Molecularly Imprinted Polymers Based Electrochemical Sensor for 2,4-Dichlorophenol Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation and Reagents
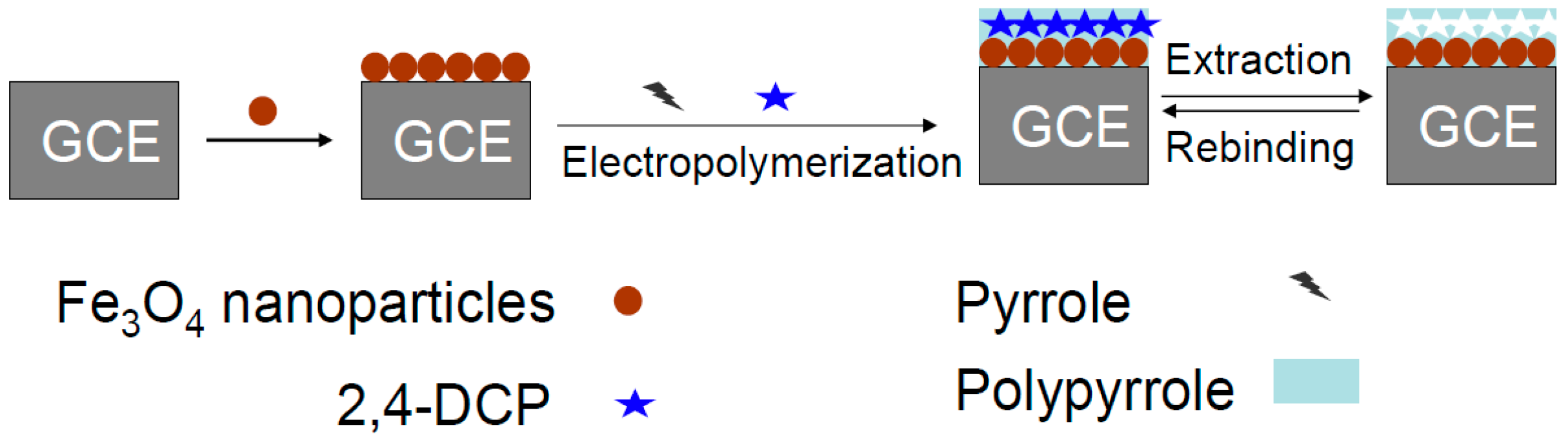
2.2. Fabrication of the Modified Electrodes
2.3. Experimental Measurements
3. Results and Discussion
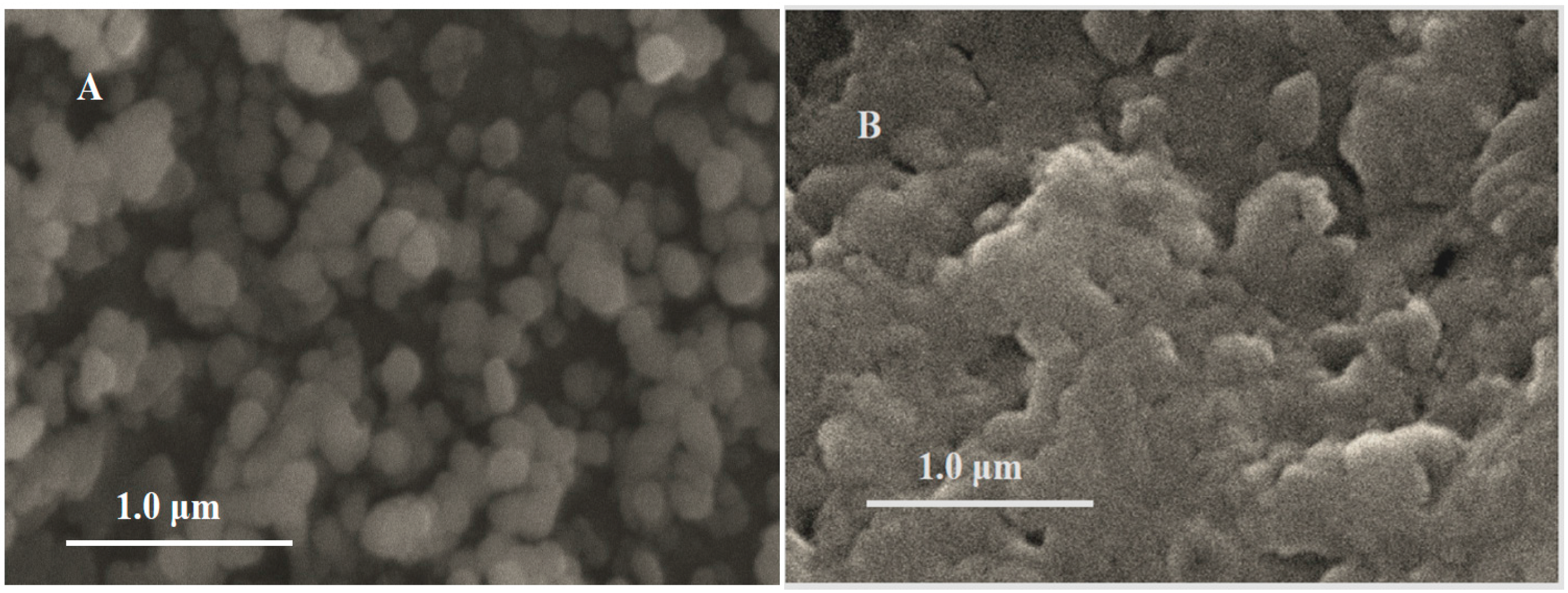
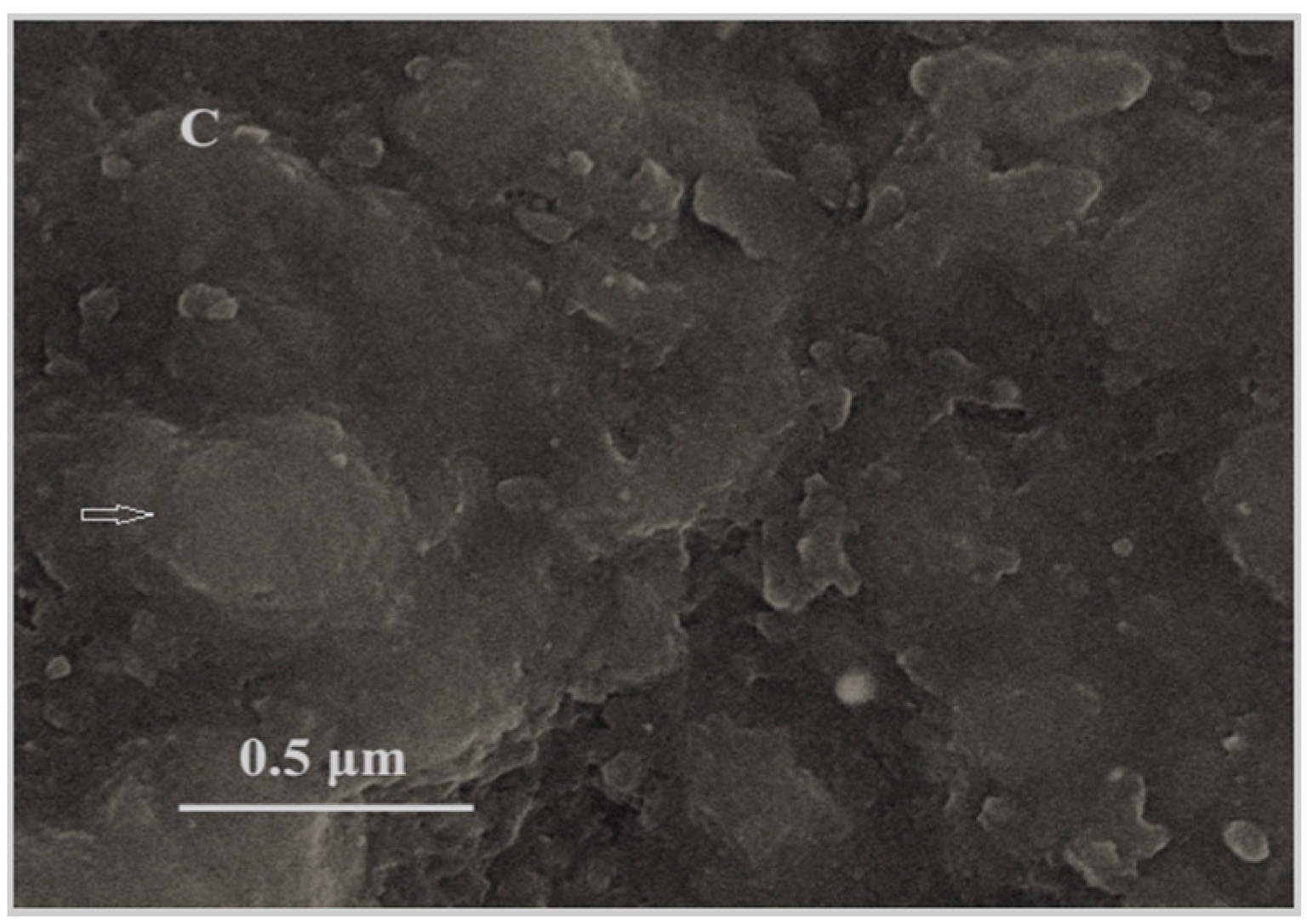
3.1. Morphology of Fe3O4 Nanoparticles and MIPs/Fe3O4
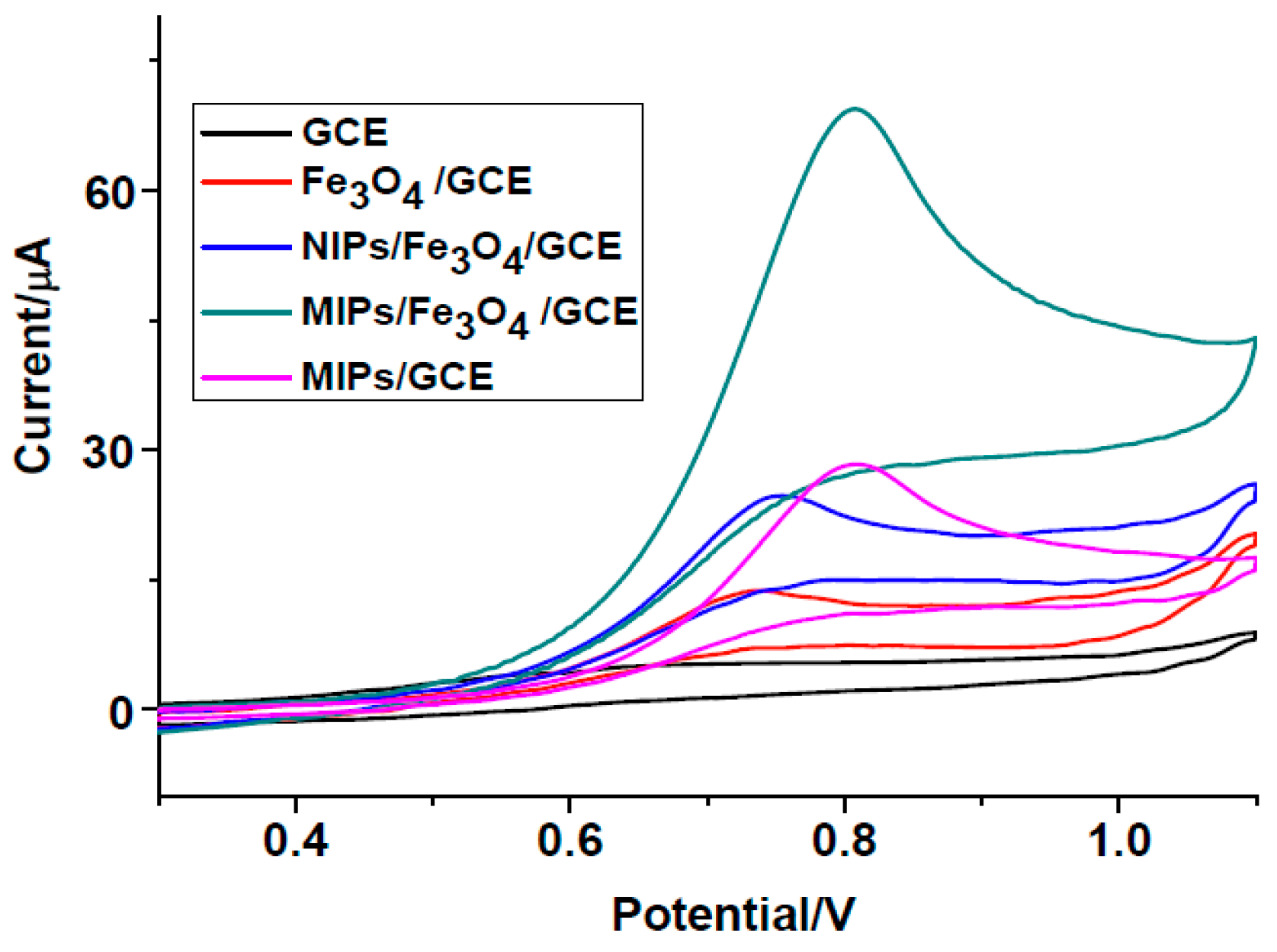
3.2. Electrochemical Behavior of 2,4-DCP at Modified Electrodes
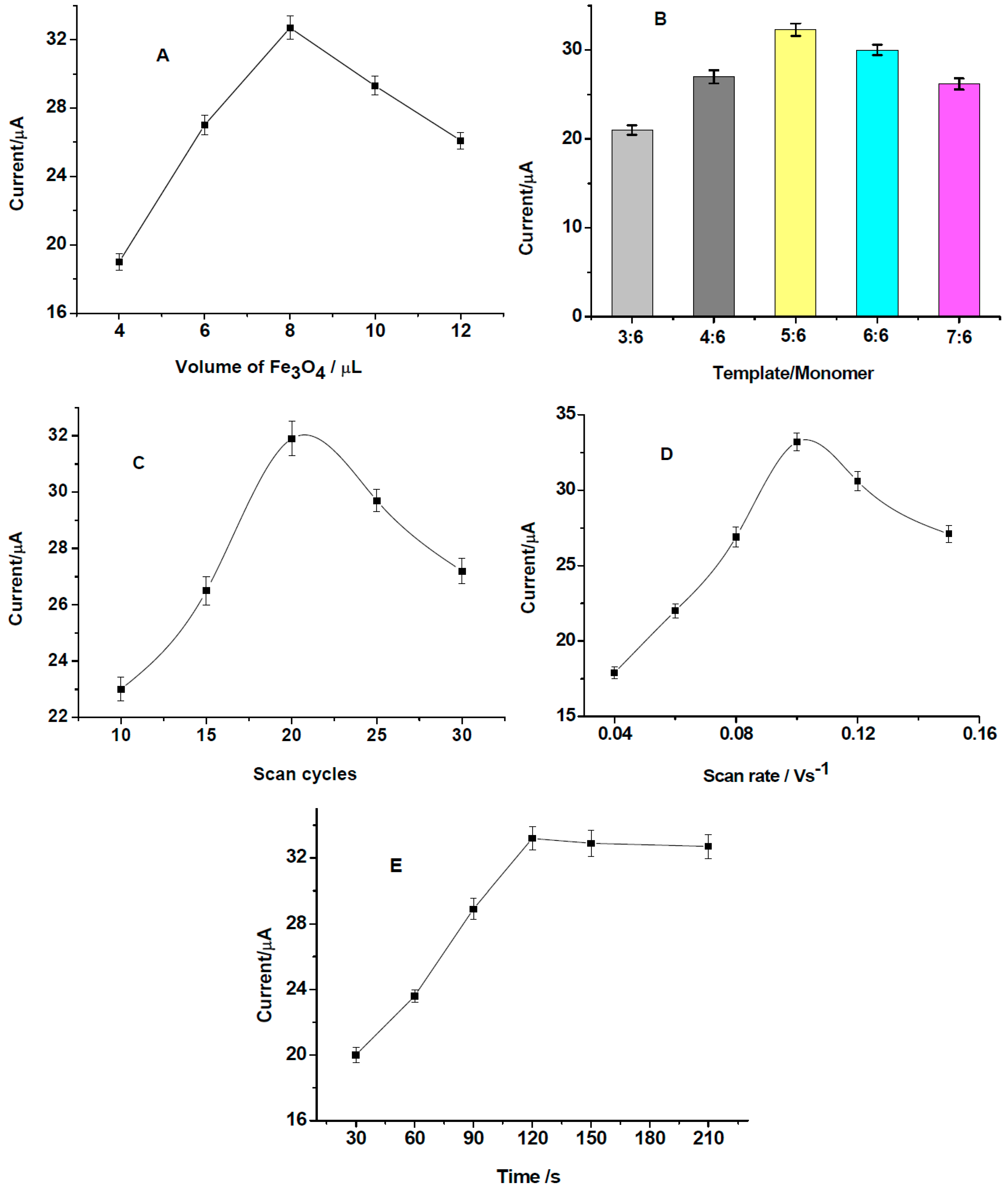
3.3. Optimization of MIPs/Fe3O4/GCE Preparation Conditions
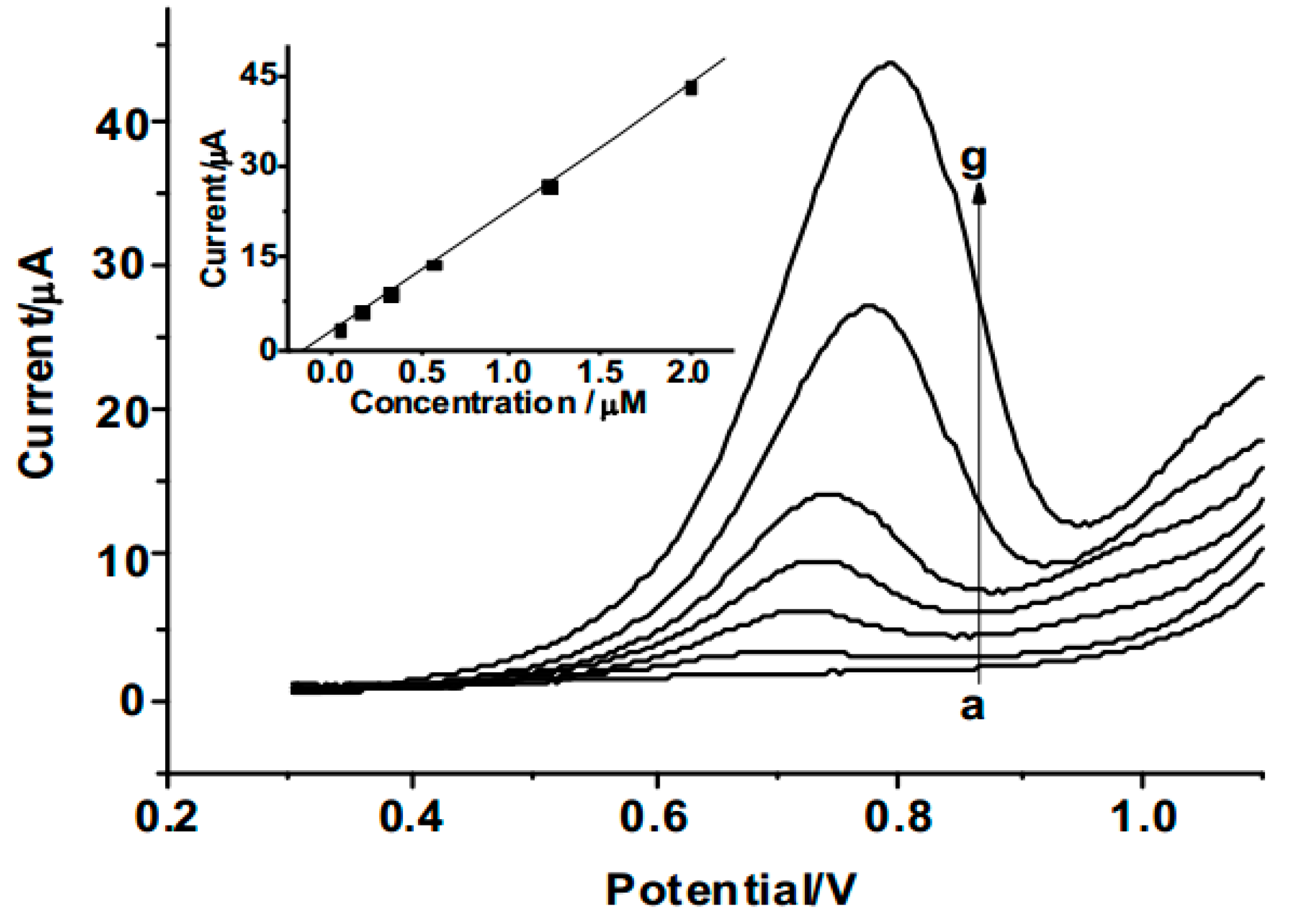
3.4. Determination of 2,4-DCP
3.5. Reproducibility and Stability
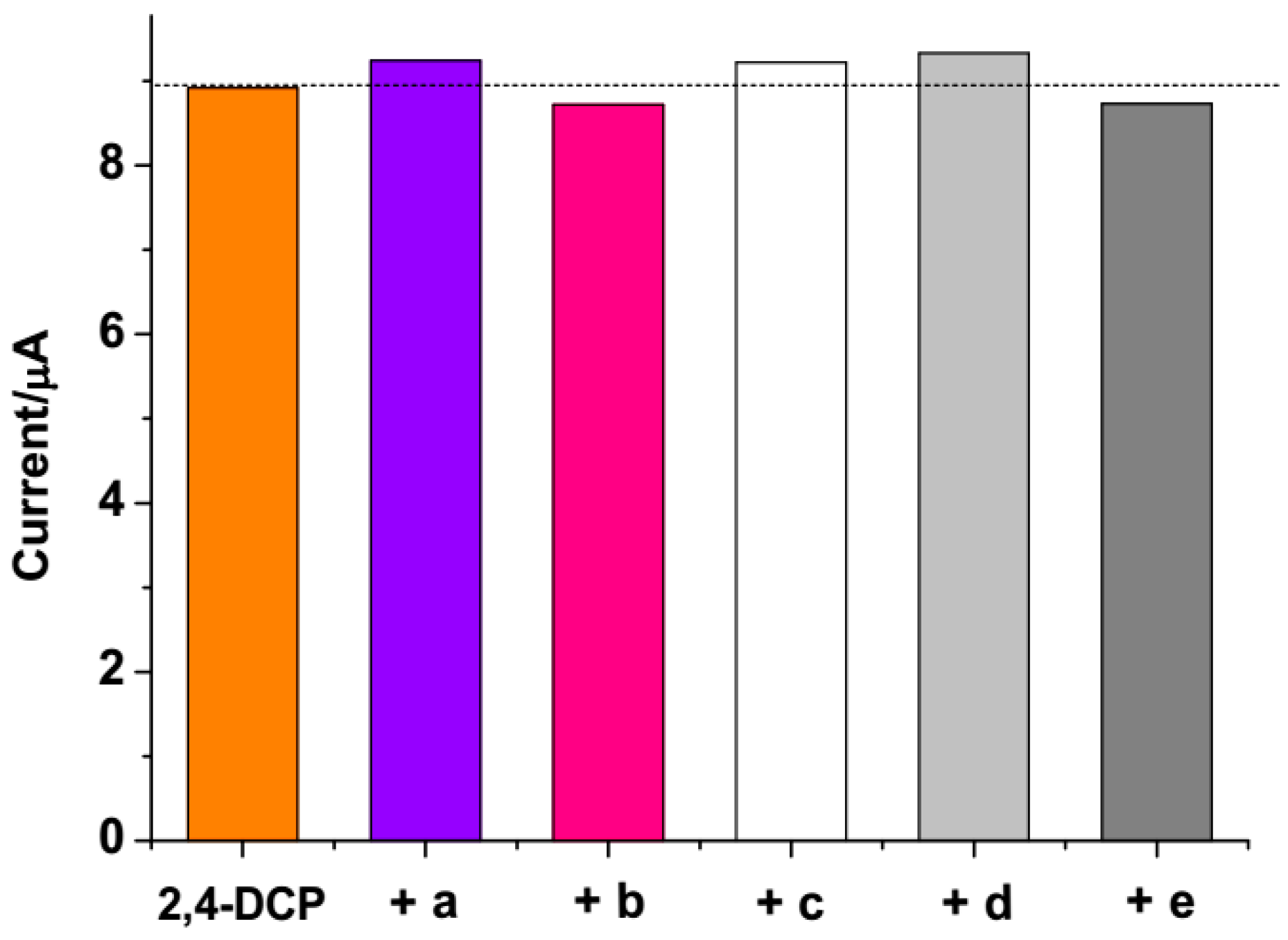
3.6. Selectivity Study
3.7. Real Water Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, Z.H.; Yu, C.; Wang, X.Y.; Wan, Y.; Li, D.; Lu, G.Z. Liquid phase hydridechlorination of chlorophenols at lower temperature on a novel Pd catalyst. J. Hazard. Mater. 2011, 186, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.Y.; Tu, Y.M.; Jian, Z.X.; Wang, H.W.; Huang, Y.L. Direct determination of chlorophenols in water samples through ultrasound-assisted hollow fiber liquid-liquid microextraction on-line coupled with high-performance liquid chromatography. J. Chromatogr. A 2013, 1271, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lee, H.K. Electro-membrane extraction followed by low-density solvent based ultrasound-assisted emulsification microextraction combined with derivatization for determining chlorophenols and analysis by gas chromatography–mass spectrometry. J. Chromatogr. A 2012, 1243, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, H.; Zhang, Z.; Lin, J. Gold nanoparticles for enhanced chemiluminescence and determination of 2,4-dichlorophenol in environmental water samples. Analyst 2011, 136, 2156–2160. [Google Scholar] [CrossRef] [PubMed]
- Arribas, A.S.; Moreno, M.; Bermejo, E.; Pérez, J.A.; Román, V.; Zapar-diel, A.; Chicharro, M. Application of carbon nanotube-modified electrodes as electrochemical sensors for the continuous monitoring of 2,4-dichlorophenol. Electroanalysis 2011, 23, 237–244. [Google Scholar] [CrossRef]
- Kong, L.M.; Huang, S.S.; Yue, Z.L.; Peng, B.; Li, M.Y.; Zhang, J. Sensitive mediator-free tyrosinase biosensor for the determination of 2,4-dichlorophenol. Microchim. Acta 2009, 165, 203–209. [Google Scholar] [CrossRef]
- Liu, J.; Niu, J.F.; Yin, L.F.; Jiang, F. In situ encapsulation of laccase in nanofibers by electrospinning for development of enzyme biosensors for chlorophenol monitoring. Analyst 2011, 136, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, X.J.; Zhou, Y.E.; Wei, H.P.; Hu, X.Y.; Wang, Y.; Yang, Z.J. An enzymatic amplified system for the detection of 2,4-dichlorophenol based on graphene membrane modified electrode. Anal. Methods 2012, 4, 3429–3435. [Google Scholar] [CrossRef]
- Reddy, S.M.; Sette, G.; Phan, Q. Electrochemical probing of selective haemoglobin binding in hydrogel-based molecularly imprinted polymers. Electrochim. Acta 2011, 56, 9203–9208. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, Z.; Li, Y. A strategy for constructing sensitive and renewable molecularly imprinted electrochemical sensors for melamine detection. Anal. Chim. Acta 2011, 706, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Teng, Y.; Pang, S.; Yan, L.; Kan, X. Pyrrole-phenylboronicacid: A novel monomer for dopamine recognition and detection based on imprinted electrochemical sensor. Biosens. Bioelectron. 2015, 64, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Pietrzyk-Le, A.; Souza, F.D.; Kutner, W. Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Han, Q.; Wang, Y.; Wu, J.; Wen, T.; Wang, R.; Hong, J.; Zhou, X.; Jiang, H. Amperometric detection of dopamine in human serumby electrochemical sensor based on gold nanoparticles doped molecularly imprinted polymers. Biosens. Bioelectron. 2013, 49, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Sun, X. Sandwich-structured graphene@Fe3O4@carbon nanocomposites with enhanced electromagnetic absorption properties. Mater. Lett. 2015, 144, 26–29. [Google Scholar] [CrossRef]
- Xing, Y.; Jin, Y.Y.; Si, J.C.; Peng, M.L.; Wang, X.F.; Chen, C.; Cui, Y.L. Controllable synthesis and characterization of Fe3O4/Au composite nanoparticles. J. Magn. Magn. Mater. 2015, 380, 150–156. [Google Scholar] [CrossRef]
- Zhang, C.; Si, S.; Yang, Z. A highly selective photoelectrochemical biosensor for uric acid based on core–shell Fe3O4@C nanoparticle and molecularly imprinted TiO2. Biosens. Bioelectron. 2015, 65, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Liu, S.; Yu, J.; Lian, W.; Huang, J. Electrochemical sensor based on molecularly imprinted film at polypyrrole-sulfonatedgraphene/hyaluronic acid-multiwalled carbon nanotubes modified electrode for determination of tryptamine. Biosens. Bioelectron. 2012, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Ramanaviciene, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer-polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Kan, X.; Zhou, H.; Li, C.; Zhu, A.; Xing, Z.; Zhao, Z. Imprinted electrochemical sensor for dopamine recognition and determination based on a carbon nanotube/polypyrrolefilm. Electrochim. Acta 2012, 63, 69–75. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Zhou, X.; Ma, P.; Wu, S.; Xu, L.; Shen, J. Ultrasensitive dopamine sensor based on novel molecularly imprinted polypyrrole coated carbon nanotubes. Biosens. Bioelectron. 2014, 58, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.J.; Pan, M.F.; Fang, G.Z.; He, X.L.; Yang, Y.K.; Dai, J.; Wang, S. Molecularly imprinted quartz crystal microbalance sensor based on poly(o-aminothiophenol) membrane and Au nanoparticles for ractopamine determination. Biosens. Bioelectron. 2014, 51, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.L.; Gong, F.C.; Zeng, G.M.; Shen, G.L.; Yu, R.Q. A novel electrosynthesized polymer applied to molecular imprinting technology. Talanta 2003, 61, 447–453. [Google Scholar] [CrossRef]
- Analytical Methods Committee. Recommendations for the definition estimation and use of the detection limit. Analyst 1987, 112, 199–204. [Google Scholar]
- Sun, Y.L.; Wang, L.P.; Liu, H.H. Myoglobin functioning as cytochrome P450 for biosensing of 2,4-dichlorophenol. Anal. Methods 2012, 4, 3358–3363. [Google Scholar] [CrossRef]
- Huang, S.S.; Qu, Y.X.; Li, R.N.; Shen, J.; Zhu, L.W. Biosensor based on horseradish peroxidase modified carbon nanotubes for determination of 2,4-dichlorophenol. Microchim. Acta 2008, 162, 261–268. [Google Scholar] [CrossRef]
| Modified electrode | Linear range (μM) | LOD (μM) | References |
|---|---|---|---|
| Nafion/MWNTs/GCE | 0.1–100 | 0.037 | [5] |
| Tyrosinase/MWNTs/GCE | 2.0–100 | 0.66 | [6] |
| Lac/PVA/F108/Au NPs/GCE | 1.0–25.0 | 0.04 | [7] |
| Mb-AG/GCE | 12.5–208 | 2.06 | [24] |
| HRP/MWNTs/GCE | 1.0–100 | 0.38 | [25] |
| MIPs/Fe3O4/GCE | 0.04–2.0 | 0.01 | this work |
| Items | Current response of sensors (μA) | RSD (%) (n = 4) | |||
|---|---|---|---|---|---|
| Sensor 1 | Sensor 2 | Sensor 3 | Sensor 4 | ||
| Reproducibility | 8.79 | 9.55 | 8.46 | 9.23 | 2.4 |
| 0 day | 3 day | 7 day | 14 day | ||
| Stability | 9.11 | 8.98 | 8.72 | 8.47 | |
| River water | Added (µM) | Found (µM) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| 0 | Not detected | – | – | |
| Sample 1 | 0.16 | 0.153 | 95.6 | 3.9 |
| 1.2 | 1.17 | 97.5 | 4.2 | |
| 0 | Not detected | – | – | |
| Sample 2 | 0.16 | 0.155 | 96.9 | 3.7 |
| 1.2 | 1.13 | 94.2 | 3.4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Cang, H.; Jin, J. Molecularly Imprinted Polymers Based Electrochemical Sensor for 2,4-Dichlorophenol Determination. Polymers 2016, 8, 309. https://doi.org/10.3390/polym8080309
Liu B, Cang H, Jin J. Molecularly Imprinted Polymers Based Electrochemical Sensor for 2,4-Dichlorophenol Determination. Polymers. 2016; 8(8):309. https://doi.org/10.3390/polym8080309
Chicago/Turabian StyleLiu, Benzhi, Hui Cang, and Jianxiang Jin. 2016. "Molecularly Imprinted Polymers Based Electrochemical Sensor for 2,4-Dichlorophenol Determination" Polymers 8, no. 8: 309. https://doi.org/10.3390/polym8080309







