Preparation and Evaluation of Green Composites from Microcrystalline Cellulose and a Soybean-Oil Derivative
Abstract
:1. Introduction
2. Materials and Methods
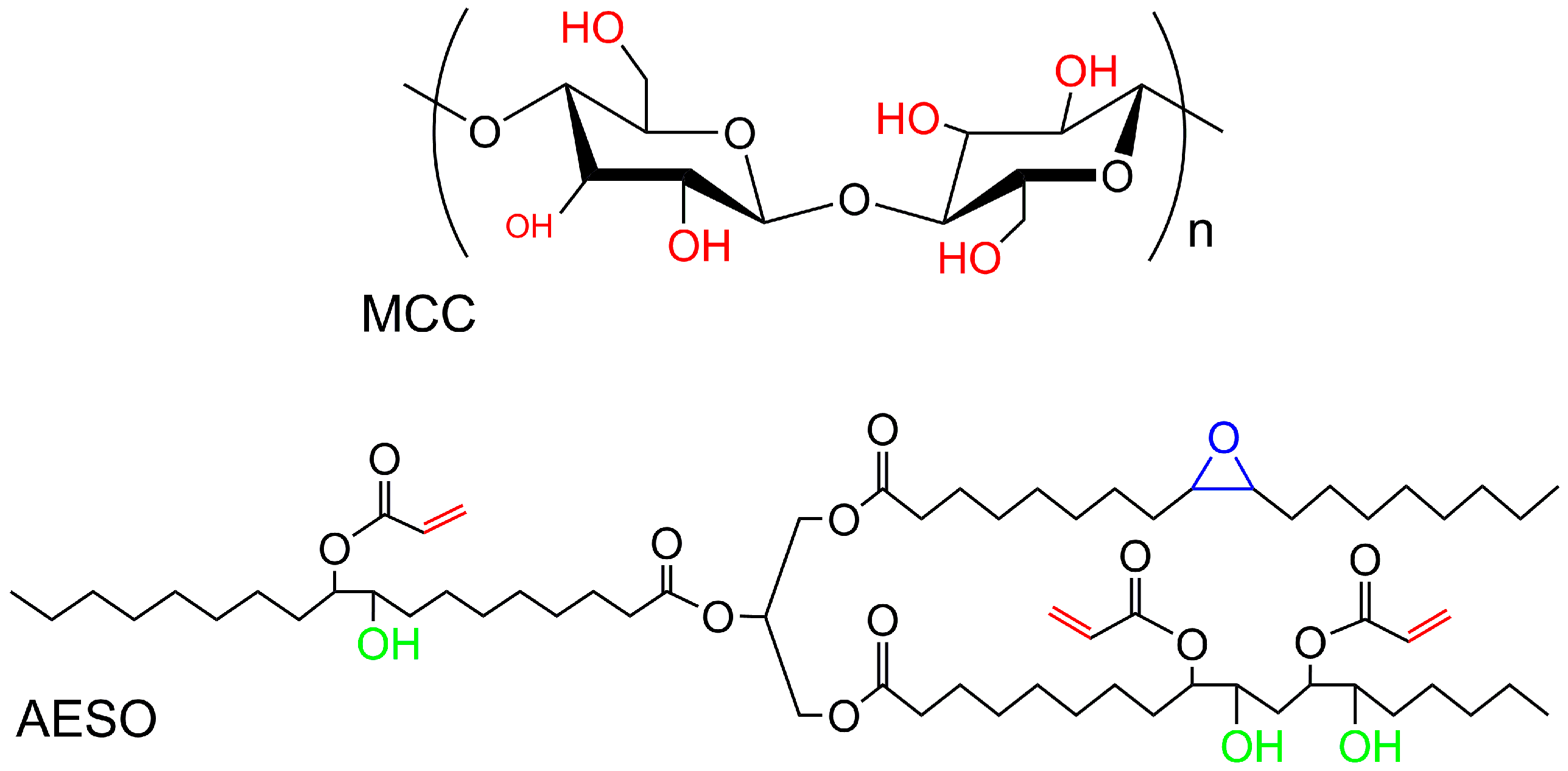
2.1. Materials
2.2. Preparation of MCC/AESO Composites
2.3. Characterization
3. Results and Discussion
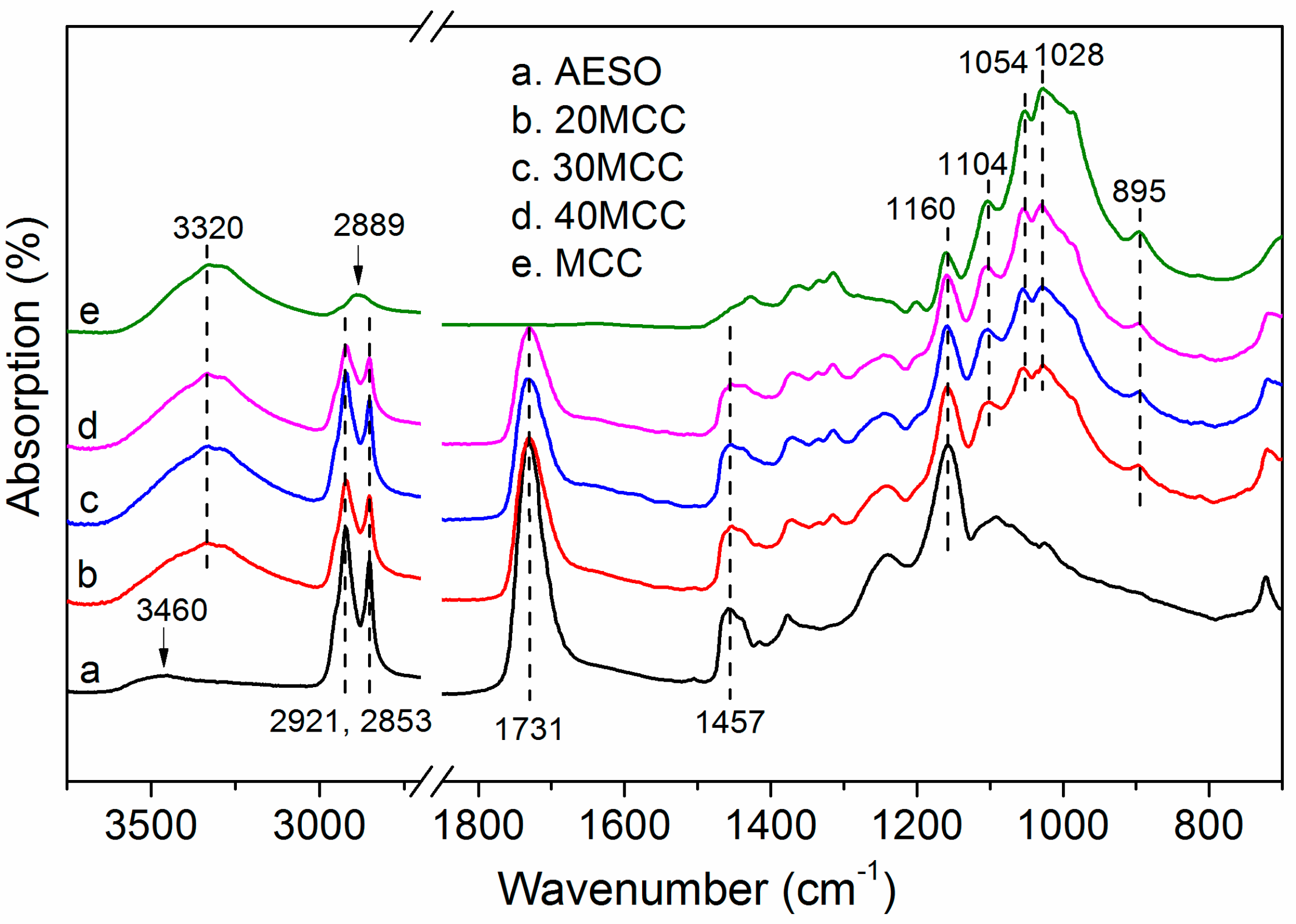
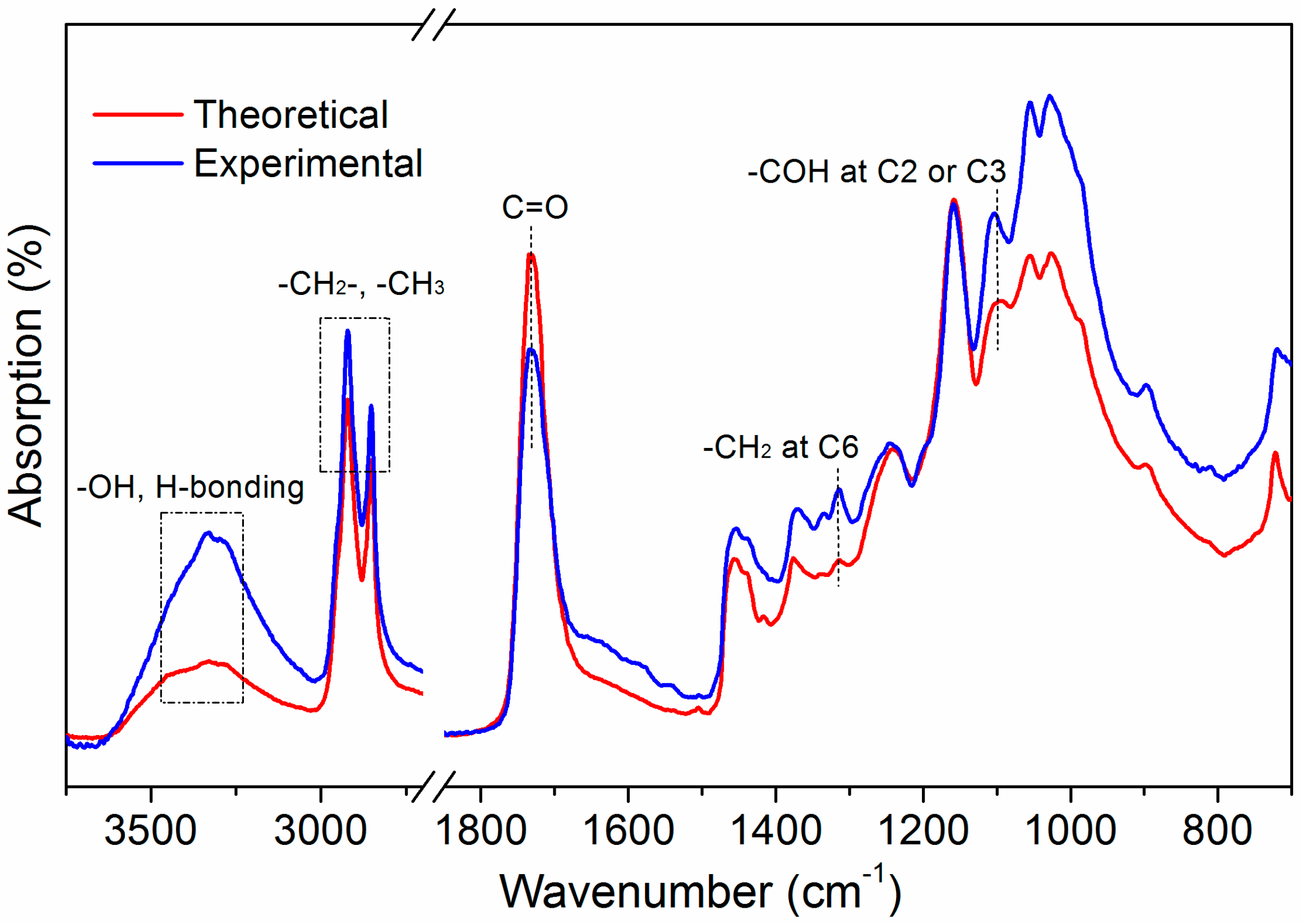
3.1. ATR-FTIR Analysis
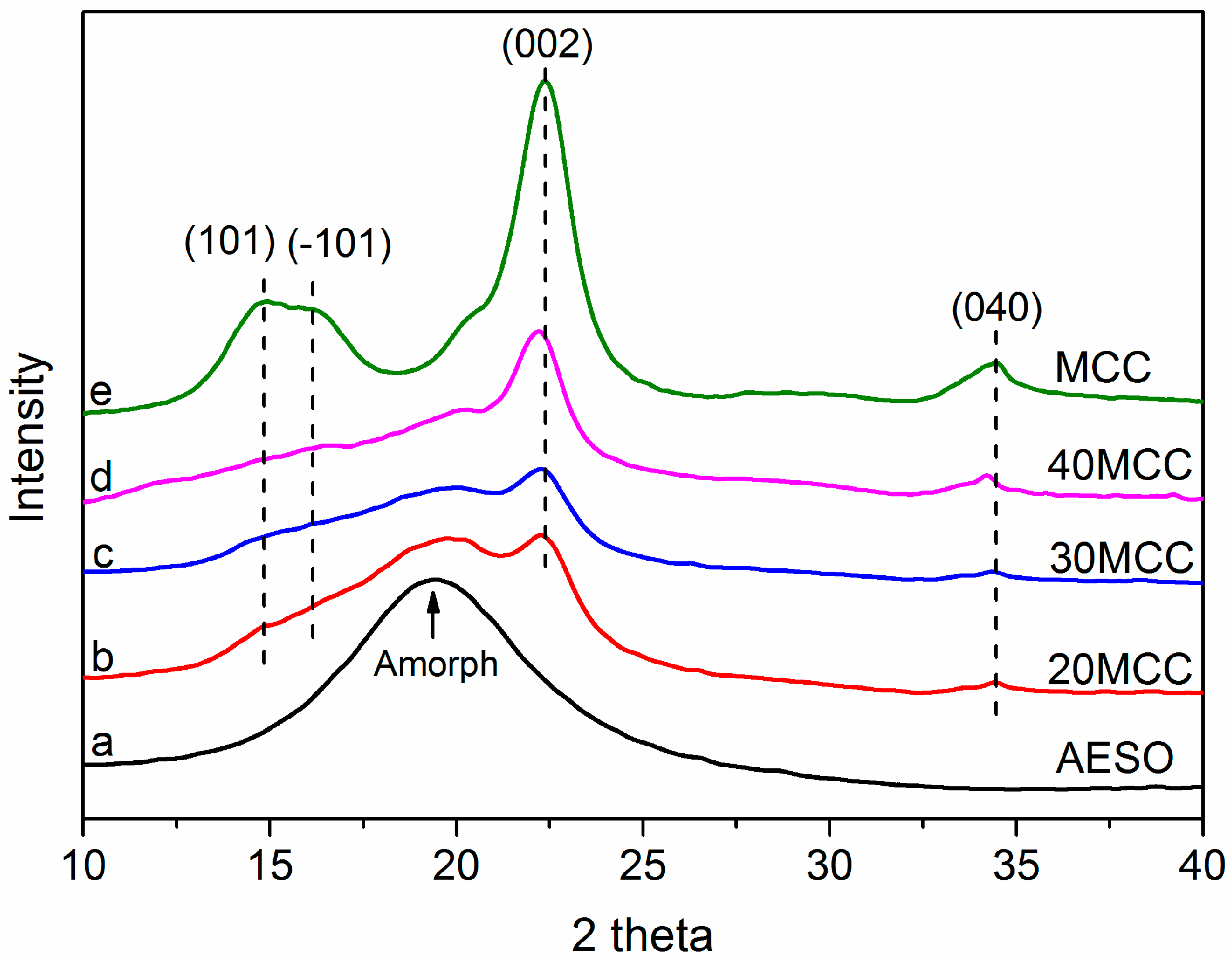
3.2. XRD Analysis
3.3. Physical Properties
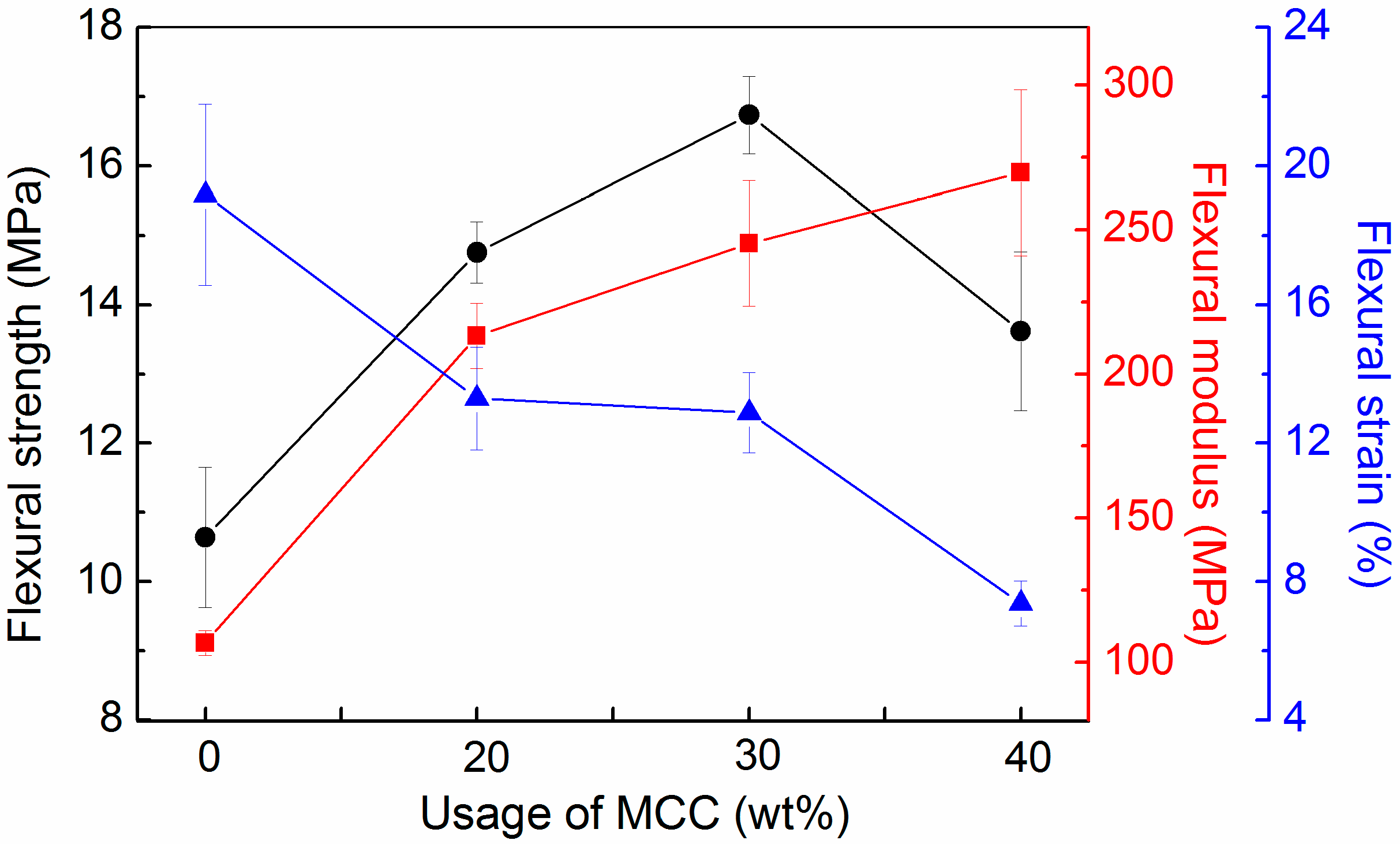
3.4. Flexural Properties
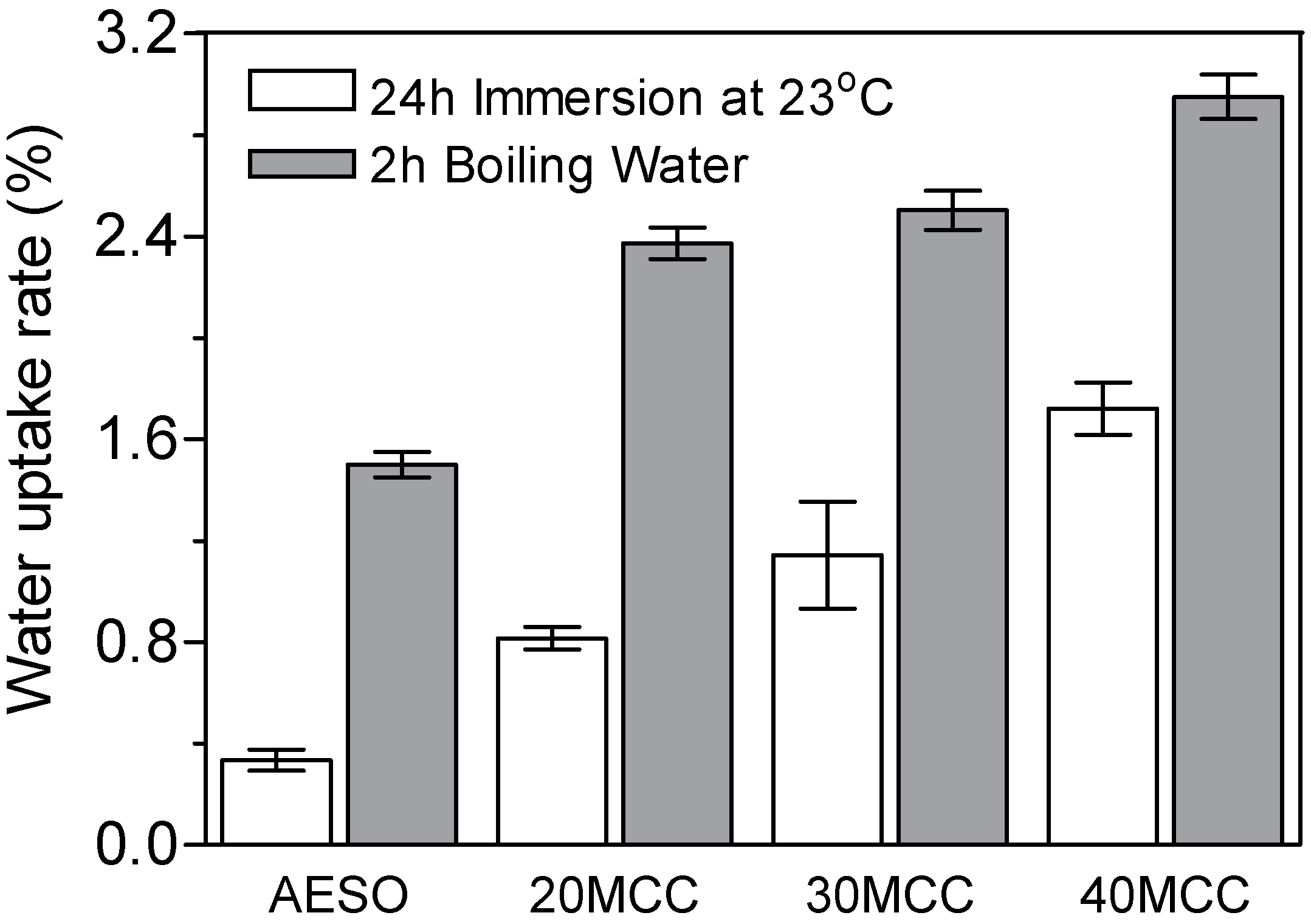
3.5. Water Absorption
3.6. Thermal Stability
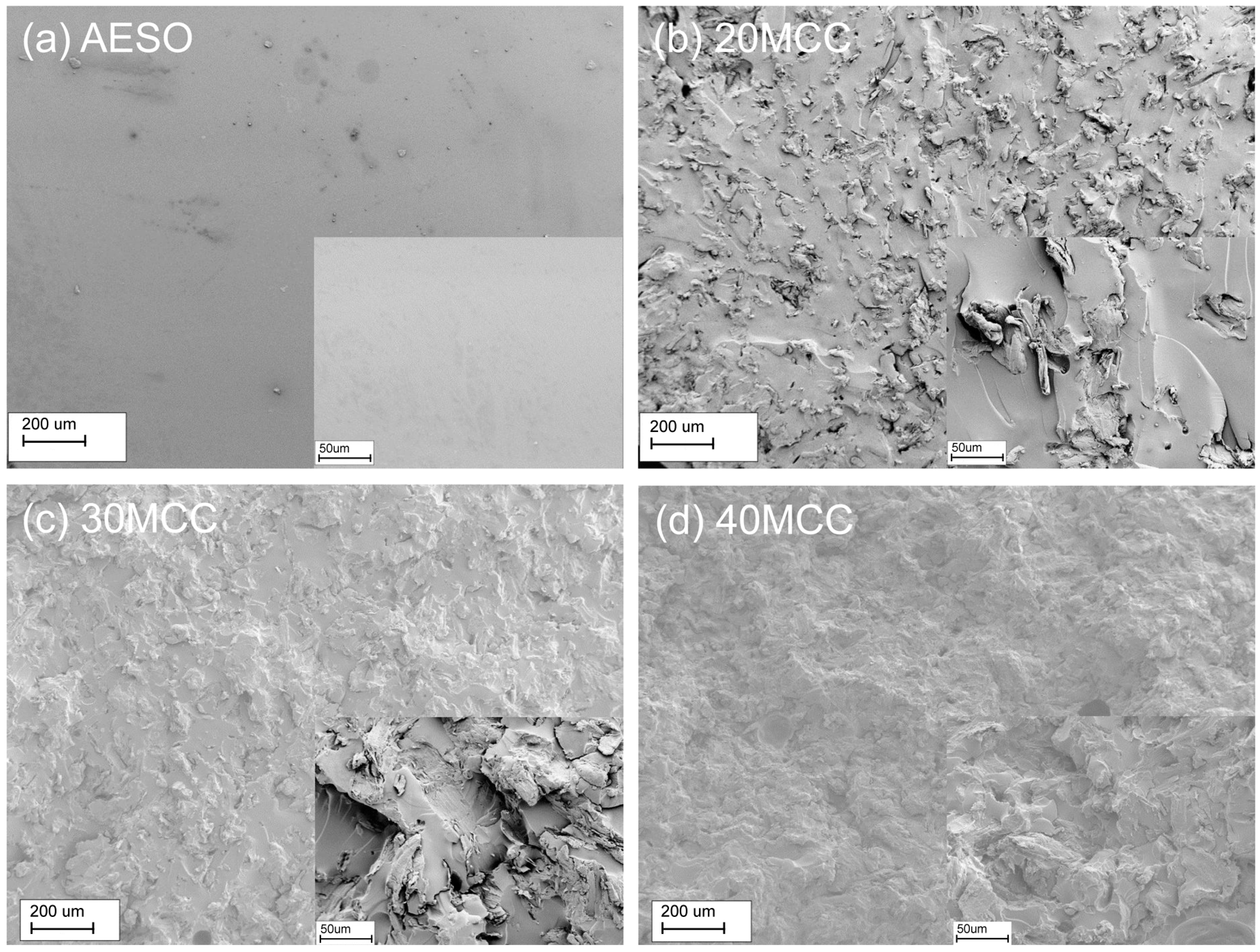
3.7. SEM Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, X.; Mohanty, A.; Misra, M. Green composites from soy-based biopolyurethane with microcrystalline cellulose. Macromol. Mater. Eng. 2013, 298, 412–418. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Y.; Ge, X.; Li, M.; Li, X.; Cho, U.R. Graft copolymers of microcrystalline cellulose as reinforcing agent for elastomers based on natural rubber. J. Appl. Polym. Sci. 2016, 133, 43087. [Google Scholar] [CrossRef]
- Zulkifli, N.I.; Samat, N.; Anuar, H.; Zainuddin, N. Mechanical properties and failure modes of recycled polypropylene/microcrystalline cellulose composites. Mater. Des. 2015, 69, 114–123. [Google Scholar] [CrossRef]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Janardhnan, S.; Sain, M.M. Isolation of cellulose microfibrils—An enzymatic approach. Bioresources 2007, 1, 176–188. [Google Scholar]
- Pinkl, S.; Veigel, S.; Colson, J.; Gindl-Altmutter, W. Nanopaper properties and adhesive performance of microfibrillated cellulose from different (ligno-) cellulosic raw materials. Polymers 2017, 9, 326. [Google Scholar] [CrossRef]
- Hua, S.; Chen, F.; Liu, Z.-Y.; Yang, W.; Yang, M.-B. Preparation of cellulose-graft-polylactic acid via melt copolycondensation for use in polylactic acid based composites: Synthesis, characterization and properties. RSC Adv. 2016, 6, 1973–1983. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, Y.; Cheng, Q.; Liu, Y.; Essien, C.; Via, B.; Wang, X.; Sun, R.; Taylor, S. Characterization of epoxy composites reinforced with wax encapsulated microcrystalline cellulose. Polymers 2016, 8, 415. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Microcrystalline-cellulose and polypropylene based composite: A simple, selective and effective material for microwavable packaging. Carbohydr. Polym. 2016, 142, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Spoljaric, S.; Genovese, A.; Shanks, R.A. Polypropylene-microcrystalline cellulose composites with enhanced compatibility and properties. Compos. A Appl. Sci. Manuf. 2009, 40, 791–799. [Google Scholar] [CrossRef]
- Yakubu, A.; Umar, T.; Mohammed, S. Chemical modification of microcrystalline cellulose: Improvement of barrier surface properties to enhance surface interactions with some synthetic polymers for biodegradable packaging material processing and applications in textile, food and pharmaceutical industry. Adv. Appl. Sci. Res. 2011, 2, 532–540. [Google Scholar]
- Cetin, N.S.; ÇETİN, N.; Harper, D.P. Vinyl acetate-modified microcrystalline cellulose-reinforced HDPE composites prepared by twin-screw extrusion. Turk. J. Agric. For. 2015, 39, 39–47. [Google Scholar] [CrossRef]
- Xiao, L.; Mai, Y.; He, F.; Yu, L.; Zhang, L.; Tang, H.; Yang, G. Bio-based green composites with high performance from poly (lactic acid) and surface-modified microcrystalline cellulose. J. Mater. Chem. 2012, 22, 15732–15739. [Google Scholar] [CrossRef]
- Deng, F.; Ge, X.; Zhang, Y.; Li, M.C.; Cho, U.R. Synthesis and characterization of microcrystalline cellulose-graft-poly (methyl methacrylate) copolymers and their application as rubber reinforcements. J. Appl. Polym. Sci. 2015, 132, 42666. [Google Scholar] [CrossRef]
- Zeng, D.; Lv, J.; Wei, C.; Yu, C. Dynamic mechanical properties of sisal fiber cellulose microcrystalline/unsaturated polyester in-situ composites. Polym. Adv. Technol. 2015, 26, 1351–1355. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J. Structure, morphology and mechanical behaviour of novel bio-based polyurethane composites with microcrystalline cellulose. Cellulose 2015, 22, 2471–2481. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J. Bio-polyetherurethane composites with high content of natural ingredients: Hydroxylated soybean oil based polyol, bio glycol and microcrystalline cellulose. Cellulose 2016, 23, 581–592. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Deléglise, M.; Lacrampe, M.-F.; Krawczak, P. Thermosetting (bio) materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Zhang, J.; Gao, Q.; Zhang, S.; Li, J. Physico-chemical properties of soybean meal-based adhesives reinforced by ethylene glycol diglycidyl ether and modified nanocrystalline cellulose. Polymers 2017, 9, 463. [Google Scholar] [CrossRef]
- In Het Panhuis, M.; Thielemans, W.; Minett, A.I.; Leahy, R.; Le Foulgoc, B.; Blau, W.J.; Wool, R.P. A composite from soy oil and carbon nanotubes. Int. J. Nanosci. 2003, 2, 185–194. [Google Scholar] [CrossRef]
- Morye, S.S.; Wool, R.P. Mechanical properties of glass/flax hybrid composites based on a novel modified soybean oil matrix material. Polym. Compos. 2005, 26, 407–416. [Google Scholar] [CrossRef]
- Lu, J.; Hong, C.K.; Wool, R.P. Bio-based nanocomposites from functionalized plant oils and layered silicate. J. Polym. Sci. B 2004, 42, 1441–1450. [Google Scholar] [CrossRef]
- Albayrak, Ö.; Şen, S.; Çaylı, G.; Ortaç, B. Bio-based polymer nanocomposites based on layered silicates having a reactive and renewable intercalant. J. Appl. Polym. Sci. 2013, 130, 2031–2041. [Google Scholar] [CrossRef]
- Adekunle, K.; Ghoreishi, R.; Ehsani, M.; Cho, S.-W.; Skrifvars, M. Jute fiber reinforced methacrylated soybean oil based thermoset composites prepared by vacuum injection molding technique. J. Biobased Mater. Bioenergy 2012, 6, 172–177. [Google Scholar] [CrossRef]
- Lee, T.S.; Choi, H.Y.; Choi, H.N.; Lee, K.-Y.; Kim, S.-H.; Lee, S.G.; Yong, D.K. Effect of surface treatment of ramie fiber on the interfacial adhesion of ramie/acetylated epoxidized soybean oil (AESO) green composite. J. Adhes. Sci. Technol. 2013, 27, 1335–1347. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Kundu, C.K.; Adekunle, K.; Bashir, T.; Skrifvars, M. Properties of green composites with regenerated cellulose fiber and soybean-based thermoset for technical applications. J. Reinf. Plast. Compos. 2014, 33, 193–201. [Google Scholar] [CrossRef]
- National Toxicology Program 14th Report on Carcinogens; U. S. Department of Health and Human Services, Public Health Services: Washington, DC, USA, 2016.
- Qiu, J.F.; Zhang, M.Q.; Rong, M.Z.; Wu, S.P.; Karger-Kocsis, J. Rigid bio-foam plastics with intrinsic flame retardancy derived from soybean oil. J. Mater. Chem. A 2013, 1, 2533–2542. [Google Scholar] [CrossRef]
- Li, A.; Li, K. Pressure-sensitive adhesives based on soybean fatty acids. RSC Adv. 2014, 4, 21521–21530. [Google Scholar] [CrossRef]
- Li, A.; Li, K. Pressure-sensitive adhesives based on epoxidized soybean oil and dicarboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2090–2096. [Google Scholar] [CrossRef]
- Oprea, S. Effects of introducing crude and modified soybean oil into polyurethane structures on the soil-burial biodegradation process. Polym. Plast. Technol. Eng. 2015, 54, 342–349. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose‒structure and characterization. Cell. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Kavkler, K.; Demsar, A. Application of FTIR and Raman spectroscopy to qualitative analysis of structural changes in cellulosic fibres. Tekstilec 2012, 55, 19–31. [Google Scholar]
- Proniewicz, L.M.; Paluszkiewicz, C.; Wesełucha-Birczyńska, A.; Majcherczyk, H.; Barański, A.; Konieczna, A. FT-IR and FT-Raman study of hydrothermally degradated cellulose. J. Mol. Struct. 2001, 596, 163–169. [Google Scholar] [CrossRef]
- Tielmann, P.; Boese, M.; Luft, M.; Reetz, M.T. A practical high-throughput screening system for enantioselectivity by using FTIR spectroscopy. Chem. Eur. J. 2003, 9, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Recent progress in Fourier transform infrared (FTIR) spectroscopy study of compositional, structural and physical attributes of developmental cotton fibers. Materials 2013, 6, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Fabrication and evaluation of acrylated epoxidized castor oil-toughened diglycidyl ether of bisphenol a nanocomposites. Can. J. Chem. Eng. 2015, 93, 2107–2116. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Chen, H.; He, J.; Li, J. A high-performance soy protein isolate-based nanocomposite film modified with microcrystalline cellulose and Cu and Zn nanoclusters. Polymers 2017, 9, 167. [Google Scholar] [CrossRef]
- De Castro, D.O.; Bras, J.; Gandini, A.; Belgacem, N. Surface grafting of cellulose nanocrystals with natural antimicrobial rosin mixture using a green process. Carbohydr. Polym. 2016, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C. True density of microcrystalline cellulose. J. Pharm. Sci. 2005, 94, 2132–2134. [Google Scholar] [CrossRef] [PubMed]
- Rajulu, A.V.; Chary, K.N.; Reddy, G.R.; Meng, Y. Void content, density and weight reduction studies on short bamboo fiber–epoxy composites. J. Reinf. Plast. Compos. 2004, 23, 127–130. [Google Scholar] [CrossRef]
- Thwe, M.M.; Liao, K. Durability of bamboo-glass fiber reinforced polymer matrix hybrid composites. Compos. Sci. Technol. 2003, 63, 375–387. [Google Scholar] [CrossRef]
- He, M.; Zhou, J.; Zhang, H.; Luo, Z.; Yao, J. Microcrystalline cellulose as reactive reinforcing fillers for epoxidized soybean oil polymer composites. J. Appl. Polym. Sci. 2015, 132, 42488. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Performance properties of microcrystalline cellulose as a reinforcing agent in wood plastic composites. Compos. B Eng. 2010, 41, 578–581. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Improvement of properties for biobased composites from modified soybean oil and hemp fibers: Dual role of diisocyanate. Compos. A Appl. Sci. Manuf. 2016, 90, 278–285. [Google Scholar] [CrossRef]
- Chen, T.; Liu, W.; Qiu, R. Mechanical properties and water absorption of hemp fibers-reinforced unsaturated polyester composites: Effect of fiber surface treatment with a heterofunctional monomer. BioResources 2013, 8, 2780–2791. [Google Scholar] [CrossRef]
- Dhakal, H.; Zhang, Z.; Richardson, M. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]

| Composites | Density (g/cm3) | Hardness (HA) |
|---|---|---|
| AESO | 1.02 ± 0.09 | 53.70 ± 3.21 |
| 20MCC | 1.09 ± 0.03 | 68.20 ± 2.31 |
| 30MCC | 1.18 ± 0.02 | 67.10 ± 1.78 |
| 40MCC | 1.13 ± 0.01 | 66.90 ± 7.50 |
| References | Maximum Weight Loss Temperatures (°C) | |
|---|---|---|
| Tmax1 | Tmax2 | |
| AESO | - | 405.8 |
| 20MCC | 326.8 | 389.5 |
| 30MCC | 325.3 | 387.7 |
| 40MCC | 325.1 | 386.2 |
| MCC | 334.0 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Fei, M.-e.; Ban, Y.; Jia, A.; Qiu, R. Preparation and Evaluation of Green Composites from Microcrystalline Cellulose and a Soybean-Oil Derivative. Polymers 2017, 9, 541. https://doi.org/10.3390/polym9100541
Liu W, Fei M-e, Ban Y, Jia A, Qiu R. Preparation and Evaluation of Green Composites from Microcrystalline Cellulose and a Soybean-Oil Derivative. Polymers. 2017; 9(10):541. https://doi.org/10.3390/polym9100541
Chicago/Turabian StyleLiu, Wendi, Ming-en Fei, Yang Ban, Anming Jia, and Renhui Qiu. 2017. "Preparation and Evaluation of Green Composites from Microcrystalline Cellulose and a Soybean-Oil Derivative" Polymers 9, no. 10: 541. https://doi.org/10.3390/polym9100541
APA StyleLiu, W., Fei, M.-e., Ban, Y., Jia, A., & Qiu, R. (2017). Preparation and Evaluation of Green Composites from Microcrystalline Cellulose and a Soybean-Oil Derivative. Polymers, 9(10), 541. https://doi.org/10.3390/polym9100541




