Berg, R.W.; Stibius, K.; Larsen, M.S.; Bohr, H.; Helix-Nielsen, K. Tailoring Properties of Biocompatible PEG-DMA Hydrogels with UV Light. Mater. Sci. Appl. 2012, 3, 425–431. [Google Scholar] [CrossRef]
- Wang, G.; Bai, Y.; Ma, X.; Wang, W.; Yin, Q.; Du, Z. Effects of the PEG length of polycarboxylate-based terpolymers on their dispersion properties. J. Mol. Liq. 2017, 225, 333–338. [Google Scholar] [CrossRef]
- Pich, A.; Berger, S.; Ornatsky, O.; Baranov, V.; Winnik, M.A. The influence of PEG macromonomers on the size and properties of thermosensitive aqueous microgels. Colloid Polym. Sci. 2009, 287, 269–275. [Google Scholar] [CrossRef]
- Lampin, M.; Warocquier-Clérout, R.; Legris, C.; Degrange, M.; Sigot-Luizard, M.F. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. 1997, 36, 99–108. [Google Scholar] [CrossRef]
- Shadpour, H.; Albritton, N.L. In-Situ Roughening of Polymeric Microstructures. ACS Appl. Mater. Interfaces 2010, 2, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Liu, D.Z.; Wang, S.Y.; Wang, S.S. Enhancement of the growth of human endothelial cells by surface roughness at the nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.W.; Kang, I.-K.; Lee, H.B. Cell behaviour on polymer surfaces with different functional groups. Biomaterials 1994, 15, 705–711. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Jeon, O.; Kwon, I.C.; Park, K. Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Zant, W.; Grijpma, D.W. Synthetic Biodegradable Hydrogels with Excellent Mechanical Properties and Good Cell Adhesion Characteristics Obtianed by the Combinatorial Synthesis of Photo-Cross-Linked Networks. Biomacromolecules 2016, 17, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Van Wachem, P.B.; Hogt, A.H.; Beugeling, T.; Feigen, J.; Bantjs, A.; Detmers, J.P.; van Aken, W.G. Adhesion of cultured human endothelial cells onto methacrylate polymers with varying surface wettability and charge. Biomaterials 1987, 8, 323–328. [Google Scholar] [CrossRef]
- Dichek, D.A.; Neville, R.F.; Zwiebel, J.A.; Freeman, S.M.; Leon, M.B.; Anderson, W.F. Seeding of intravascular stents with genetically engineered endothelial cells. Circulation 1989, 80, 1347–1353. [Google Scholar] [CrossRef] [PubMed]

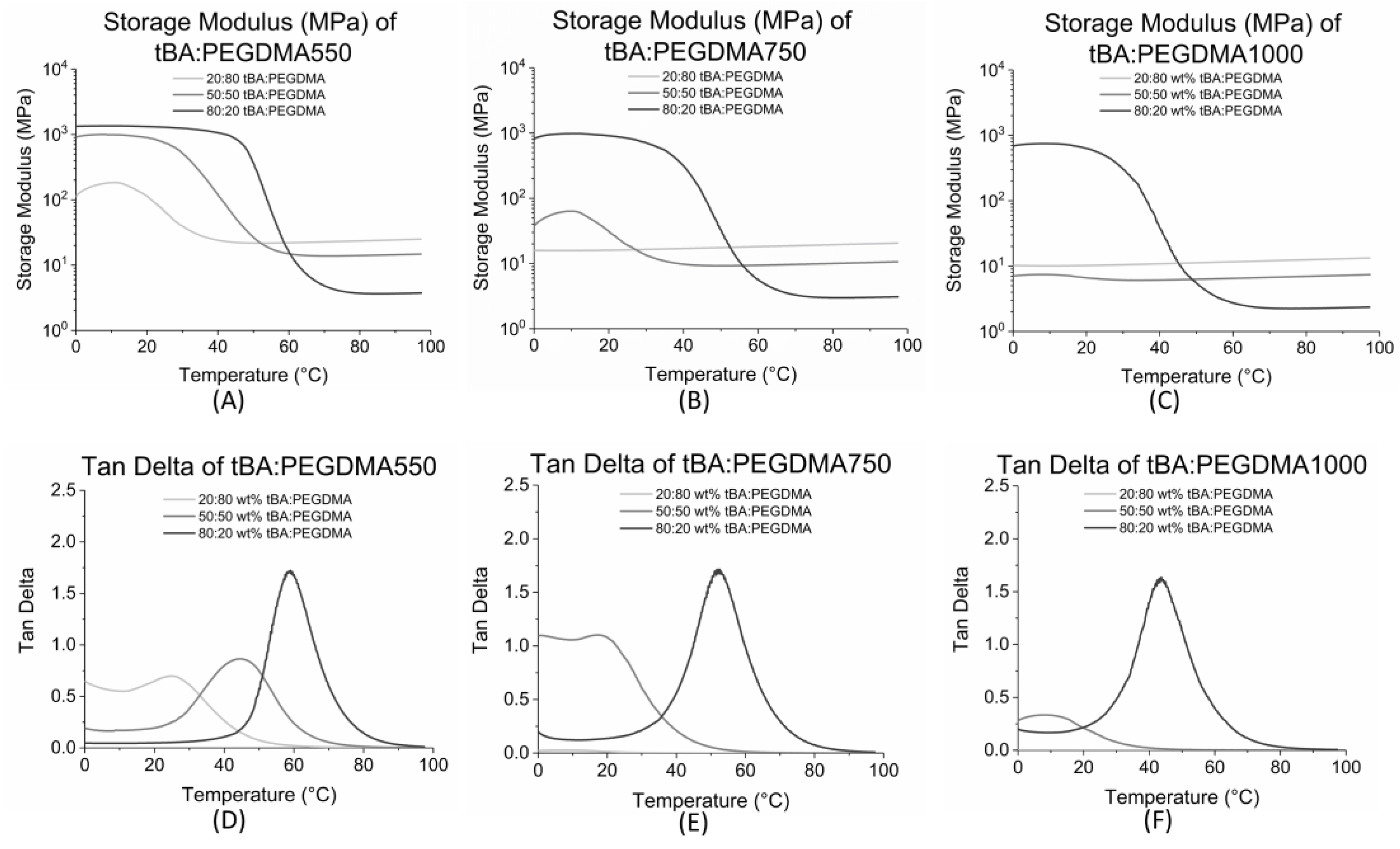
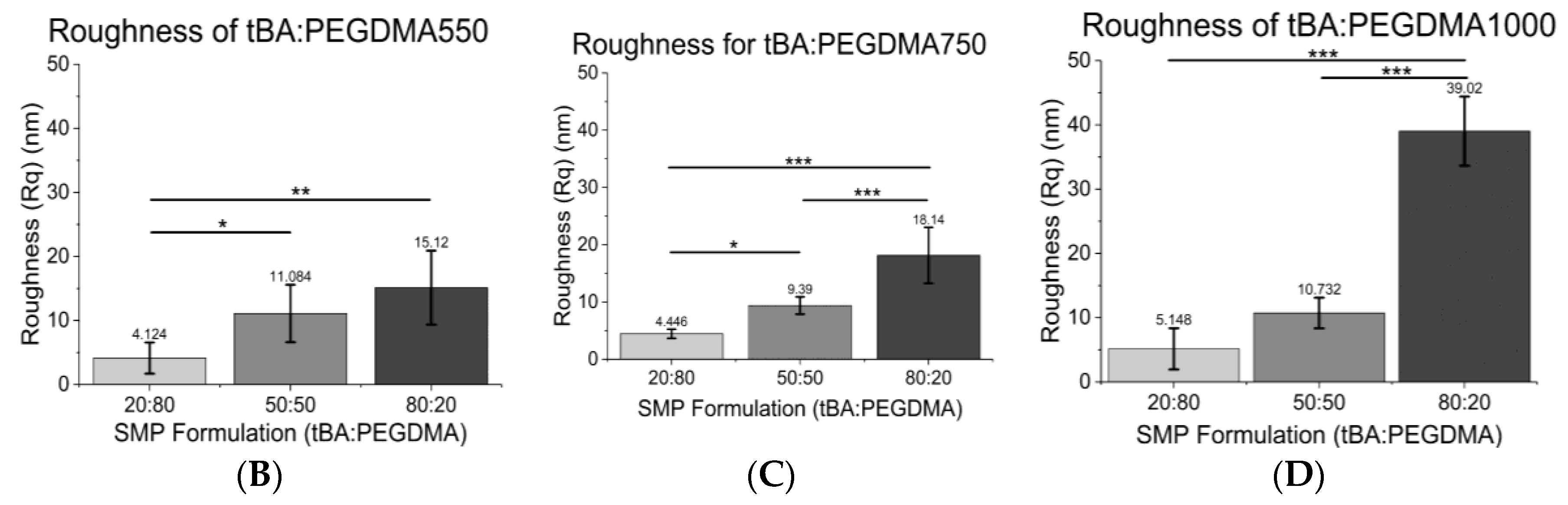
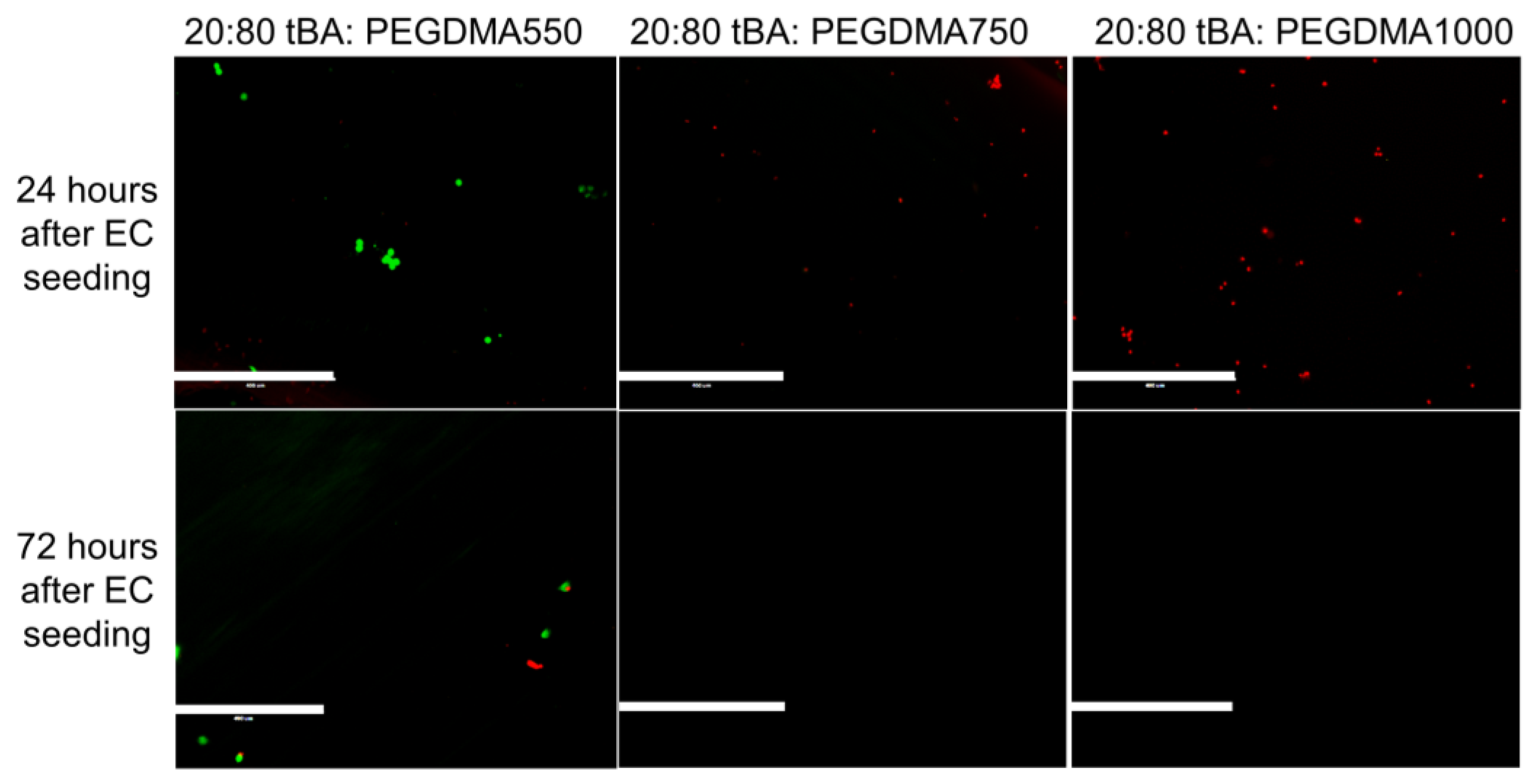
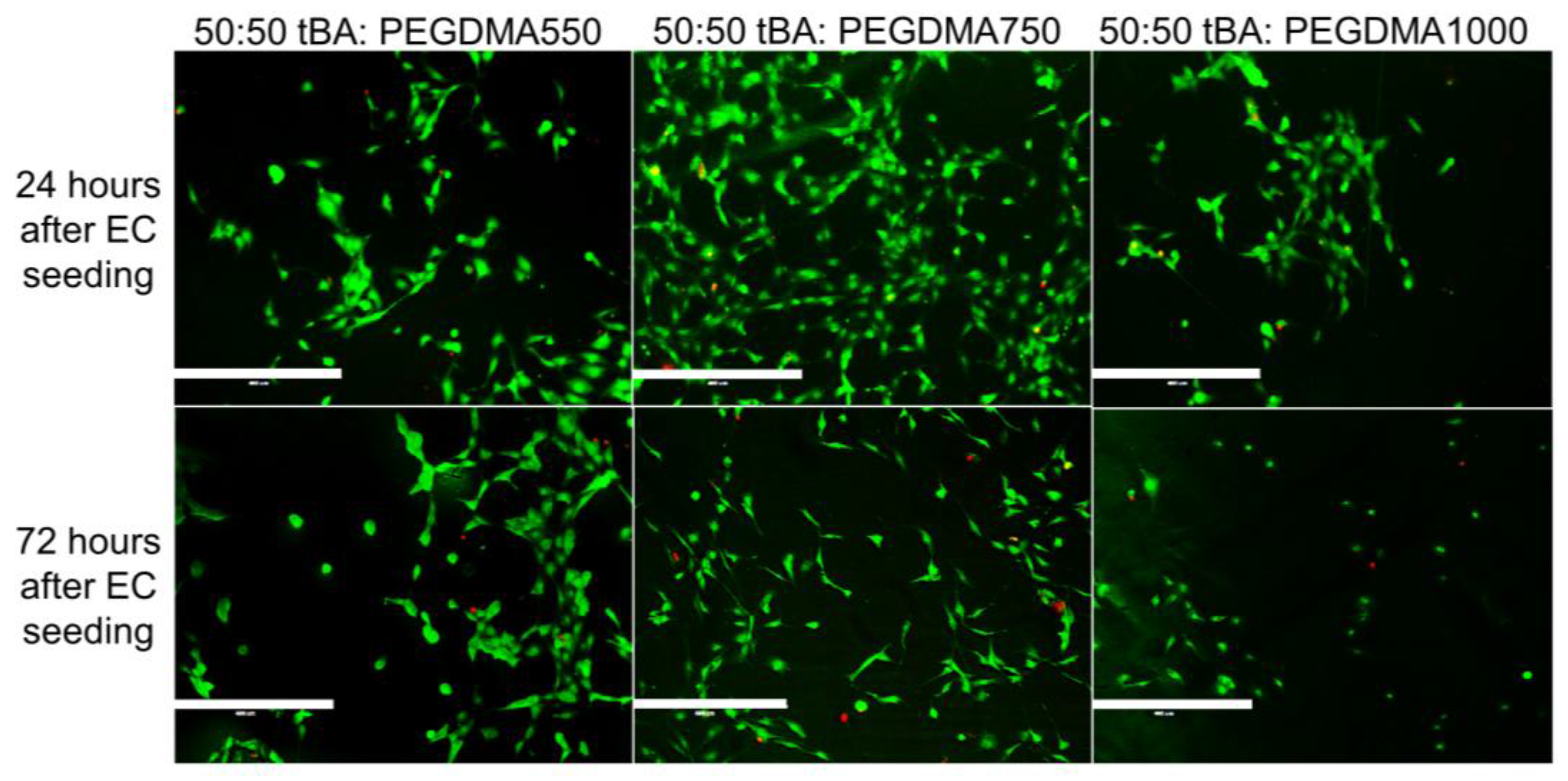
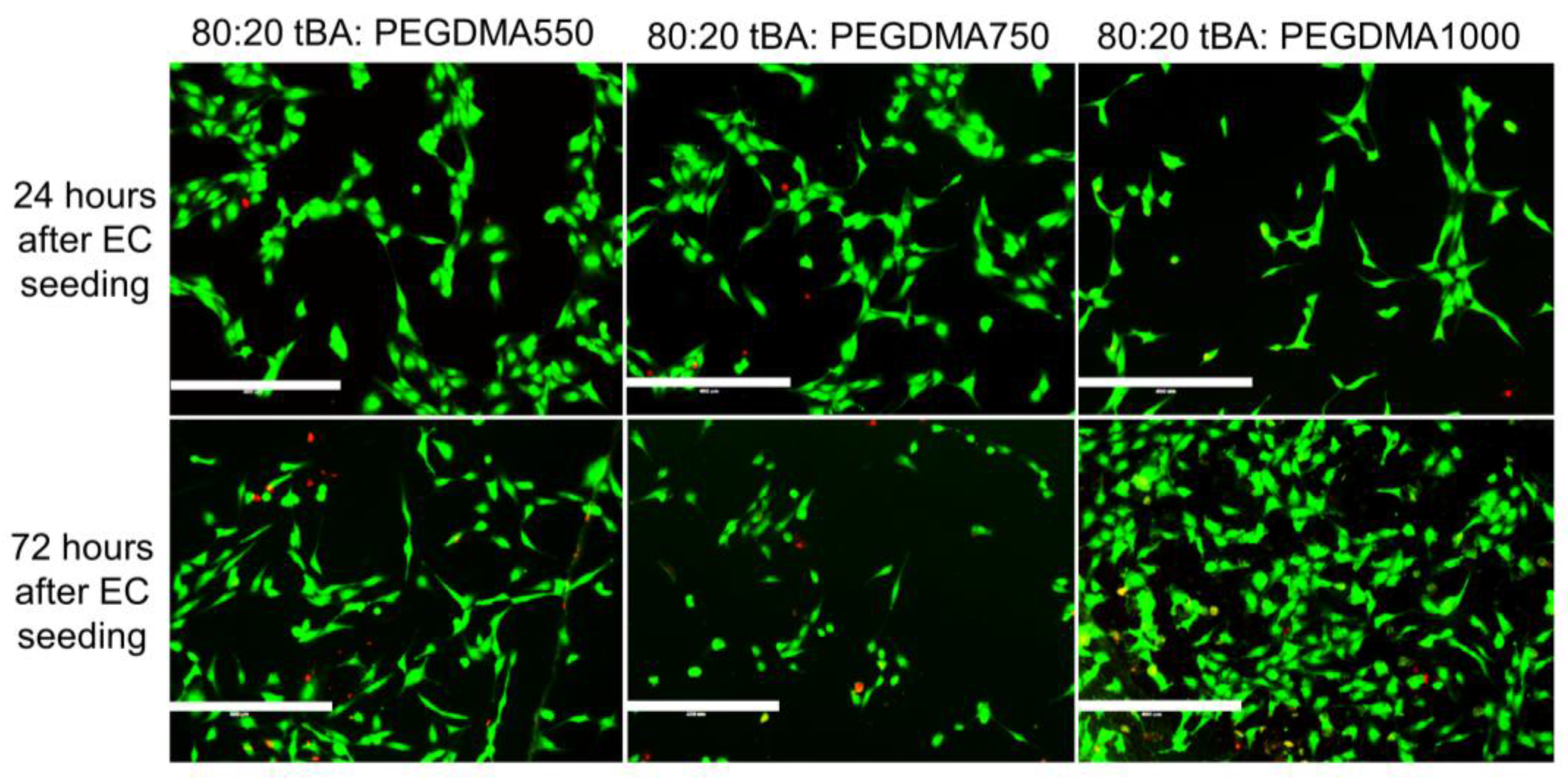

| Formulation | Tg (°C) | Tonset (°C) | Tg Range (°C) |
|---|---|---|---|
| 20:80 tBA:PEGDMA1000 | 6 ± 2 | - | - |
| 20:80 tBA:PEGDMA750 | 11 ± 1 | - | - |
| 20:80 tBA:PEGDMA550 | 25 ± 1 | 15 ± 2 | 19 ± 5 |
| 50:50 tBA:PEGDMA1000 | 10 ± 1 | 8 ± 3 | 3 ± 5 |
| 50:50 tBA:PEGDMA750 | 19 ± 2 | 12 ± 1 | 13 ± 4 |
| 50:50 tBA:PEGDMA550 | 44 ± 1 | 25 ± 3 | 38 ± 7 |
| 80:20 tBA:PEGDMA1000 | 44 ± 1 | 26 ± 3 | 37 ± 4 |
| 80:20 tBA:PEGDMA750 | 52 ± 1 | 35 ± 1 | 32 ± 1 |
| 80:20 tBA:PEGDMA550 | 60 ± 3 | 47 ± 3 | 24 ± 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindarajan, T.; Shandas, R. Shape Memory Polymers Containing Higher Acrylate Content Display Increased Endothelial Cell Attachment. Polymers 2017, 9, 572. https://doi.org/10.3390/polym9110572
Govindarajan T, Shandas R. Shape Memory Polymers Containing Higher Acrylate Content Display Increased Endothelial Cell Attachment. Polymers. 2017; 9(11):572. https://doi.org/10.3390/polym9110572
Chicago/Turabian StyleGovindarajan, Tina, and Robin Shandas. 2017. "Shape Memory Polymers Containing Higher Acrylate Content Display Increased Endothelial Cell Attachment" Polymers 9, no. 11: 572. https://doi.org/10.3390/polym9110572
APA StyleGovindarajan, T., & Shandas, R. (2017). Shape Memory Polymers Containing Higher Acrylate Content Display Increased Endothelial Cell Attachment. Polymers, 9(11), 572. https://doi.org/10.3390/polym9110572