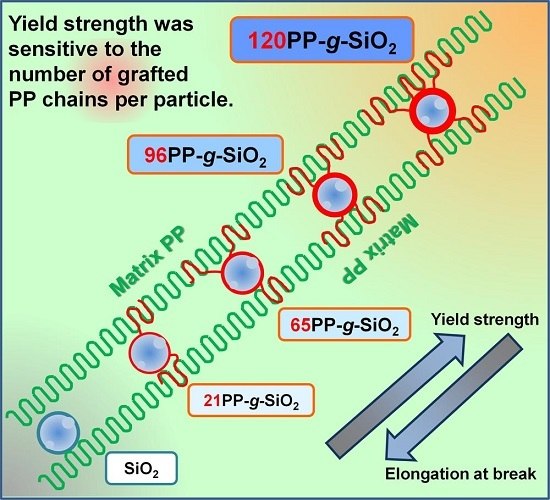
Well-Defined Polypropylene/Polypropylene-Grafted Silica Nanocomposites: Roles of Number and Molecular Weight of Grafted Chains on Mechanistic Reinforcement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydroxylated Isotactic Polypropylene (PP-t-OH)
2.3. Synthesis of Polypropylene-Grafted SiO2 (PP-g-SiO2)
2.4. Synthesis of PP/PP-g-SiO2 Nanocomposites
2.5. Analyses
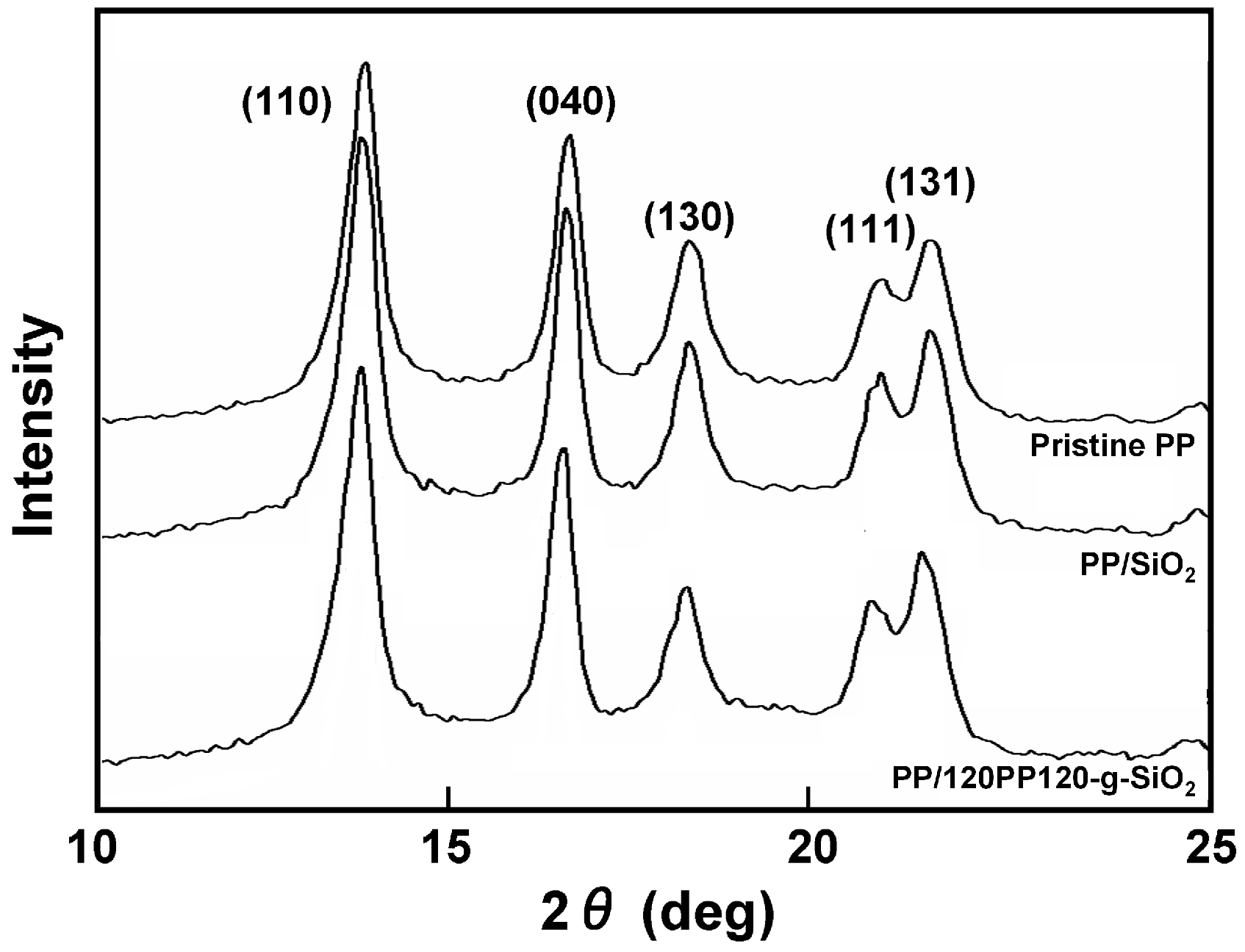
3. Results and Discussion
4. Conclusions
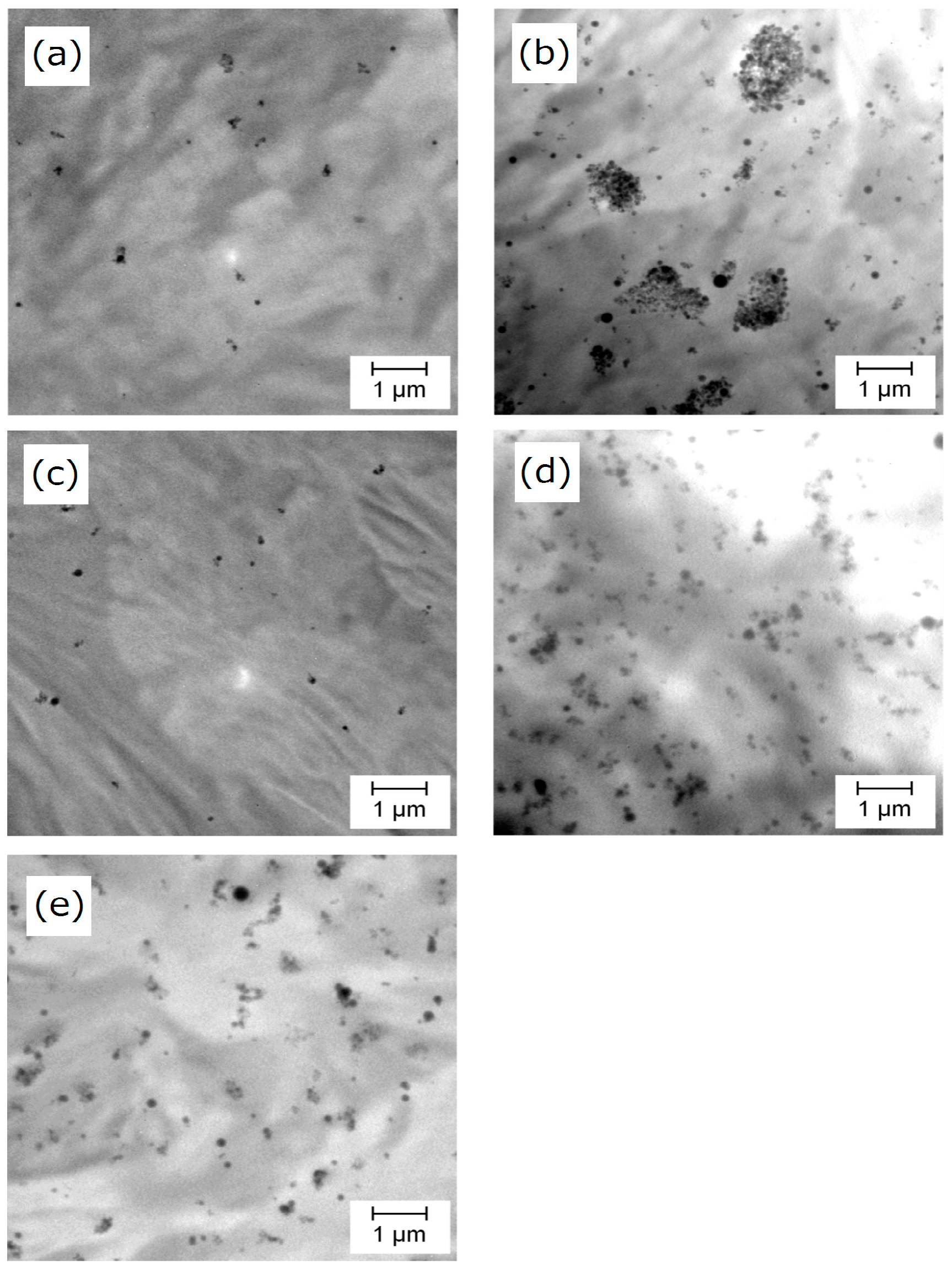
- As long as filler surfaces are mostly covered by grafted chains, the number of grafted chains per particle is not important for improving the dispersion of nanoparticles. 21 chain/particle (corresponding to 120% coverage) was sufficient in our system.
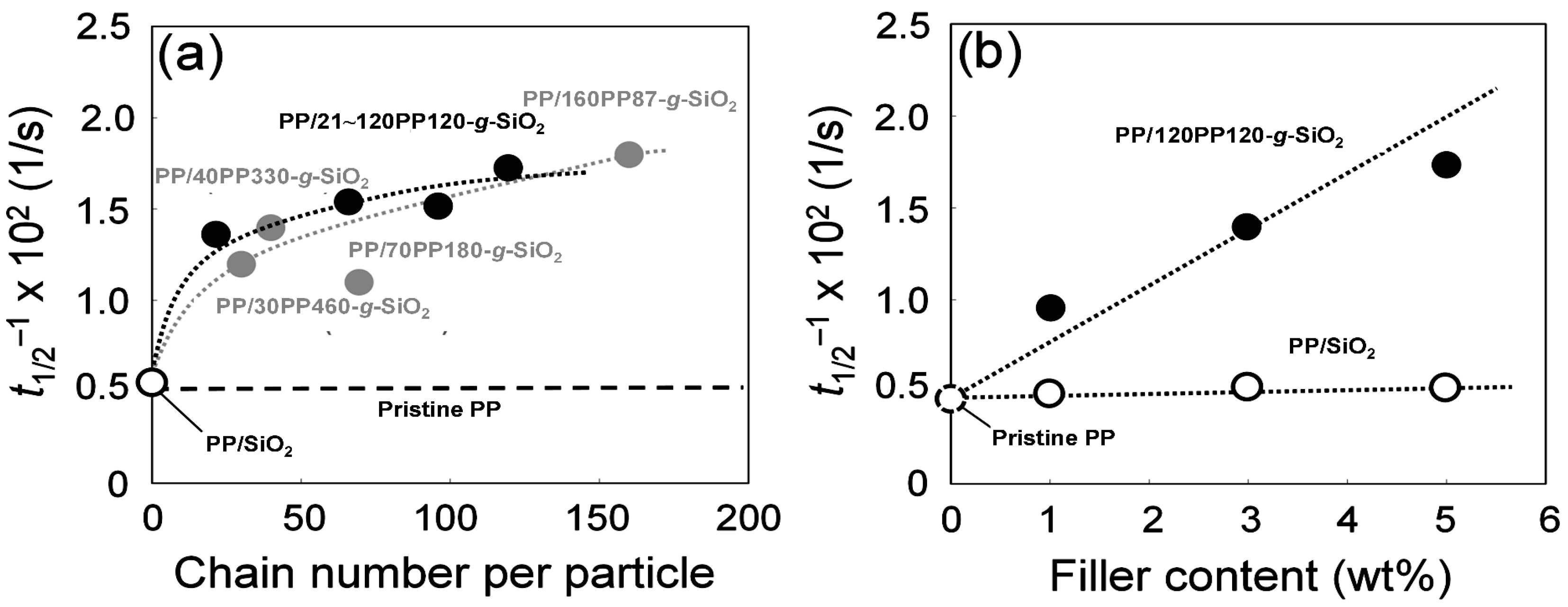
- The presence of grafted chains is essential to endow a nucleating ability to SiO2 nanoparticles. The density of dispersed PP-g-SiO2 nanoparticles in PP is important to enhance the crystallization rate, rather than the number of grafted chains.
- The Young’s modulus is not sensitive to the interfacial bonding as long as nanoparticles are well dispersed. Meanwhile, both the number and the molecular weight of grafted chains are crucial for improving the yield strength.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Usuki, A.; Kawasumi, M.; Kojima, Y.; Fukushima, Y.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Usuki, A.; Kawasumi, M.; Kojima, Y.; Fukushima, Y.; Okada, A.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Wu, J.; Li, J.X.; Cheung, Y.K. Polypropylene/calcium carbonate nanocomposites. Polymer 2002, 43, 2981–2992. [Google Scholar] [CrossRef]
- Gilman, J.W. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Yong, T.; Yuan, H.; Lei, S.; Ruowen, Z.; Zhou, G.; Zuyao, C.; Weicheng, F. Preparation and thermal stability of polypropylene/montmorillonite nanocomposites. Polym. Degrad. Stab. 2003, 82, 127–131. [Google Scholar]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Cuesta, J.M.L.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Du, M.; Guo, B.; Jia, D. Thermal stability and flame retardant effects of halloysite nanotubes on poly(propylene). Eur. Polym. J. 2006, 42, 1362–1369. [Google Scholar] [CrossRef]
- Du, F.; Scogna, R.C.; Zhou, W.; Brand, S.; Fischer, J.E.; Winey, K.I. Nanotube Networks in Polymer Nanocomposites: Rheology and Electrical Conductivity. Macromolecules 2004, 37, 9048–9055. [Google Scholar] [CrossRef]
- Tjong, S.C.; Liang, G.D.; Bao, S.P. Electrical behavior of polypropylene/multiwalled carbon nanotube nanocomposites with low percolation threshold. Scr. Mater. 2007, 57, 461–464. [Google Scholar] [CrossRef]
- Thomassin, J.M.; Pardoen, C.J.T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R 2013, 74, 211–232. [Google Scholar] [CrossRef]
- Li, N.; Huang, Y.; Du, F.; He, X.; Lin, X.; Gao, H.; Ma, Y.; Li, F.; Chen, Y.; Eklund, P.C. Electromagnetic Interference (EMI) Shielding of Single-Walled Carbon Nanotube Epoxy Composites. Nano Lett. 2006, 6, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.P. Optical Properties of Functional Hybrid Organic–Inorganic Nanocomposites. Adv. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Paiva, L.B.; Morales, A.R.; Guimaraes, T.R. Structural and optical properties of polypropylene–montmorillonite nanocomposites. Mater. Sci. Eng. A 2007, 447, 261–265. [Google Scholar] [CrossRef]
- Bharadwaj, R.K. Modeling the Barrier Properties of Polymer-Layered Silicate Nanocomposites. Macromolecules 2001, 34, 9189–9192. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Saujanya, C.; Radhakrishnan, S. Structure development and crystallization behaviour of PP/nanoparticulate composite. Polymer 2001, 42, 6723–6731. [Google Scholar] [CrossRef]
- Asuka, K.; Liu, B.; Terano, M.; Nitta, K. Homogeneously Dispersed Poly(propylene)/SiO2 Nanocomposites with Unprecedented Transparency. Macromol. Rapid Commun. 2006, 27, 910–913. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Lee, H.J.; Jeong, S.H. Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci. 2003, 38, 2143–2147. [Google Scholar] [CrossRef]
- Calvo, M.E.; Smirnov, J.R.C.; Míguez, H. Novel approaches to flexible visible transparent hybrid films for ultraviolet protection. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 945–956. [Google Scholar] [CrossRef]
- Mazrouaa, A.M. Polypropylene Nanocomposites. In Polypropylene, 1st ed.; Dogan, F., Ed.; InTech: Rijeka, Croatia, 2012; Volume 14, pp. 265–287. [Google Scholar]
- Palza, H.; Vergara, R.; Zapata, P. Composites of polypropylene melt blended with synthesized silica nanoparticles. Compos. Sci. Technol. 2011, 71, 535–540. [Google Scholar] [CrossRef]
- Bikiaris, D.; Papageorgiou, G.; Pavlidou, E.; Vouroutzis, N.; Palatzoglou, P.; Karayannidis, G. Preparation by melt mixing and characterization of isotactic polypropylene/SiO2 nanocomposites containing untreated and surface-treated nanoparticles. J. Appl. Polym. Sci. 2006, 100, 2684–2696. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. A new compounding method for exfoliated graphite–polypropylene nanocomposites with enhanced flexural properties and lower percolation threshold. Compos. Sci. Technol. 2007, 67, 2045–2051. [Google Scholar] [CrossRef]
- Scharlach, K.; Kaminsky, W. New Polyolefin-Nanocomposites by In Situ Polymerization with Metallocene Catalysts. Macromol. Symp. 2008, 261, 10–17. [Google Scholar] [CrossRef]
- Guo, N.; DiBenedetto, S.A.; Kwon, D.K.; Wang, L.; Russell, M.T.; Lanagan, M.T.; Facchetti, A.; Marks, T.J. Supported Metallocene Catalysis for In Situ Synthesis of High Energy Density Metal Oxide Nanocomposites. J. Am. Chem. Soc. 2007, 129, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Balhoul, W.; Legaré, V.B.; David, L.; Cassagnau, P. Morphology and viscoelasticity of PP/TiO2 nanocomposites prepared by in situ sol–gel method. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1213–1222. [Google Scholar] [CrossRef]
- Kaneko, K.; Yadav, N.; Takeuchi, K.; Maira, B.; Terano, M.; Taniike, T. Versatile strategy for fabrication of polypropylene nanocomposites with inorganic network structures based on catalyzed in-situ sol–gel reaction during melt mixing. Compos. Sci. Technol. 2014, 102, 120–125. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. In situ Polymerization Approach to Graphene-Reinforced Nylon-6 Composites. Macromolecules 2010, 43, 6716–6723. [Google Scholar] [CrossRef]
- Cheng, H.K.F.; Sahoo, N.G.; Tan, Y.P.; Pan, Y.; Bao, H.; Li, L.; Chan, S.H.; Zhao, J. Poly(vinyl alcohol) Nanocomposites Filled with Poly(vinyl alcohol)-Grafted Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Q.; Rong, M.Z.; Yu, S.L.; Wetzel, B.; Friedrich, K. Effect of particle surface treatment on the tribological performance of epoxy based nanocomposites. Wear 2002, 253, 1086–1093. [Google Scholar] [CrossRef]
- Goel, V.; Pietrasik, J.; Dong, H.; Sharma, J.; Matyjaszewski, K.; Krishnamoorti, R. Structure of Polymer Tethered Highly Grafted Nanoparticles. Macromolecules 2011, 44, 8129–8135. [Google Scholar] [CrossRef]
- Zhu, A.; Cai, A.; Zhou, W.; Shi, Z. Effect of flexibility of grafted polymer on the morphology and property of nanosilica/PVC composites. Appl. Surf. Sci. 2008, 254, 3745–3752. [Google Scholar] [CrossRef]
- Lin, N.; Chen, G.; Huang, J.; Dufresne, A.; Chang, P.R. Effects of polymer-grafted natural nanocrystals on the structure and mechanical properties of poly(lactic acid): A case of cellulose whisker-graft-polycaprolactone. J. Appl. Polym. Sci. 2009, 113, 3417–3425. [Google Scholar] [CrossRef]
- Kumar, S.K.; Jouault, N.; Benicewicz, B.; Neely, T. Nanocomposites with Polymer Grafted Nanoparticles. Macromolecules 2013, 46, 3199–3214. [Google Scholar] [CrossRef]
- Wang, Z.M.; Hong, H.; Chung, T.C. Synthesis of Maleic Anhydride Grafted Polypropylene with High Molecular Weight Using Borane/O2 Radical Initiator and Commercial PP Polymers. Macromolecules 2005, 38, 8966–8970. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Zheng, X.Y.; Zeng, H.M.; Friedrich, K. Improvement of tensile properties of nano-SiO2/PP composites in relation to percolation mechanism. Polymer 2001, 42, 3301–3304. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Tensile performance improvement of low nanoparticles filled-polypropylene composites. Compos. Sci. Technol. 2002, 62, 1327–1340. [Google Scholar] [CrossRef]
- Cai, L.F.; Huang, X.B.; Rong, M.Z.; Ruan, W.H.; Zhang, M.Q. Effect of grafted polymeric foaming agent on the structure and properties of nano-silica/polypropylene composites. Polymer 2006, 47, 7043–7050. [Google Scholar] [CrossRef]
- Ma, C.G.; Rong, M.Z.; Zhang, M.Q.; Friedrich, K. Irradiation-induced surface graft polymerization onto calcium carbonate nanoparticles and its toughening effects on polypropylene composites. Polym. Eng. Sci. 2005, 45, 529–538. [Google Scholar] [CrossRef]
- Castel, C.D.; Pelegrini, T.; Barbosa, R.V.; Liberman, S.A.; Mauler, R.S. Properties of silane grafted polypropylene/montmorillonite nanocomposites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 185–191. [Google Scholar] [CrossRef]
- Lin, O.H.; Akil, H.M.; Ishak, Z.A.M. Surface-activated nanosilica treated with silane coupling agents/polypropylene composites: Mechanical, morphological, and thermal studies. Polym. Compos. 2011, 32, 1568–1583. [Google Scholar] [CrossRef]
- Umemori, M.; Taniike, T.; Terano, M. Influences of polypropylene grafted to SiO2 nanoparticles on the crystallization behavior and mechanical properties of polypropylene/SiO2 nanocomposites. Polym. Bull. 2012, 68, 1093–1108. [Google Scholar] [CrossRef]
- Taniike, T.; Toyonaga, M.; Terano, M. Polypropylene-grafted nanoparticles as a promising strategy for boosting physical properties of polypropylene-based nanocomposites. Polymer 2014, 55, 1012–1019. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Zhang, Y.X.; Zeng, H.M.; Walter, R.; Friedrich, K. Structure–property relationships of irradiation grafted nano-inorganic particle filled polypropylene composites. Polymer 2001, 42, 167–183. [Google Scholar] [CrossRef]
- Bartholome, C.; Beyou, E.; Bourgeat-Lami, E.; Cassagnau, P.; Chaumont, P.; David, L.; Zydowicz, N. Viscoelastic properties and morphological characterization of silica/polystyrene nanocomposites synthesized by nitroxide-mediated polymerization. Polymer 2005, 46, 9965–9973. [Google Scholar] [CrossRef]
- Han, C.J.; Lee, M.S.; Byun, D.J.; Kim, S.Y. Synthesis of Hydroxy-Terminated Polyethylene via Controlled Chain Transfer Reaction and Poly(ethylene-b-caprolactone) Block Copolymer. Macromoleclues 2002, 35, 8923–8925. [Google Scholar] [CrossRef]
- Naga, N.; Mizumura, K. Chain transfer reaction by trialkylaluminum (AIR3) in the stereospecific polymerization of propylene with metallocene—AIR3/Ph3CB(C6F5)4. Polymer 1998, 39, 5059–5067. [Google Scholar] [CrossRef]
- Yamada, K.; Hikosaka, M.; Toda, A.; Yamazaki, S.; Tagashira, K. Equilibrium Melting Temperature of Isotactic Polypropylene with High Tacticity: 1. Determination by Differential Scanning Calorimetry. Macromolecules 2003, 36, 4790–4801. [Google Scholar] [CrossRef]
- Toda, A.; Taguchi, K.; Sato, K.; Nozaki, K.; Murayama, M.; Tagashira, K.; Konishi, M. Melting kinetics of it-polypropylene crystals over wide heating rates. J. Therm. Anal. Calorim. 2013, 113, 1231–1237. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Müller, A.J.; Lin, M.C.; Chen, H.L. SAXS/DSC Analysis of the Lamellar Thickness Distribution on a SSA Thermally Fractionated Model Polyethylene. Macromol. Chem. Physic. 2011, 212, 2009–2016. [Google Scholar] [CrossRef]
- Chen, S.H.; Su, C.H.; Su, A.C.; Sun, Y.S.; Jeng, U.; Chen, S.A. Gibbs–Thomson analysis of crystalline poly(9,9-di-n-octyl-2,7-fluorene). J. Appl. Crystallogr. 2007, 40, 573–576. [Google Scholar] [CrossRef]
- Liu, Y.; Bo, S.; Zhu, Y.; Zhang, W. Determination of molecular weight and molecular sizes of polymers by high temperature gel permeation chromatography with a static and dynamic laser light scattering detector. Polymer 2003, 44, 7209–7220. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Kawai, T.; Kuroda, S.; Toyonaga, M.; Taniike, T.; Terano, M. The effect of the addition of polypropylene grafted SiO2 nanoparticle on the crystallization behavior of isotactic polypropylene. J. Therm. Anal. Calorim. 2013, 113, 1511–1519. [Google Scholar] [CrossRef]
- Loos, J.; Hückert, A.; Petermann, J. On the crystallization behavior of cold-drawn syndiotactic polypropylene. Colloid Polym. Sci. 1996, 274, 1006–1011. [Google Scholar] [CrossRef]
- Vladimirov, V.; Betchev, C.; Vassiliou, A.; Papageorgiou, G.; Bikiaris, D. Dynamic mechanical and morphological studies of isotactic polypropylene/fumed silica nanocomposites with enhanced gas barrier properties. Compos. Sci. Technol. 2006, 66, 2935–2944. [Google Scholar] [CrossRef]
- Ranade, A.; Nayak, K.; Fairbrother, D.; D’Souz, N.A. Maleated and non-maleated polyethylene–montmorillonite layered silicate blown films: Creep, dispersion and crystallinity. Polymer 2005, 46, 7323–7333. [Google Scholar] [CrossRef]
- Stern, C.; Frick, A.; Weickert, G. Relationship between the structure and mechanical properties of polypropylene: Effects of the molecular weight and shear-induced structure. J. Appl. Polym. Sci. 2007, 103, 519–533. [Google Scholar] [CrossRef]
- Garcia, M.; Vliet, G.; Jahin, S.; Schrauwen, B.A.G.; Sarkissov, A.; Zyl, W.E.; Boukamp, B. Polypropylene/SiO2 Nanocomposites with Improved Mechanical Properties. Rev. Adv. Mater. Sci. 2004, 2, 169–175. [Google Scholar]
- McNally, T.; McShane, P.; Nally, G.M.; Murphy, W.R.; Cook, M.; Miller, A. Rheology, phase morphology, mechanical, impact and thermal properties of polypropylene/metallocene catalysed ethylene 1-octene copolymer blends. Polymer 2002, 43, 3785–3793. [Google Scholar] [CrossRef]

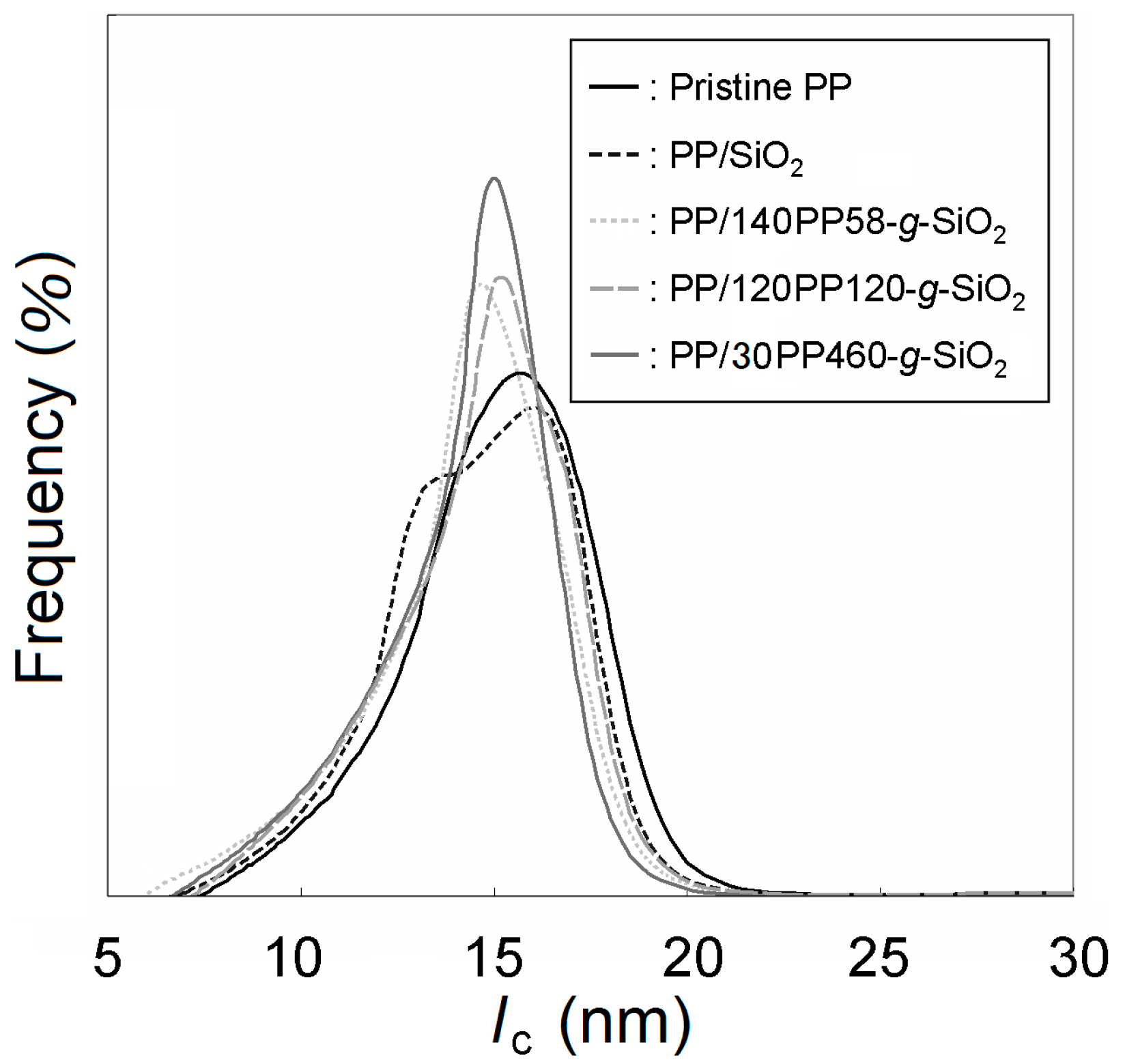
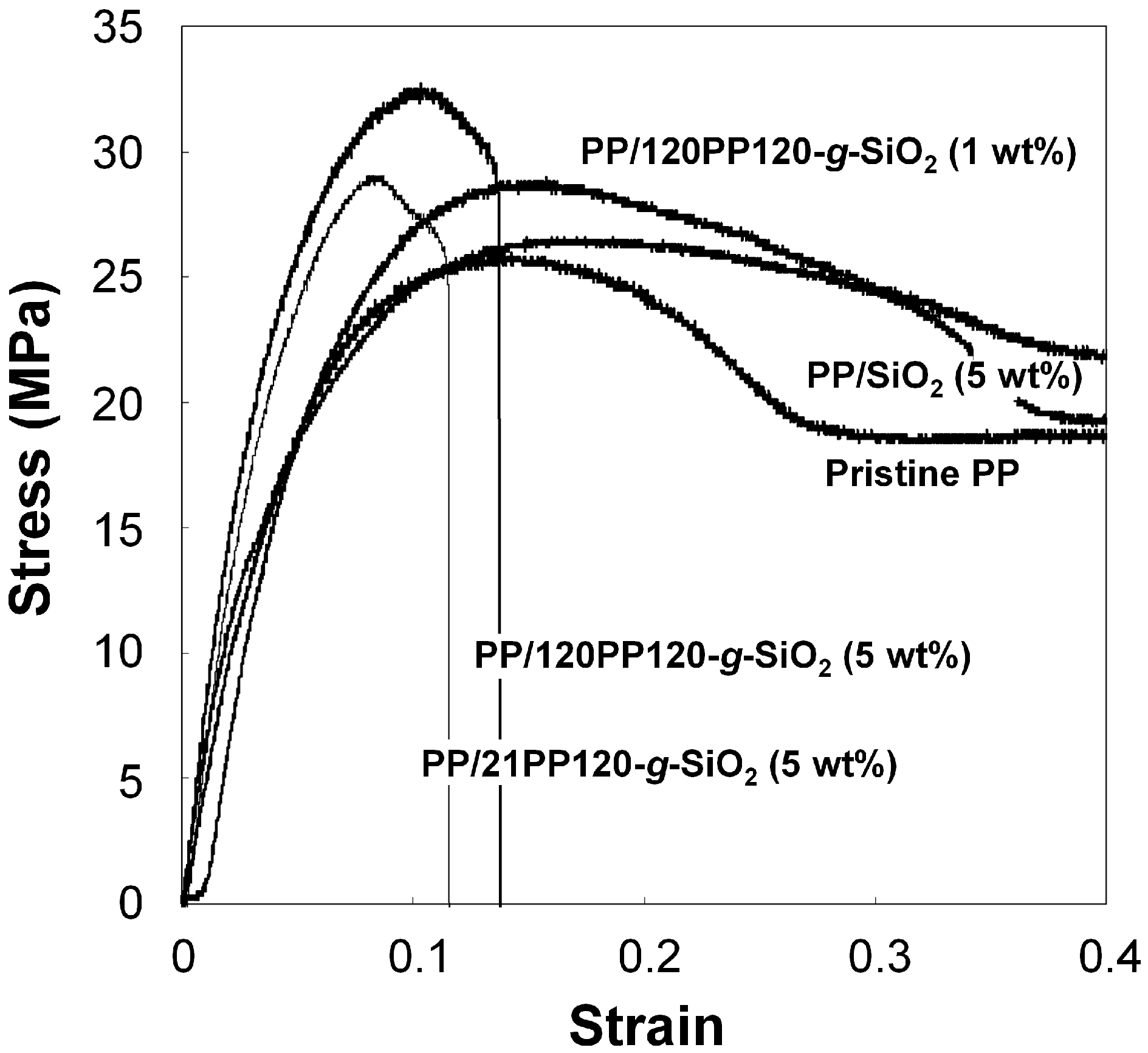

| Sample a | PP-t-OH amount (μmol per gram of SiO2) b | Grafted amount (wt %) c | Chain number per particle d |
|---|---|---|---|
| 21PP120-g-SiO2 | 16.7 | 2.2 | 21 |
| 65PP120-g-SiO2 | 83.3 | 6.3 | 65 |
| 96PP120-g-SiO2 | 333 | 9.1 | 96 |
| 120PP120-g-SiO2 | 667 | 11.4 | 120 |
| 140PP58-g-SiO2 e | 667 | 6.4 | 140 |
| 160PP87-g-SiO2 e | 667 | 10.7 | 160 |
| 70PP180-g-SiO2 e | 667 | 9.6 | 70 |
| 40PP330-g-SiO2 e | 667 | 10.4 | 40 |
| 30PP460-g-SiO2 e | 667 | 10.9 | 30 |
| Sample | Tm (°C) a | Xc (%) a | t1/2−1 × 102 (1/s) b |
|---|---|---|---|
| Pristine PP | 163 | 50 | 0.46 |
| PP/SiO2 (1 wt %) | 160 | 49 | 0.48 |
| PP/SiO2 (3 wt %) | 161 | 49 | 0.52 |
| PP/SiO2 (5 wt %) | 162 | 47 | 0.51 |
| PP/140PP58-g-SiO2 (5 wt %) c | 159 | 50 | 2.1 |
| PP/140PP87-g-SiO2 (5 wt %) c | 162 | 51 | 1.8 |
| PP/21PP120-g-SiO2 (5 wt %) | 163 | 51 | 1.3 |
| PP/65PP120-g-SiO2 (5 wt %) | 162 | 51 | 1.5 |
| PP/96PP120-g-SiO2 (5 wt %) | 161 | 52 | 1.4 |
| PP/120PP120-g-SiO2 (1 wt %) | 161 | 50 | 0.90 |
| PP/120PP120-g-SiO2 (3 wt %) | 162 | 49 | 1.4 |
| PP/120PP120-g-SiO2 (5 wt %) | 160 | 51 | 1.7 |
| PP/70PP180-g-SiO2 (5 wt %) c | 163 | 48 | 1.1 |
| PP/40PP330-g-SiO2 (5 wt %) c | 162 | 51 | 1.4 |
| PP/30PP460-g-SiO2 (5 wt %) c | 161 | 53 | 1.2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyonaga, M.; Chammingkwan, P.; Terano, M.; Taniike, T. Well-Defined Polypropylene/Polypropylene-Grafted Silica Nanocomposites: Roles of Number and Molecular Weight of Grafted Chains on Mechanistic Reinforcement. Polymers 2016, 8, 300. https://doi.org/10.3390/polym8080300
Toyonaga M, Chammingkwan P, Terano M, Taniike T. Well-Defined Polypropylene/Polypropylene-Grafted Silica Nanocomposites: Roles of Number and Molecular Weight of Grafted Chains on Mechanistic Reinforcement. Polymers. 2016; 8(8):300. https://doi.org/10.3390/polym8080300
Chicago/Turabian StyleToyonaga, Masahito, Patchanee Chammingkwan, Minoru Terano, and Toshiaki Taniike. 2016. "Well-Defined Polypropylene/Polypropylene-Grafted Silica Nanocomposites: Roles of Number and Molecular Weight of Grafted Chains on Mechanistic Reinforcement" Polymers 8, no. 8: 300. https://doi.org/10.3390/polym8080300
APA StyleToyonaga, M., Chammingkwan, P., Terano, M., & Taniike, T. (2016). Well-Defined Polypropylene/Polypropylene-Grafted Silica Nanocomposites: Roles of Number and Molecular Weight of Grafted Chains on Mechanistic Reinforcement. Polymers, 8(8), 300. https://doi.org/10.3390/polym8080300