Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion
Abstract
:1. Introduction
2. Optically Transparent Polyimides
2.1. Factors Influencing the Transparency of PI Films
- (a)
- Chemical factors
- CT interactions and electronic conjugation originating from the PI chain structures
- Partial decomposition of terminal amino groups, aliphatic units in the main chains and the side groups
- (b)
- Physical factors
- Chain aggregation influenced by imidization methods (film preparation routes) and heating programs for film formation
- (c)
- Processing factors
- Temperatures, atmosphere, and type of solvent(s) used for film preparation
- Unknown colored impurities originally contained in the monomers, particularly aromatic diamines.
2.2. Influences of Processing Conditions
2.3. Influence of the Chain Structure on the PI Film Transparency
3. Low-CTE Polyimides
3.1. Necessity of Low-CTE Materials
3.2. Points to Note in the CTE Measurements
- (1)
- Release of residual strain.
- (2)
- Desorption of adsorbed water.
- (3)
- Evaporation of the residual solvents.
- (4)
- Imidization of the non-cyclized portion on using imperfectly imidized samples.
- (5)
- Crystallization.
- (6)
- Crosslinking reactions.
- (7)
- Thermal decomposition.
4. Approaches to Simultaneously Achieve High Transparency, Low CTE, and Other Required Properties
4.1. A Transition of Development of Low-CTE, Transparent PIs
4.2. PIs Derived from Cycloaliphatic Diamines
4.2.1. Problems in the PAA Polymerization
- (a)
- Choice of higher-Mw cycloaliphatic diamines with bulkier and more distorted structures.
- (b)
- Choice of a higher-Mw tetracarboxylic dianhydride with bulkier and more distorted structures.
- (c)
- Optimization of the reaction conditions.
- (d)
- One-pot polymerization method (only for highly soluble PI systems).
- (e)
- Addition of acetic acid.
- (f)
- Use of silylated cycloaliphatic diamines.
4.2.2. Properties of t-CHDA-Derived PIs
4.3. PIs Derived from Cycloaliphatic Tetracarboxylic Dianhydrides
4.3.1. Problems in the Polymerizability of Cycloaliphatic Tetracarboxylic Dianhydrides with Aromatic Diamines and other Problems
4.3.2. Properties of PIs Derived from Cycloaliphatic Tetracarboxylic Dianhydrides
4.4. Cycloaliphatic PIs Showing High Transparency, Low CTE, and Sufficient Ductility by Simple Drying of Coated PI Solutions
4.4.1. A Favorable Property Accompanying the Solubility Improvement of PIs
4.4.2. An Approach to Improve the Poor Solubility of CBDA-based PIs while Maintaining Their High Transparency and Low-CTE Property
4.5. Attempts at Fulfilling the Target Properties without Relying on Cycloaliphatic Monomers
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lee, D.; Lim, Y.W.; Im, H.G.; Jeong, S.; Ji, S.; Kim, Y.H.; Choi, G.M.; Park, J.U.; Lee, J.Y.; Jin, J.; et al. Bioinspired transparent laminated composite film for flexible green optoelectronics. ACS Appl. Mater. Interfaces 2017, 9, 24161–24168. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.; Ji, S.; Shin, S.H.; Kim, S.Y.; Kim, Y.C.; Kim, J.Y.; Park, J.U. Fully-integrated, bezel-less transistor arrays using reversibly foldable interconnects and stretchable origami substrates. Nanoscale 2016, 8, 9504–9510. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo Chemical. Available online: http://www.sumitomo-chem.co.jp/sep/english/products/pes/index.html (accessed on 16 October 2017).
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook, 4th ed.; John Wiley: New York, NY, USA, 1999. [Google Scholar]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. (Eds.) Polyimides: Thermally Stable Polymers; Plenum: New York, NY, USA, 1987. [Google Scholar]
- Bessonov, M.I.; Zubkov, V.A. Polyamic acid and Polyimides: Synthesis, Transformation and Structure; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Ghosh, M.K.; Mittal, K.L. (Eds.) Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Ando, S.; Ueda, M.; Kakimoto, M.; Kochi, M.; Takeichi, T.; Hasegawa, M.; Yokota, R. (Eds.) The Latest Polyimides: Fundamentals and Applications, 2nd ed.; NTS: Tokyo, Japan, 2010. [Google Scholar]
- Sroog, C.E. Polyimide. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hoshino, Y.; Katsura, N.; Ishii, J. Superheat resistant polymers with low coefficients of thermal expansion. Polymer 2017, 111, 91–102. [Google Scholar] [CrossRef]
- Ni, H.; Liu, J.; Wang, Z.; Yang, S. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- St. Clair, A.K.; Slemp, W.S. Evaluation of colorless polyimide film for thermal control coating applications. SAMPE J. 1985, 21, 28–33. [Google Scholar]
- Hasegawa, M.; Ishigami, T.; Ishii, J.; Sugiura, K.; Fujii, M. Solution-processable transparent polyimides with low coefficient of thermal expansion and self-orientation behavior induced by solution casting. Eur. Polym. J. 2013, 49, 3657–3672. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kurosaki, T. Soluble and colorless polyimides from bicyclo[2.2.2]octane-2,3,5,6-tetracarboxylic 2,3:5,6-dianhydrides. Macromolecules 1997, 30, 993–1000. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Wada, Y. Polyimides containing trans-1,4-cyclohexane unit (II). Low-K and Low-CTE semi- and wholly cycloaliphatic polyimides. High Perform. Polym. 2007, 19, 175–193. [Google Scholar] [CrossRef]
- Takahashi, T.; Takabayashi, S.; Inoue, H. Preparation of polyimide films from a-BPDA/ODA by one-pot and two-step imidization methods. High Perform. Polym. 1998, 10, 33–44. [Google Scholar] [CrossRef]
- Hasegawa, M.; Shi, Z.; Yokota, R.; He, F.; Ozawa, H. Thermo-processable polyimides with high Tg and high thermo-oxidative stability as derived from 2,3,3′,4′-biphenyltetracarboxylic dianhydride. High Perform. Polym. 2001, 13, 355–364. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. Ed. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishigami, T.; Ishii, J. Optically transparent aromatic poly(ester imide)s with low coefficients of thermal expansion (1). Self-orientation behavior during solution casting process and substituent effect. Polymer 2015, 74, 1–15. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tanaka, Y.; Tominaga, A. γ-Butyrolactone-processable high-modulus poly(ester imide)s. Polym. Int. 2012, 61, 466–476. [Google Scholar] [CrossRef]
- Mulliken, R.S. Molecular compounds and their spectra. II. J. Am. Chem. Soc. 1952, 74, 811–824. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kochi, M.; Mita, I.; Yokota, R. Molecular aggregation and fluorescence spectra of aromatic polyimides. Eur. Polym. J. 1989, 25, 349–354. [Google Scholar] [CrossRef]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of aromatic polyimides and electronic properties of their source materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef]
- Fukuda, M.; Takao, Y.; Tamai, Y. Formation of a 6FDA-based ring polyimide with nanoscale cavity evaluated by DFT calculations. J. Mol. Struct. 2005, 739, 105–115. [Google Scholar] [CrossRef]
- Matsuura, T.; Hasuda, Y.; Nishi, S.; Yamada, N. Polyimide derived from 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl. 1. Synthesis and characterization of polyimides prepared with 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 1991, 24, 5001–5005. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sensui, N.; Shindo, Y.; Yokota, R. Structure and properties of novel asymmetric biphenyl type polyimides. Homo- and copolymers and blends. Macromolecules 1999, 32, 387–396. [Google Scholar] [CrossRef]
- Suzuki, H.; Abe, T.; Takaishi, K.; Narita, M.; Hamada, F. The synthesis and X-ray structure of 1,2,3,4-cyclobutane tetracarboxylic dianhydride and the preparation of a new type of polyimide showing excellent transparency and heat resistance. J. Polym. Sci. Part A Polym. Chem. Ed. 2000, 38, 108–116. [Google Scholar] [CrossRef]
- Volksen, W.; Cha, H.J.; Sanchez, M.I.; Yoon, D.Y. Polyimides derived from nonaromatic monomers: Synthesis, characterization and potential applications. React. Funct. Polym. 1996, 30, 61–69. [Google Scholar] [CrossRef]
- Matsumoto, T. Nonaromatic polyimides derived from cycloaliphatic monomers. Macromolecules 1999, 32, 4933–4939. [Google Scholar] [CrossRef]
- Seino, H.; Sasaki, T.; Mochizuki, A.; Ueda, M. Synthesis of fully aliphatic polyimides. High Perform. Polym. 1999, 11, 255–262. [Google Scholar] [CrossRef]
- Li, J.; Kato, J.; Kudo, K.; Shiraishi, S. Synthesis and properties of novel soluble polyimides having an unsymmetric spiro tricyclic dianhydride unit. Macromol. Chem. Phys. 2000, 201, 2289–2297. [Google Scholar] [CrossRef]
- Mathews, A.S.; Kim, I.; Ha, C.S. Synthesis, characterization, and properties of fully aliphatic polyimides and their derivatives for microelectronics and optoelectronics applications. Macromol. Res. 2007, 15, 114–128. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kasamatsu, K.; Koseki, K. Colorless poly(ester imide)s derived from hydrogenated trimellitic anhydride. Eur. Polym. J. 2012, 48, 483–498. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Kumakura, K.; Koyama, J. Colorless polyimides with low coefficient of thermal expansion derived from alkyl-substituted cyclobutanetetracarboxylic dianhydrides. Polym. Int. 2014, 63, 486–500. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Ishii, J.; Yamaguchi, S.; Takezawa, E.; Kagayama, T.; Ishikawa, A. Colorless polyimides derived from 1S,2S,4R,5R-cyclohexanetetracarboxylic dianhydride, self-orientation behavior during solution casting, and their optoelectronic applications. Polymer 2014, 55, 4693–4708. [Google Scholar] [CrossRef]
- Jou, J.H.; Chen, L.J. Relaxation modulus and thermal expansion coefficient of polyimide films coated on substrates. Appl. Phys. Lett. 1991, 59, 46–47. [Google Scholar] [CrossRef]
- Hasegawa, M.; Morita, K.; Ishii, J. Ultra-low-modulus polyimides and their applications. J. Photopolym. Sci. Technol. 2010, 23, 495–499. [Google Scholar] [CrossRef]
- Hasegawa, M.; Matano, T.; Shindo, Y.; Sugimura, T. Spontaneous molecular orientation of polyimides induced by thermal imidization (2). In-plane orientation. Macromolecules 1996, 29, 7897–7909. [Google Scholar] [CrossRef]
- Coburn, J.C.; Pottiger, M.T. Thermal curing in polyimide films and coatings. In Polyimides: Fundamentals and Applications; Ghosh, M.K., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 207–247. [Google Scholar]
- Numata, S.; Oohara, S.; Fujisaki, K.; Imaizumi, J.; Kinjo, N. Thermal expansion behavior of various aromatic polyimides. J. Appl. Polym. Sci. 1986, 31, 101–110. [Google Scholar] [CrossRef]
- Sensui, N.; Ishii, J.; Takata, A.; Oami, Y.; Hasegawa, M.; Yokota, R. Ultra-low CTE and improved toughness of PMDA/PDA polyimide-based molecular composites containing asymmetric BPDA-type polyimides. High Perform. Polym. 2009, 21, 709–728. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uejima, K.; Sato, H. Some properties of polyimides prepared from p- or m-terphenyltetracarboxylic dianhydride. Polym. Prepr. Jpn. 1991, 40, 3748–3750. [Google Scholar]
- Hasegawa, M.; Koyanaka, M. Polyimides containing trans-1,4-cyclohexane unit. Polymerizability of their precursors and low-CTE and low-K and high-Tg properties. High Perform. Polym. 2003, 15, 47–64. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horii, S. Low-CTE polyimides derived from 2,3,6,7-naphthalenetetracarboxylic dianhydride. Polym. J. 2007, 39, 610–621. [Google Scholar] [CrossRef]
- Hasegawa, M.; Koseki, K. Poly(ester imide)s possessing low CTE and low water absorption. High Perform. Polym. 2006, 18, 697–717. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tsujimura, Y.; Koseki, K.; Miyazaki, T. Poly(ester imide)s possessing low CTE and low water absorption (II). Effect of substituents. Polym. J. 2008, 40, 56–67. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sakamoto, Y.; Tanaka, Y.; Kobayashi, Y. Poly(ester imide)s possessing low coefficients of thermal expansion (CTE) and low water absorption (III). Use of bis(4-aminophenyl)terephthalate and effect of substituents. Eur. Polym. J. 2010, 46, 1510–1524. [Google Scholar] [CrossRef]
- Ishii, J.; Takata, A.; Oami, Y.; Yokota, R.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (6). Mechanism of negative in-plane CTE generation in non-stretched polyimide films. Eur. Polym. J. 2010, 46, 681–693. [Google Scholar] [CrossRef]
- Hasegawa, M.; Watanabe, Y.; Tsukuda, S.; Ishii, J. Solution-processable colorless polyimides with ultralow coefficients of thermal expansion for optoelectronic applications. Polym. Int. 2016, 65, 1063–1073. [Google Scholar] [CrossRef]
- Feiring, A.E.; Auman, B.C.; Wonchoba, E.R. Synthesis and properties of fluorinated polyimides from novel 2,2′-bis(fluoroalkoxy)benzidines. Macromolecules 1993, 26, 2779–2784. [Google Scholar] [CrossRef]
- Trofimenko, S.; Auman, B.C. Polyimides based on 9,9-disubstituted xanthene dianhydrides. Macromolecules 1994, 27, 1136–1146. [Google Scholar] [CrossRef]
- Auman, B.C. Low dielectric constant, low moisture absorption and low thermal expansion coefficient polyimides based on new rigid fluorinated monomers. In Advances in Polyimide Science and Technology; Feger, C., Khojasteh, M.M., Htoo, M.S., Eds.; Technomic: Lancaster, OA, USA, 1993; pp. 15–32. [Google Scholar]
- Choi, M.C.; Wakita, J.; Ha, C.S.; Ando, S. Highly transparent and refractive polyimides with controlled molecular structure by chlorine side groups. Macromolecules 2009, 42, 5112–5120. [Google Scholar] [CrossRef]
- Russel, T.P.; Gugger, H.; Swalen, J.D. In-plane orientation of polyimide. J. Polym. Sci. Part B Polym. Phys. Ed. 1983, 21, 1745–1756. [Google Scholar] [CrossRef]
- Takahashi, N.; Yoon, D.Y.; Parrish, W. Molecular order in condensed states of semiflexible poly(amic acid) and polyimide. Macromolecules 1984, 17, 2583–2588. [Google Scholar] [CrossRef]
- Boese, D.; Lee, H.; Yoon, D.Y.; Swalen, J.D.; Rabolt, J.F.J. Chain orientation and anisotropies in optical and dielectric properties in thin films of stiff polyimides. J. Polym. Sci. Part B Polym. Phys. Ed. 1992, 30, 1321–1327. [Google Scholar] [CrossRef]
- Ishii, J.; Shimizu, N.; Ishihara, N.; Ikeda, Y.; Sensui, N.; Matano, T.; Hasegawa, M. Spontaneous molecular orientation of polyimide induced by thermal imidization (4). Casting- and melt-induced in-plane orientation. Eur. Polym. J. 2010, 46, 69–80. [Google Scholar] [CrossRef]
- Terui, Y.; Matsuda, S.; Ando, S. Molecular structure and thickness dependence of chain orientation in aromatic polyimide films. J. Polym. Sci. Part B Polym. Phys. Ed. 2005, 43, 2109–2120. [Google Scholar] [CrossRef]
- Hasegawa, M.; Okuda, K.; Horimoto, M.; Shindo, Y.; Yokota, R.; Kochi, M. Spontaneous molecular orientation of polyimides induced by thermal imidization. 3. Component chain orientation in binary polyimide blends. Macromolecules 1997, 30, 5745–5752. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hasegawa, M. Highly tough and highly transparent soluble polybenzoxazoles. High Perform. Polym. 2007, 19, 243–269. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hasegawa, M. Highly tough and highly transparent soluble polybenzoxazoles (II). Effect of sulfone group. High Perform. Polym. 2009, 21, 219–244. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tominaga, A. Fluorene-containing poly(ester imide)s and application to positive-type photosensitive heat-resistant materials. Macromol. Mater. Eng. 2011, 296, 1002–1017. [Google Scholar] [CrossRef]
- Jin, Q.; Yamashita, T.; Horie, K.; Yokota, R.; Mita, I. Polyimides with alicyclic diamines. I. Syntheses and thermal properties. J. Polym. Sci. Part A Polym. Chem. Ed. 1993, 31, 2345–2351. [Google Scholar] [CrossRef]
- Ogura, T.; Ueda, M. Facile synthesis of semiaromatic poly(amic acid)s from trans-1,4-cyclohexanediamine and aromatic tetracarboxylic dianhydrides. Macromolecules 2007, 40, 3527–3529. [Google Scholar] [CrossRef]
- Oishi, Y.; Ogasawara, K.; Hirahara, H.; Mori, K. Synthesis of alicyclic polyimides by the silylation method. J. Photopolym. Sci. Technol. 2001, 14, 37–40. [Google Scholar] [CrossRef]
- Fukukawa, K.; Okazaki, M.; Sakata, Y.; Urakami, T.; Yamashita, W.; Tamai, S. Synthesis and properties of multi-block semi-alicyclic polyimides for thermally stable transparent and low CTE film. Polymer 2013, 54, 1053–1063. [Google Scholar] [CrossRef]
- Matsumoto, T. Effect of the radio frequency O2 plasma treatment at polyimide surface on the adhesion of the vacuum deposited thin Fe film. In The Latest Polyimides: Fundamentals and Applications, 2nd ed.; Ando, S., Ueda, M., Kakimoto, M., Kochi, M., Takeichi, T., Hasegawa, M., Yokota, R., Eds.; NTS: Tokyo, Japan, 2010; pp. 231–246. [Google Scholar]
- Shiotani, A.; Shimazaki, H.; Matsuo, M. Preparation of transparent polyimides derived from cis- and trans-dicyclohexyl-3,3′,4,4′--tetracarboxylic dianhydrides. Macromol. Mater. Eng. 2001, 286, 434–441. [Google Scholar] [CrossRef]
- Tsuda, Y.; Etou, K.; Hiyoshi, N.; Nishikawa, M.; Matsuki, Y.; Bessho, N. Soluble copolyimides based on 2,3,5-tricarboxycyclopentyl acetic dianhydride and conventional aromatic tetracarboxylic dianhydrides. Polym. J. 1998, 30, 222–228. [Google Scholar] [CrossRef]
- Guo, Y.; Song, H.; Zhai, L.; Liu, J.; Yang, S. Synthesis and characterization of novel semi-alicyclic polyimides from methyl-substituted tetralin dianhydride and aromatic diamines. Polym. J. 2012, 44, 718–723. [Google Scholar] [CrossRef]
- Fang, X.; Yang, Z.; Zhang, S.; Gao, L.; Ding, M. Synthesis and properties of polyimides derived from cis- and trans-1,2,3,4-cyclohexanetetracarboxylic dianhydrides. Polymer 2004, 45, 2539–2549. [Google Scholar] [CrossRef]
- Uchida, A.; Hasegawa, M.; Manami, H. cis, cis, cis-1,2,4,5-Cyclohexanetetracarboxylic acid and its dianhydride. Acta Cryst. 2003, C59, o1–o4. [Google Scholar] [CrossRef]
- Kiso, T.; Ishii, J.; Hasegawa, M. Colorless polyimides derived from hydrogenated trimellitic anhydride (6). Effect of steric structure control. Polym. Prepr. Jpn. 2014, 63, 2791–2792. [Google Scholar]
- Tokita, Y.; Fujii, M.; Hasegawa, M.; Kishimoto, S.; Isogai, Y. Cycloaliphatic poly(ester imide)s derived from 2,3,5-norbornanetricarboxylic anhydride. Polym. Prepr. Jpn. 2008, 57, 1490. [Google Scholar]
- Matsumoto, T.; Ozawa, H.; Ishiguro, E.; Komatsu, S. Properties of alicyclic polyimides with bis-spironorbornane structure prepared in various solvents. J. Photopolym. Sci. Technol. 2016, 29, 237–242. [Google Scholar] [CrossRef]
- Fujii, M.; Hasegawa, M. Highly tough and highly transparent polyimides (10). Cycloaliphatic polyimides derived from bicyclo[2.2.2]octane-2,3,5,6-tetracarboxylic dianhydride. Polym. Prepr. Jpn. 2007, 56, 1561. [Google Scholar]
- Hasegawa, M.; Fujii, M.; Wada, Y. Approaches to improve the film ductility of colorless cycloaliphatic polyimides. Polym. Adv. Technol. 2017, in press. [Google Scholar] [CrossRef]
- Baklagina, Y.G.; Milevskaya, I.S.; Efanova, N.V.; Sidorovich, A.V.; Zubkov, V.A. Structures of rigid-chain polyimides from pyromellitic dianhydride. Vysokomol. Soedin. 1976, 18, 1235–1242. [Google Scholar]
- Obata, Y.; Okuyama, K.; Kurihara, S.; Kitano, Y.; Jinda, T. X-ray structure analysis of an aromatic polyimide. Macromolecules 1995, 28, 1547–1551. [Google Scholar] [CrossRef]
- Ishii, J.; Makimura, R.; Shindo, N.; Hasegawa, M. Transparent thermoplastic polyimides (10). Thermal and UV-stability. Polym. Prepr. Jpn. 2016, 65, 3M09. [Google Scholar]
- Hirai, T.; Ishii, J.; Hasegawa, M. In-plane orientation in colorless polyimides as induced by solution casting from polyimide varnishes (16). Applications of aromatic poly(ester imide)s to low-CTE and transparent plastic substrates. Polym. Prepr. Jpn. 2014, 63, 2787–2788. [Google Scholar]
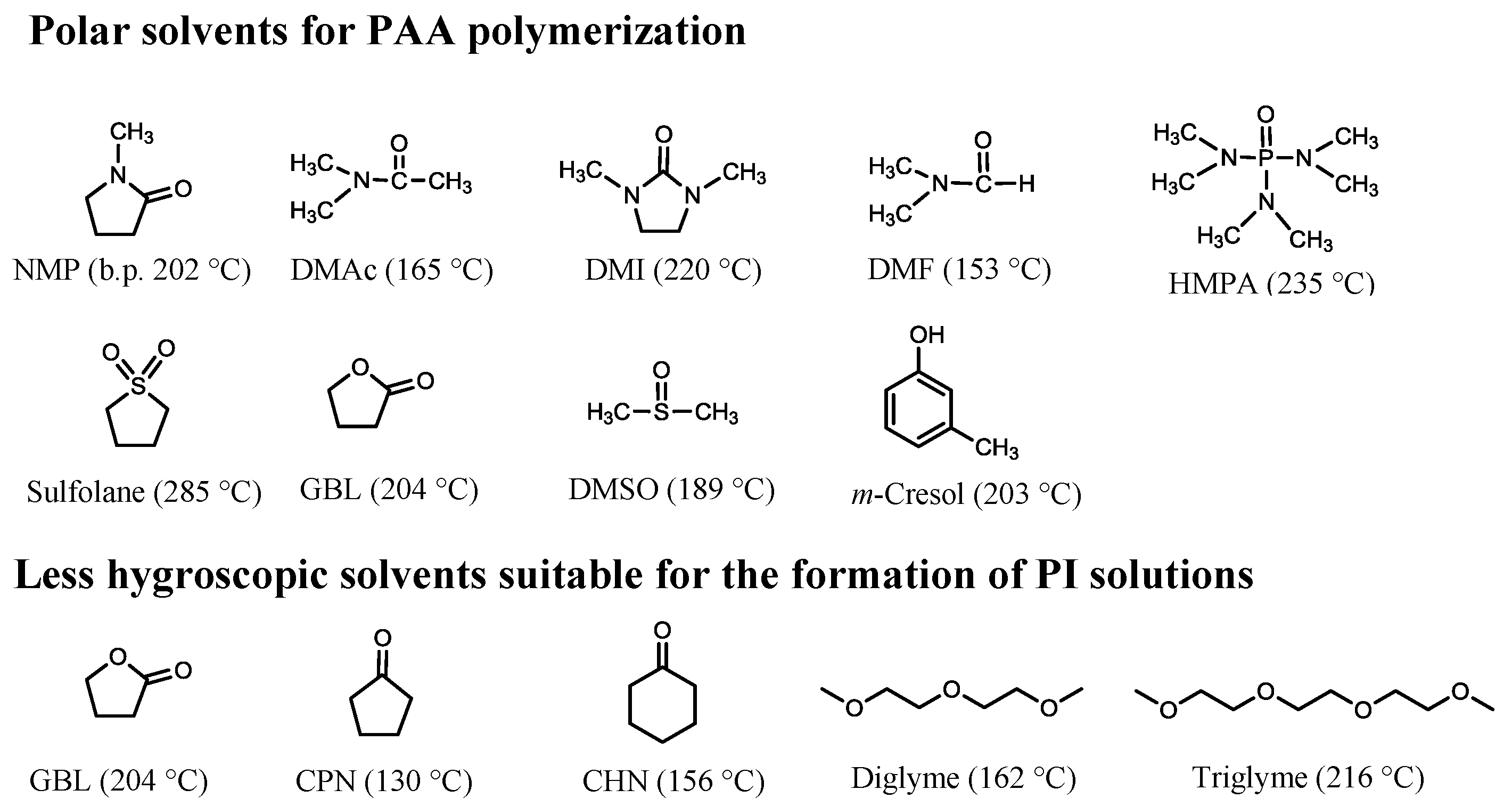

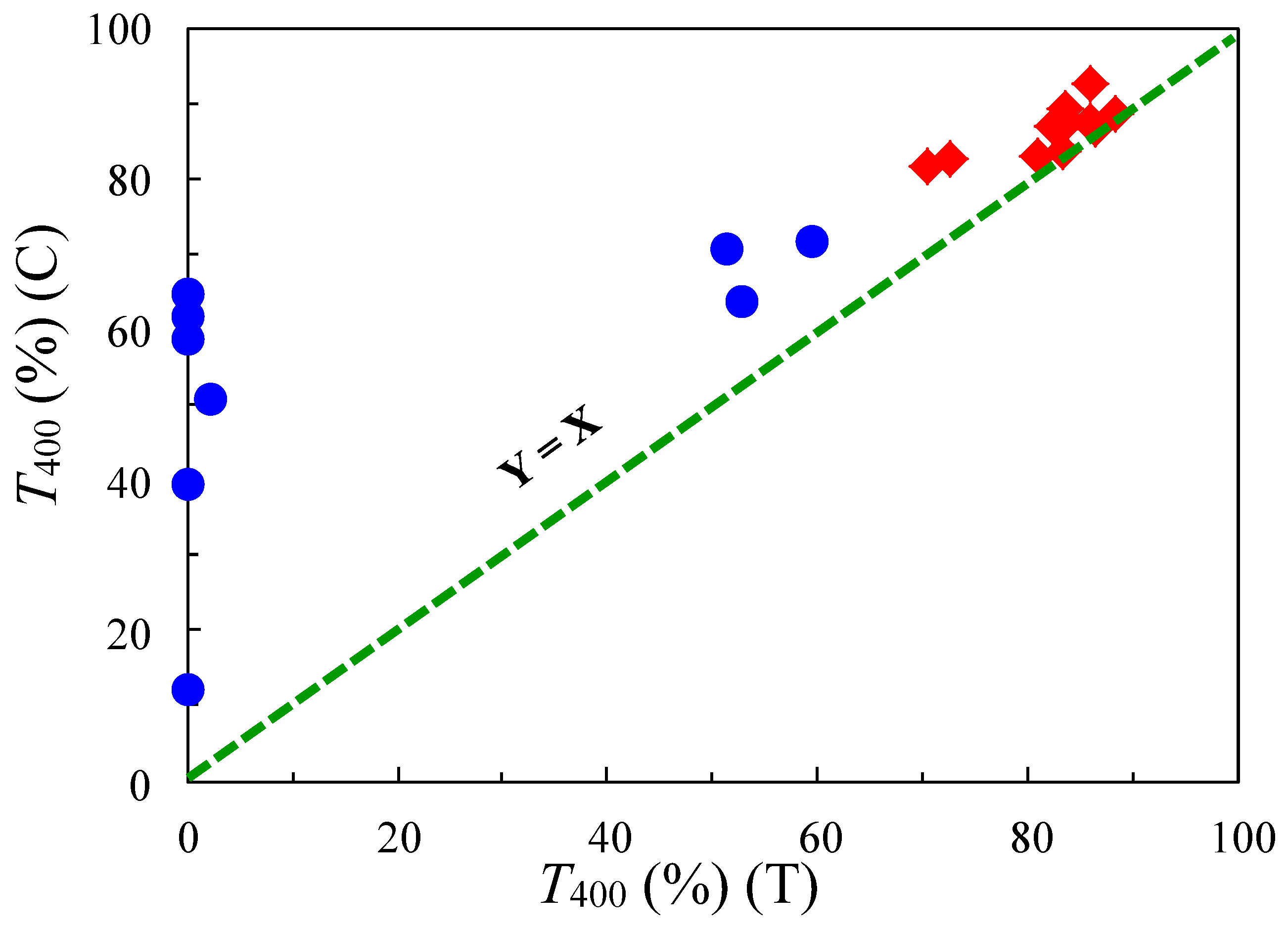

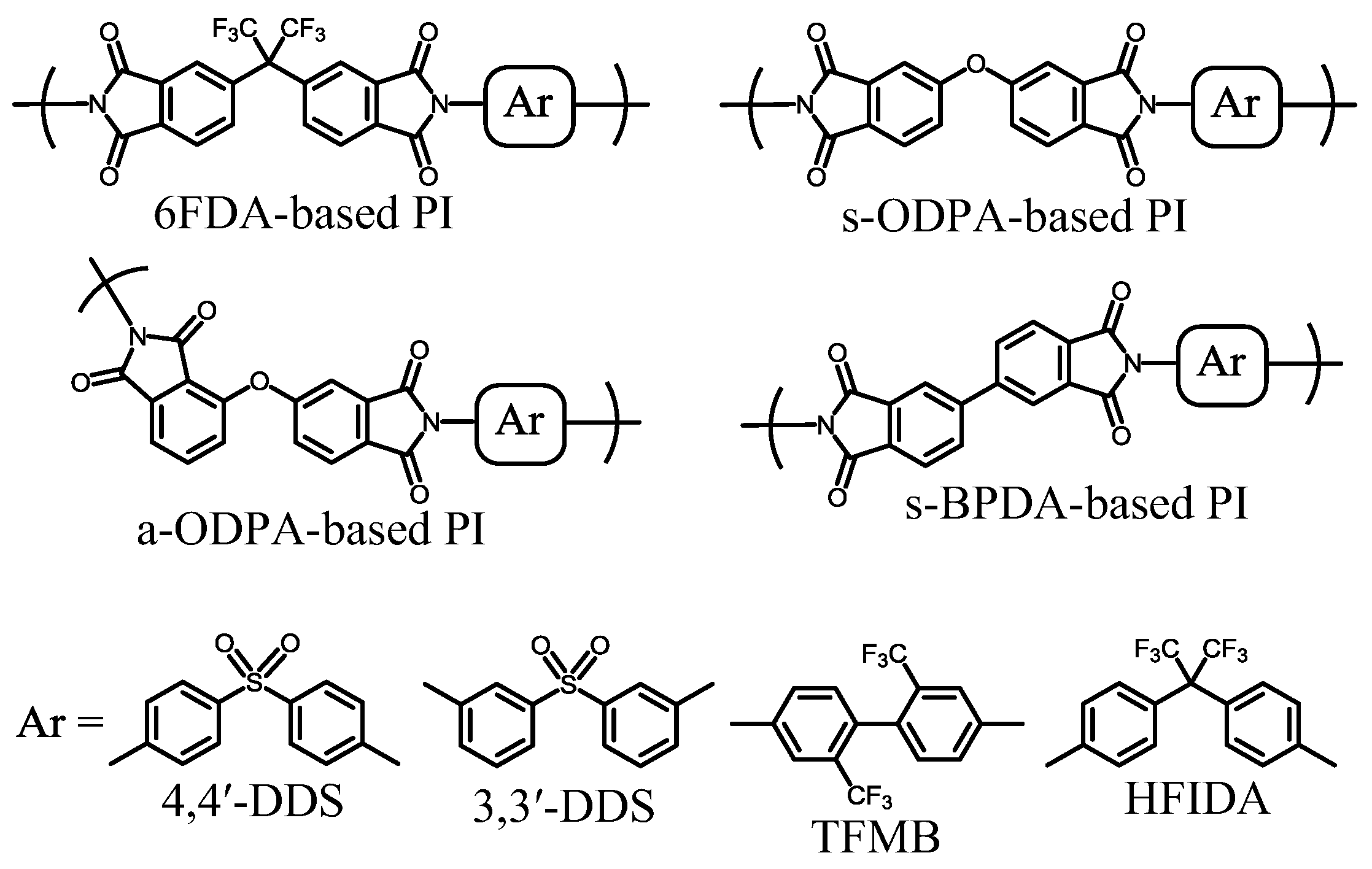
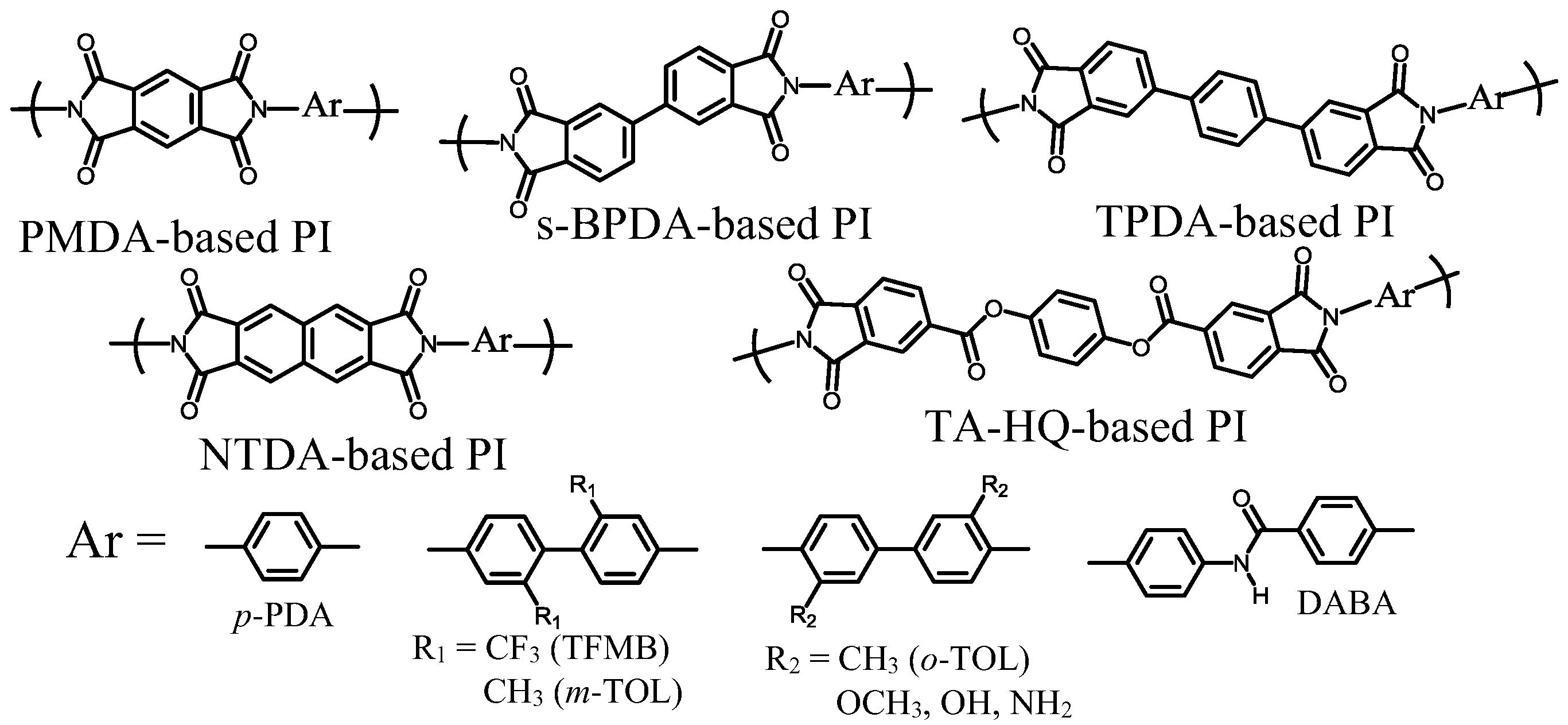
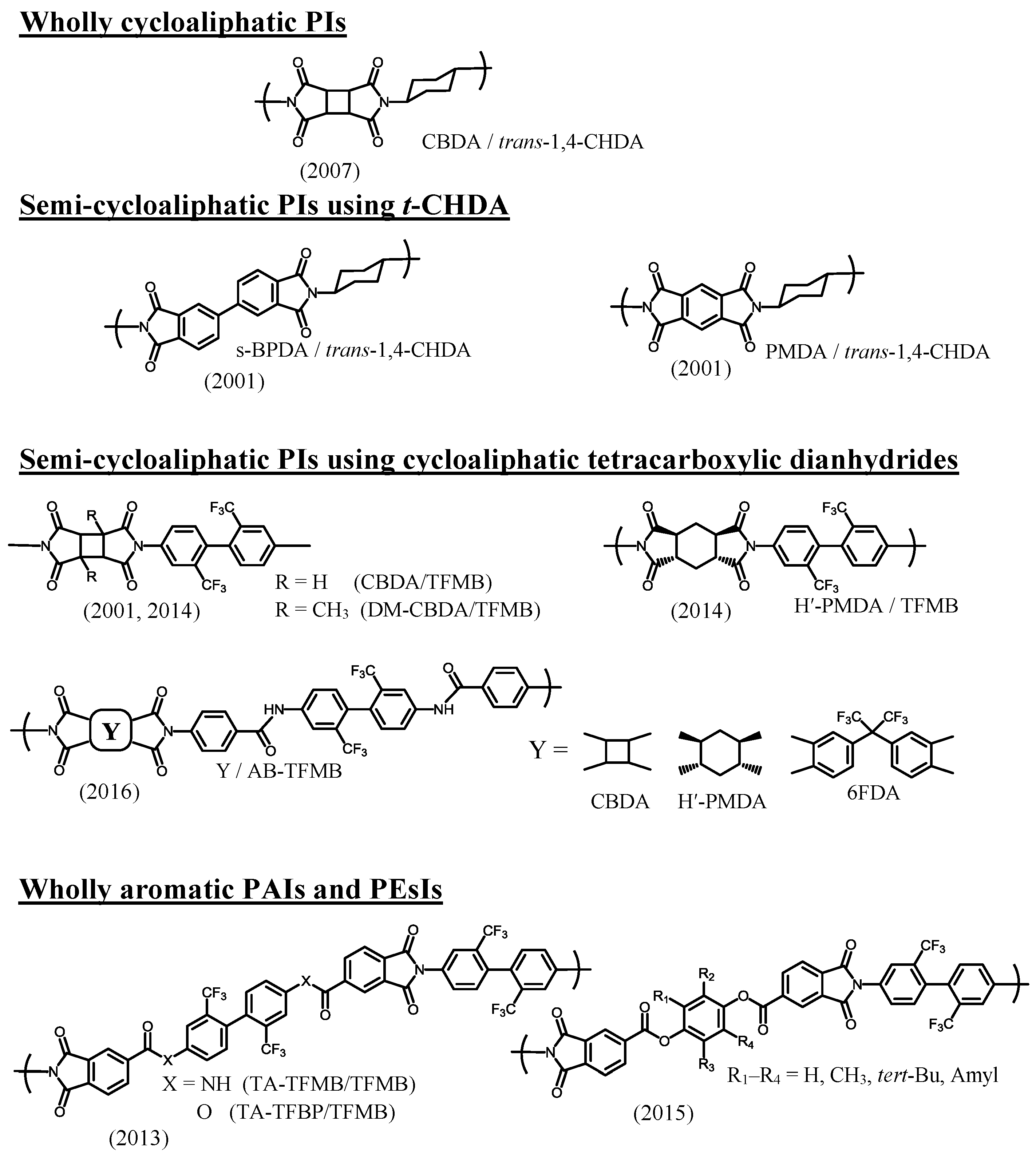
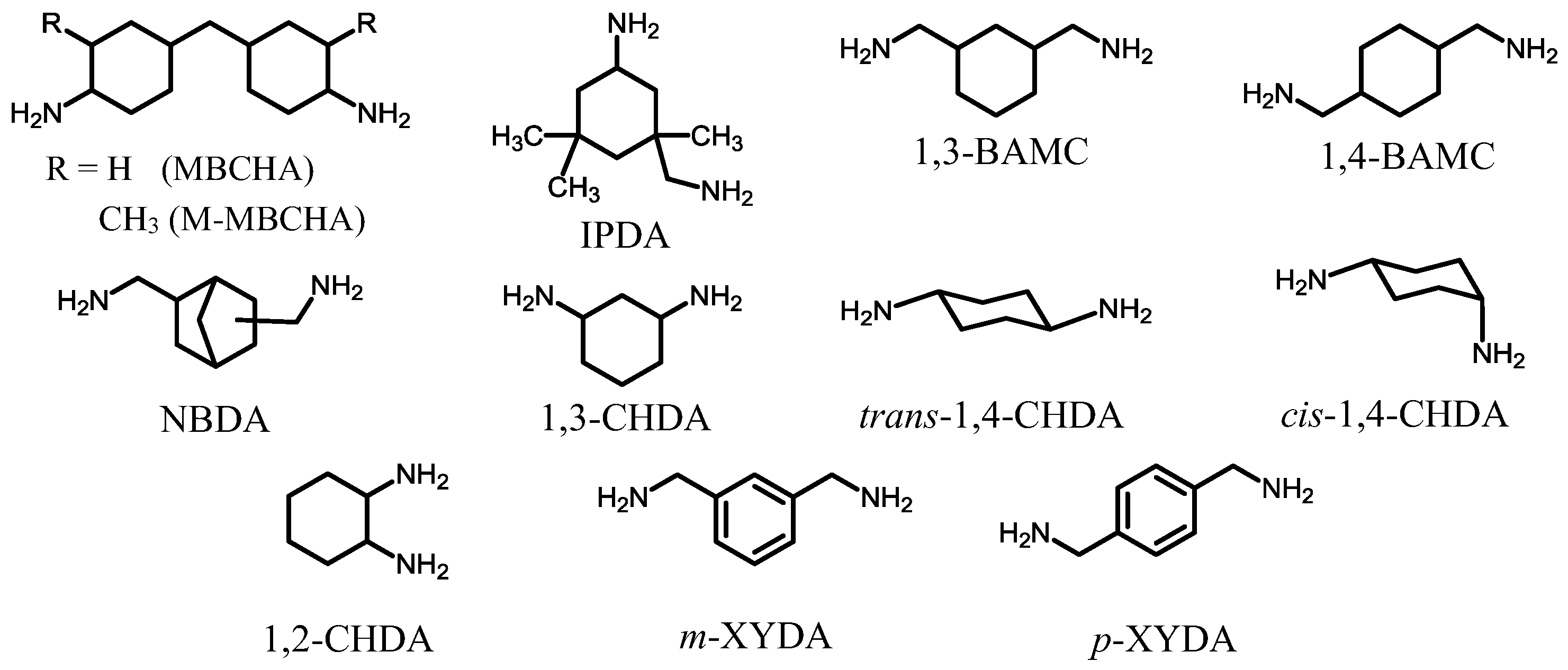

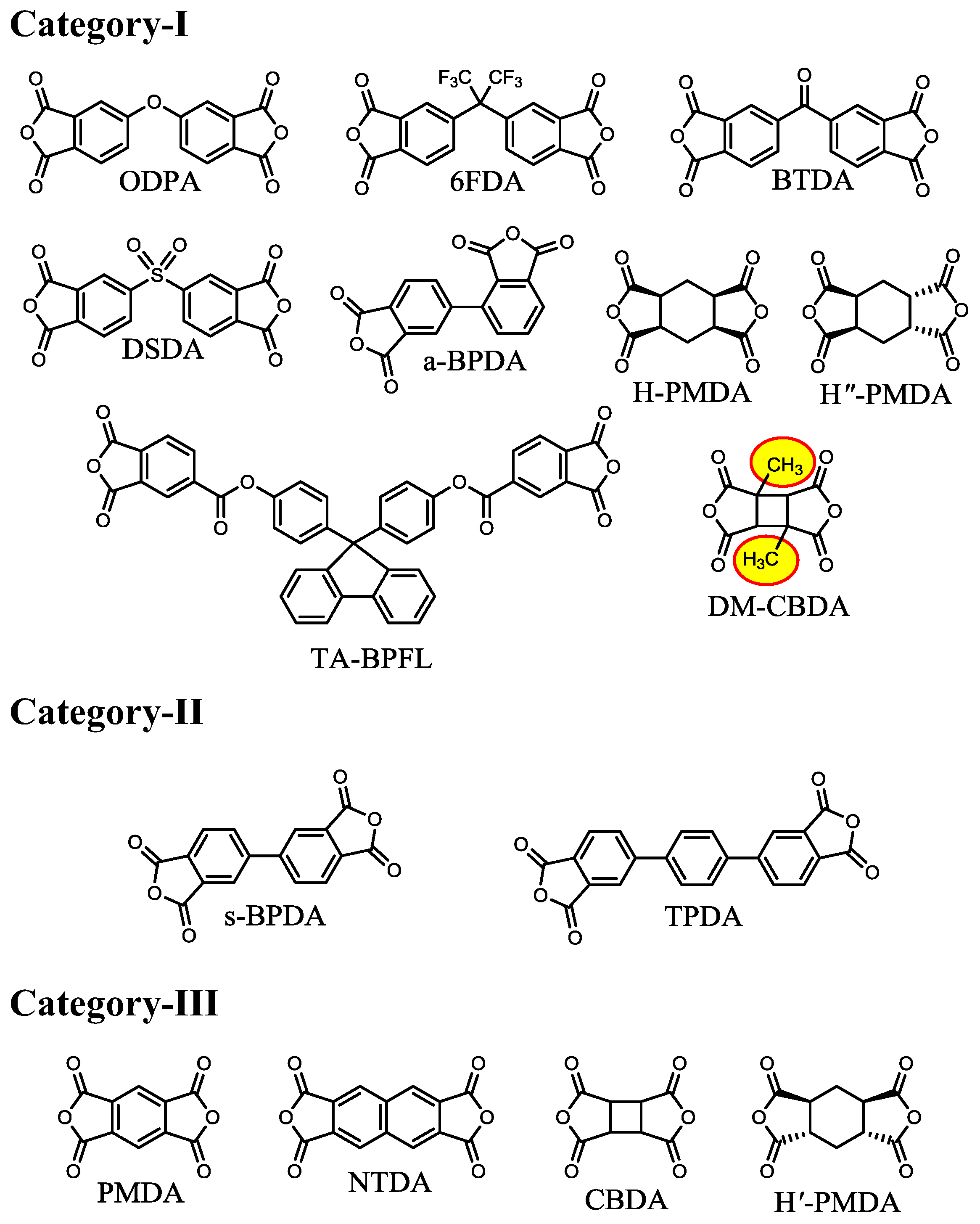
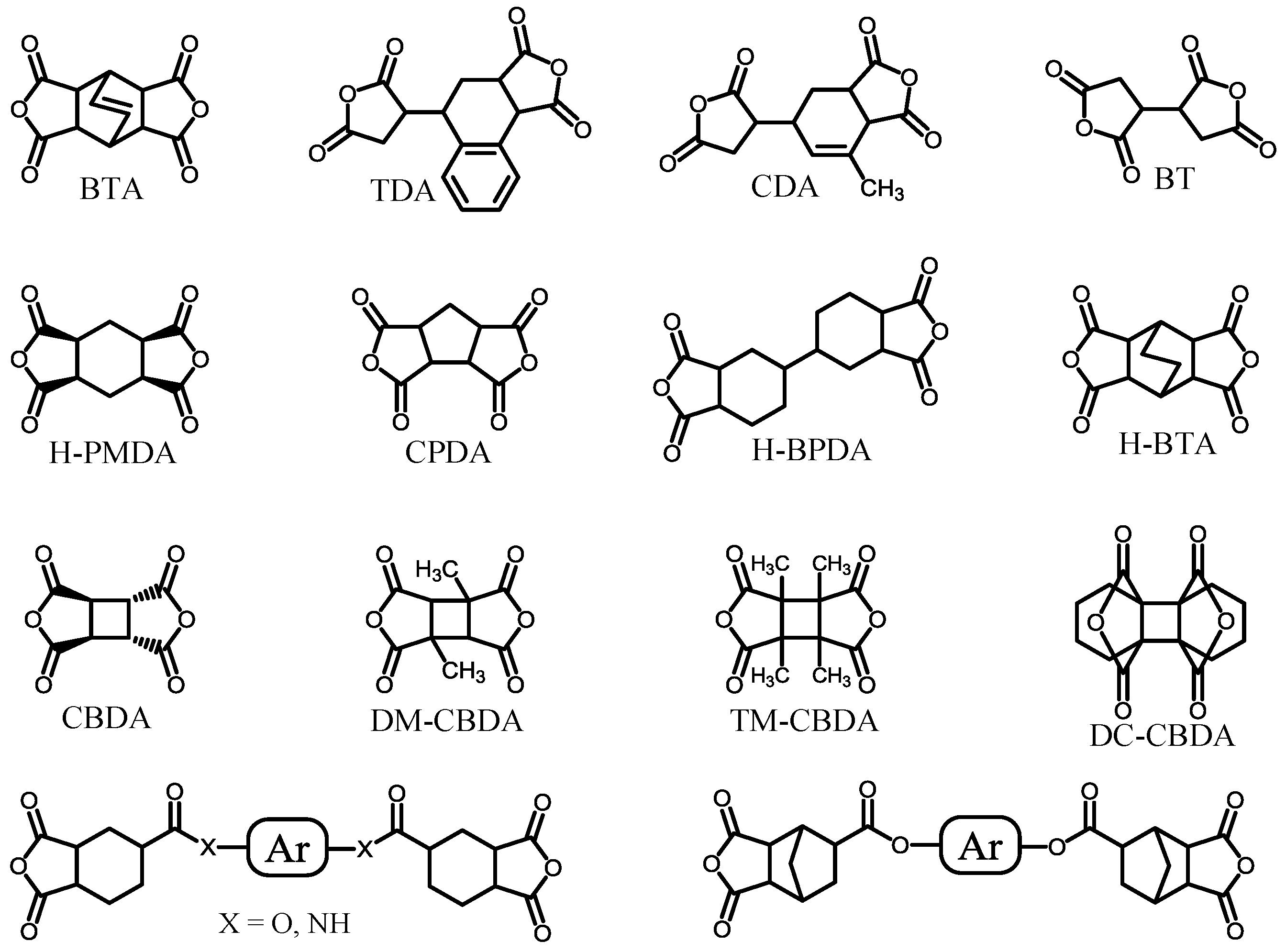
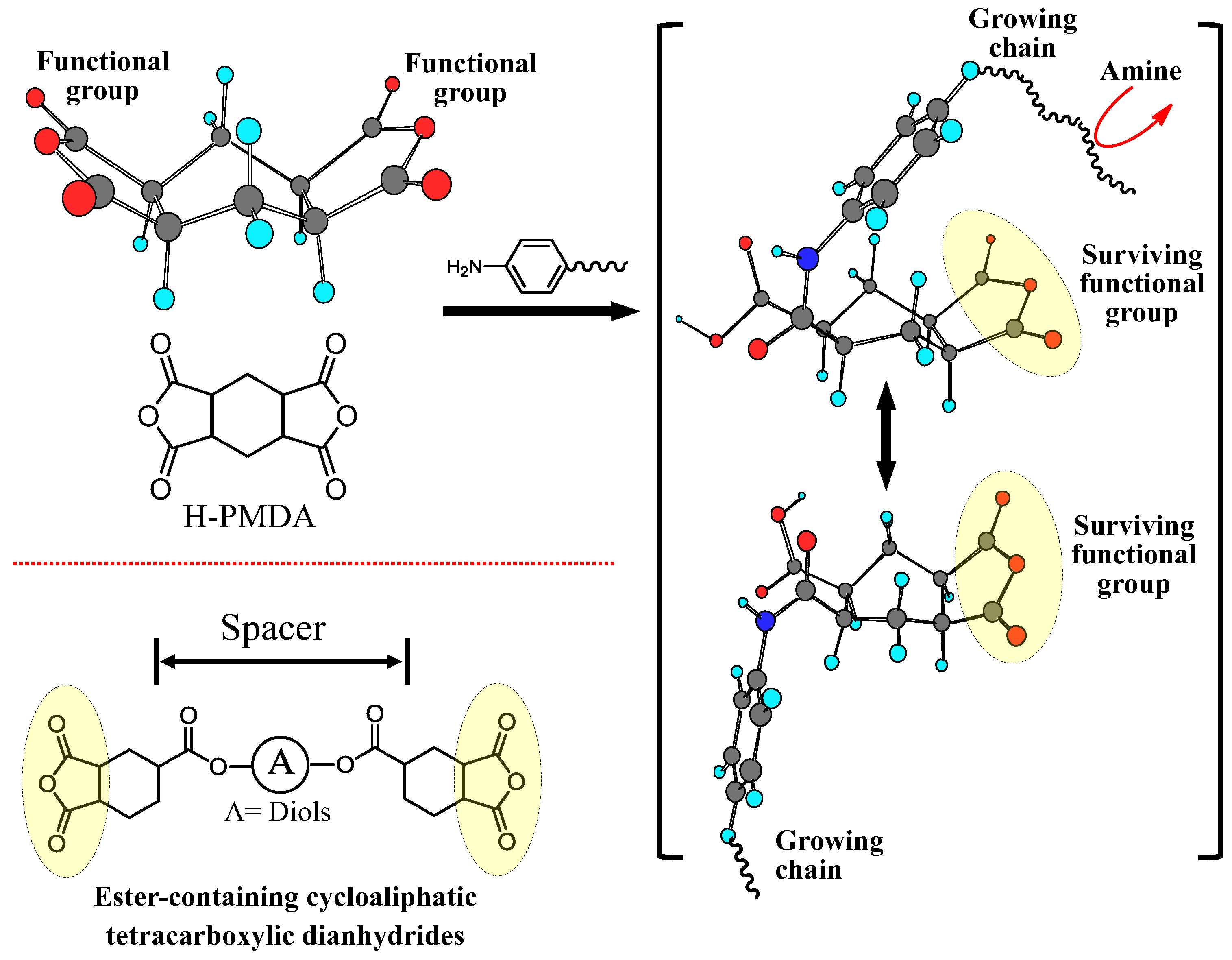
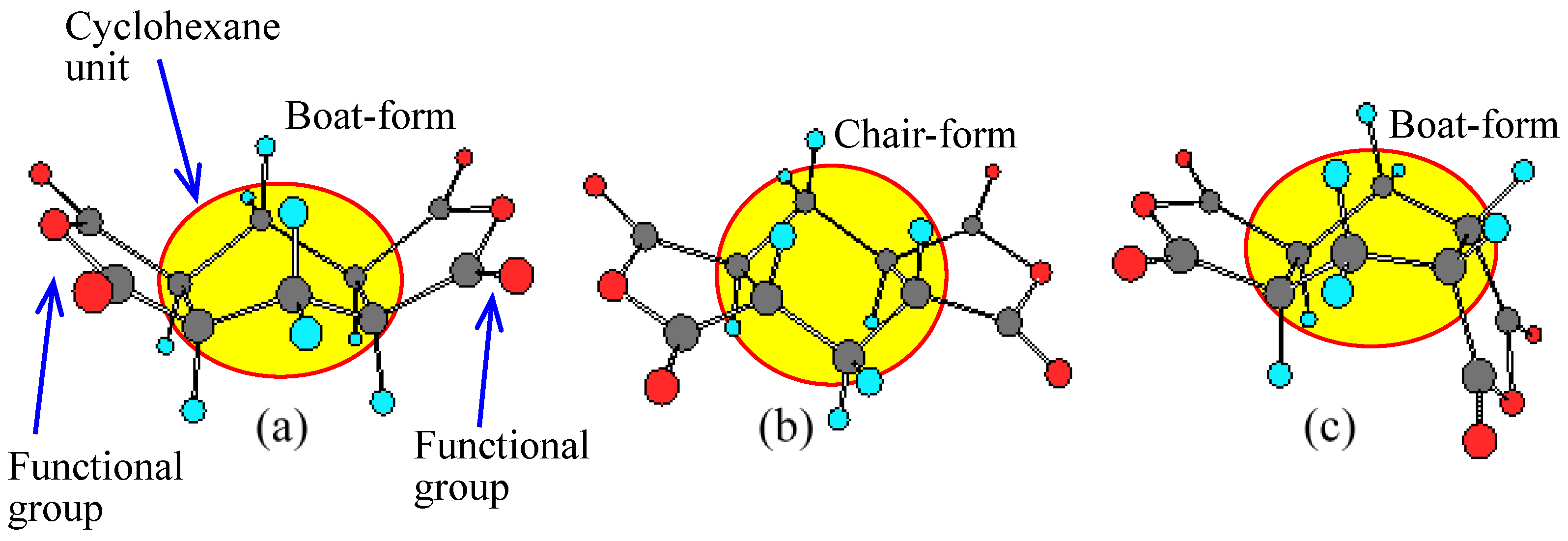
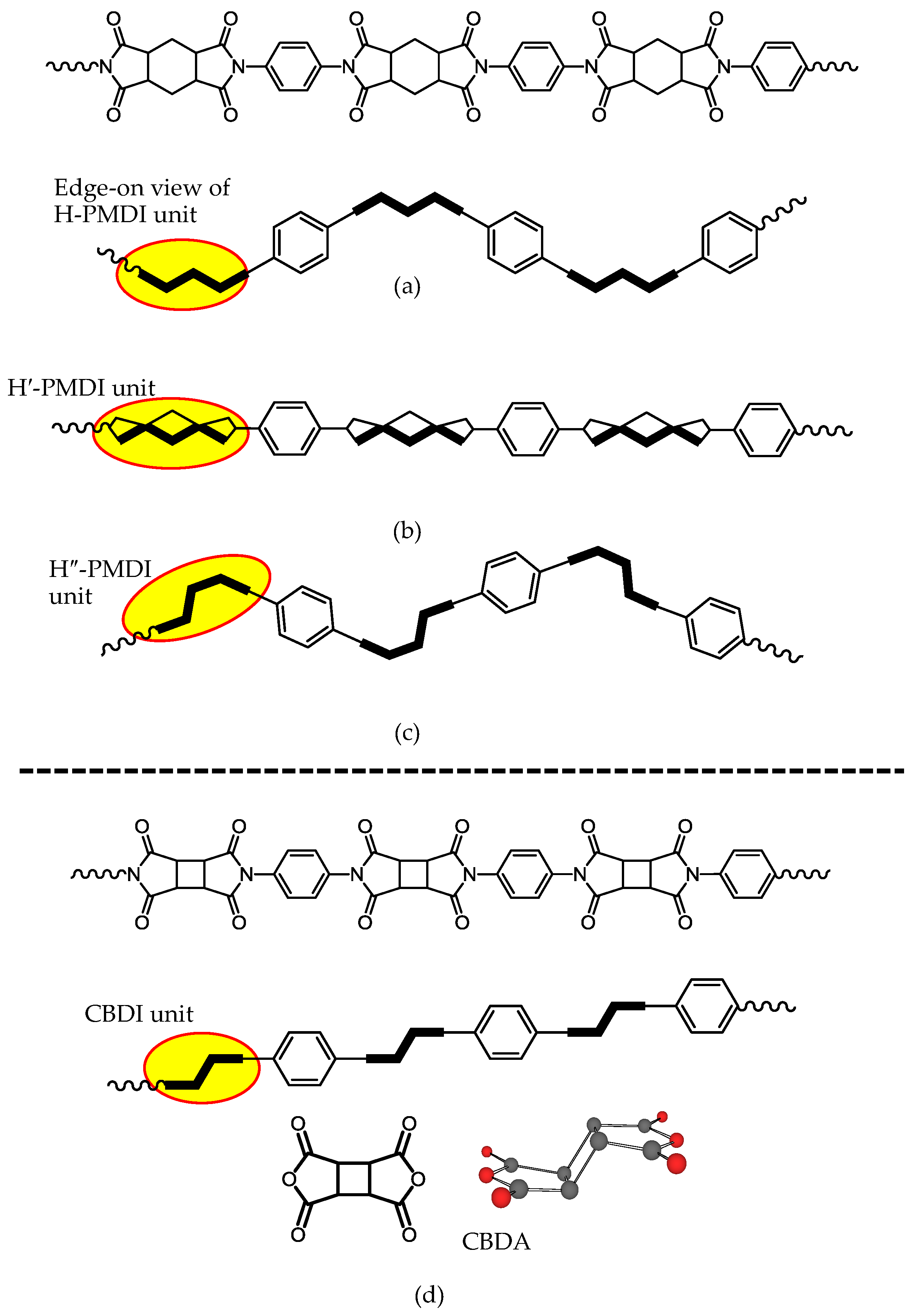


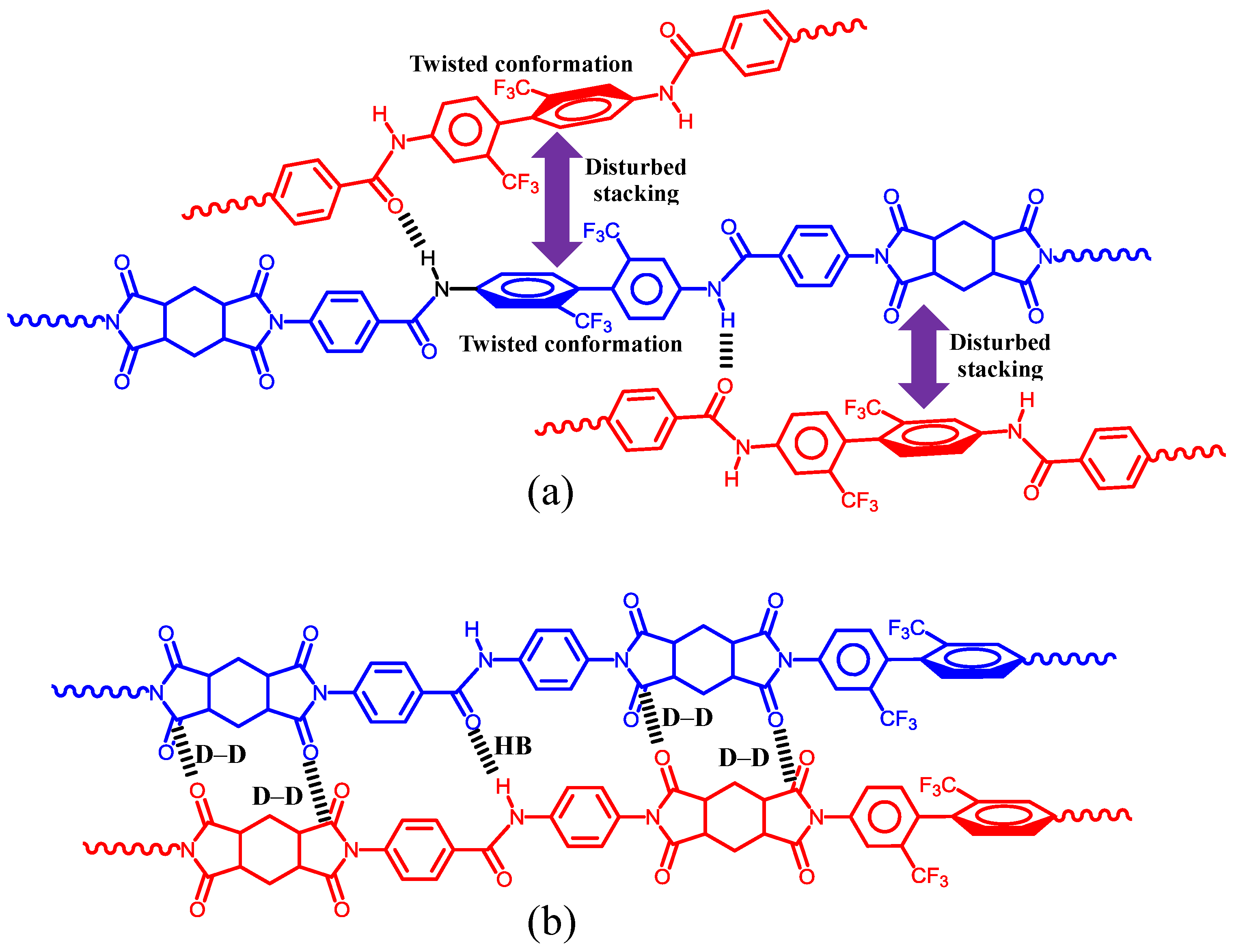
| No. | System a | Salt formation | Chemical imidization process | Appearance of PI films | CTE b (ppm·K−1) | Tg (°C) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | PMDA/TFMB | None | Non-applicable | Highly colored | –5 (T) | 400 | [27,46,50] |
| 2 | 6FDA/TFMB | None | Applicable | Colorless | 53 (C) 64 (T) | 324–335 | [15,27,45] |
| 3 | s-BPDA/TFMB | None | Non-applicable | Slightly colored | 34 (T) | 314 | [45,46] |
| 4 | CBDA/t-CHDA | Very strong | Non-applicable | Colorless | 26 (T) | ND c | [17] |
| 5 | s-BPDA/t-CHDA | Strong | Non-applicable | Colorless | 10 (T) | 360 | [45] |
| 6 | PMDA/t-CHDA | Very strong | Non-applicable | Colorless | 10 (T) | ND c | [17] |
| 7 | CBDA/TFMB | None | Non-applicable | Colorless | 21 (T) | 356 | [36,45] |
| 8 | DM-CBDA/TFMB | None | Non-applicable | Colorless | 28 (T) | 341 | [36] |
| 9 | HTA-44BP/o-TOL | None | Applicable | Colorless | 63 (C) | 295 | [35] |
| 10 | H′-PMDA/TFMB | None | Applicable | Colorless | 30–46 (C) | 357 | [37] |
| 11 | H′-PMDA/AB-TFMB | None | Applicable | Colorless | 25 (C) | 340 | [51] |
| 12. | CBDA(70); 6FDA(30)/AB-TFMB | None | Applicable | Colorless | 7.3 (C) | 329 | [51] |
| 13 | CBDA(80); 6FDA(20)/AB-TFMB | None | Applicable | Colorless | 4.2 (C) | 335 | [51] |
| 14 | TA-HQ/TFMB | None | Non-applicable | Slightly colored | 23 (T) | 370 | [21,48] |
| 15 | TA-TMHQ/TFMB | None | Applicable | Almost colorless | 12 (C) | 276 | [21] |
| 16 | TA-TFMB/TFMB | None | Applicable | Almost colorless | 10 (C) | 328 | [15] |
| 17 | TA-TFBP/TFMB | None | Applicable | Almost colorless | 20 (C) | 261 | [15] |
| 18 | TA-TFBP(70); NTDA(30)/TFMB | None | Applicable | Almost colorless | 13 (C) | 277 | [15] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, M. Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion. Polymers 2017, 9, 520. https://doi.org/10.3390/polym9100520
Hasegawa M. Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion. Polymers. 2017; 9(10):520. https://doi.org/10.3390/polym9100520
Chicago/Turabian StyleHasegawa, Masatoshi. 2017. "Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion" Polymers 9, no. 10: 520. https://doi.org/10.3390/polym9100520
APA StyleHasegawa, M. (2017). Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion. Polymers, 9(10), 520. https://doi.org/10.3390/polym9100520




