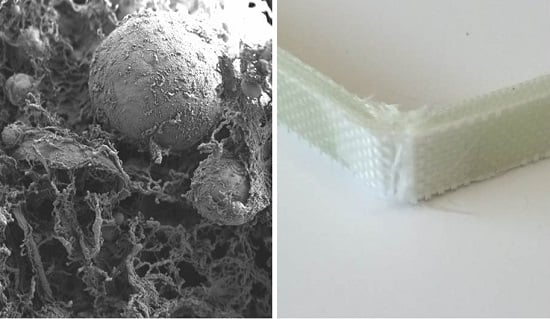
Microfibrillated Lignocellulose Enables the Suspension-Polymerisation of Unsaturated Polyester Resin for Novel Composite Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Microfibrillated Lignocellulose
2.2. Suspension Polymerisation
2.3. Fibre-Reinforced Composites
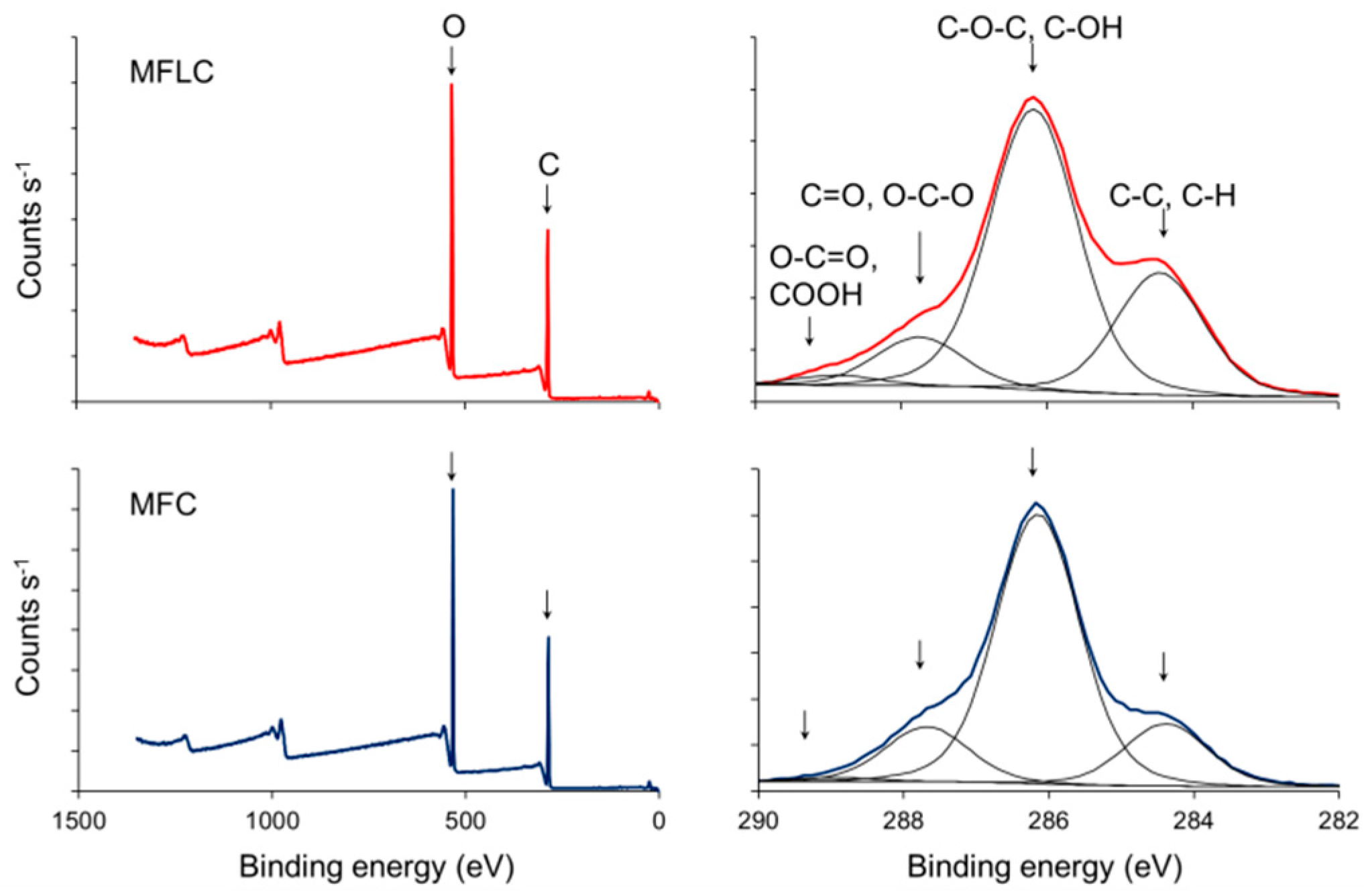
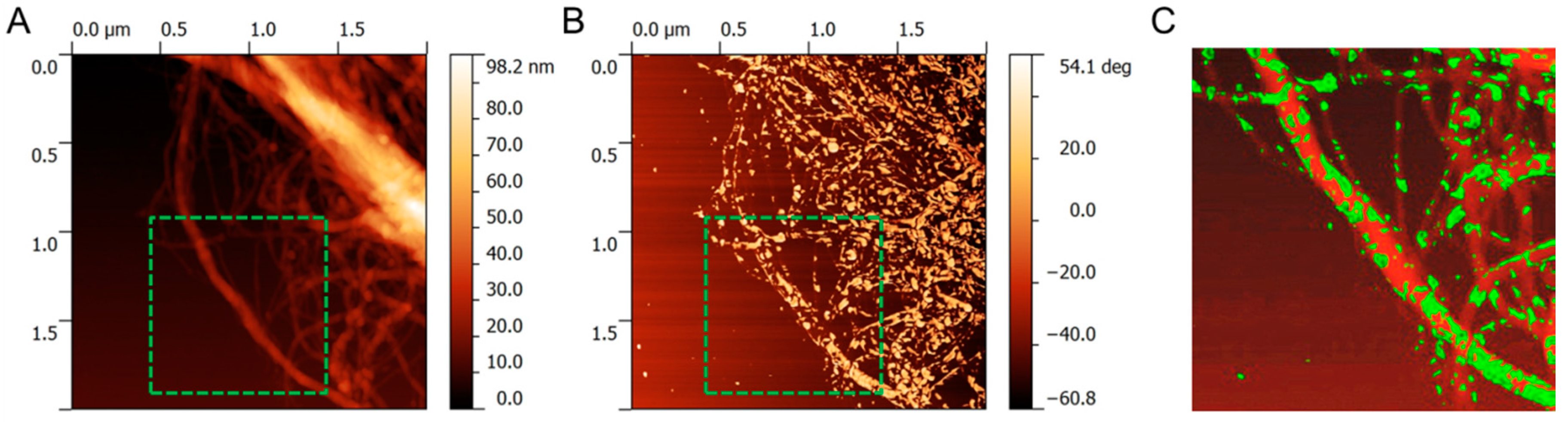
2.4. Characterisation
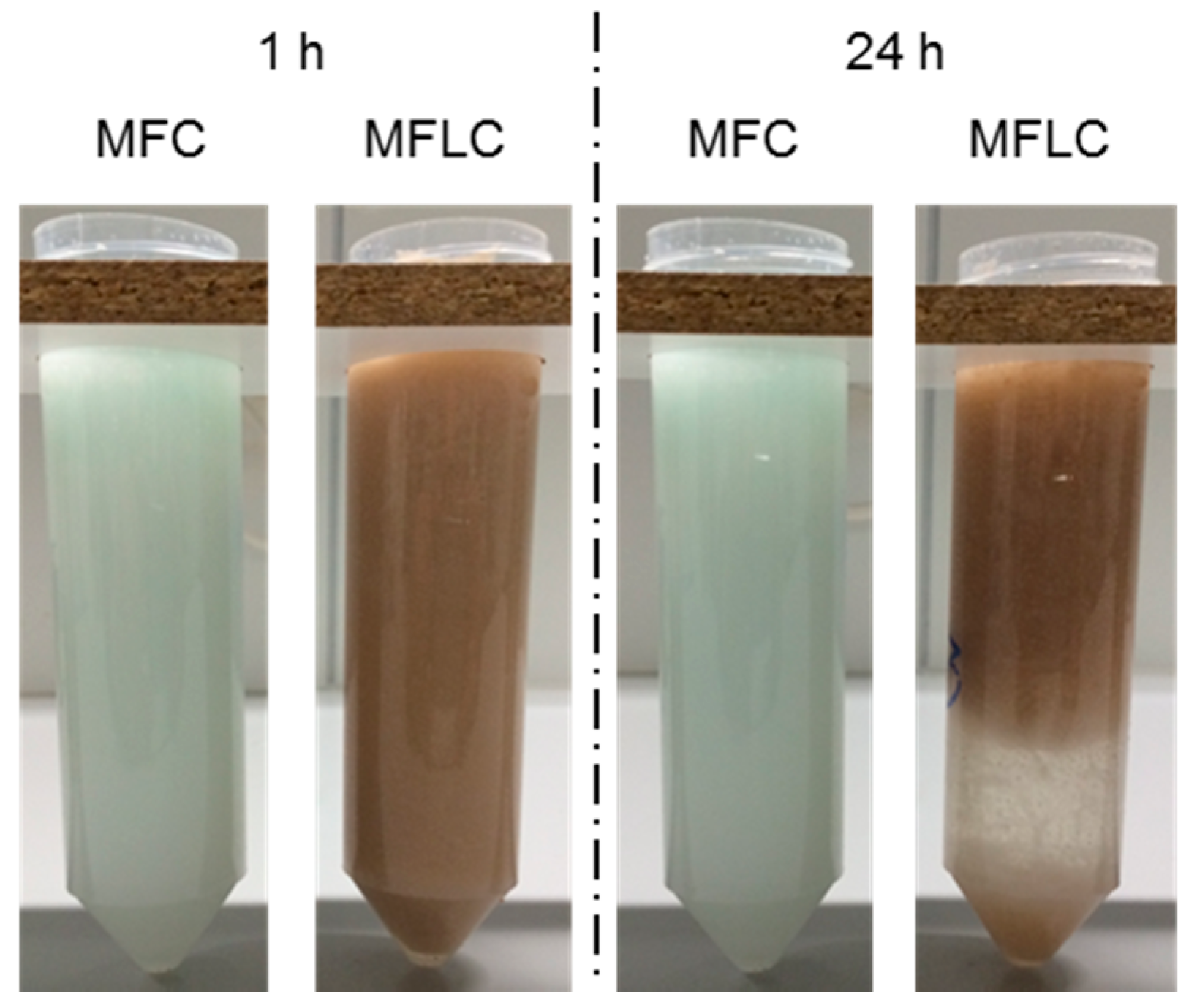
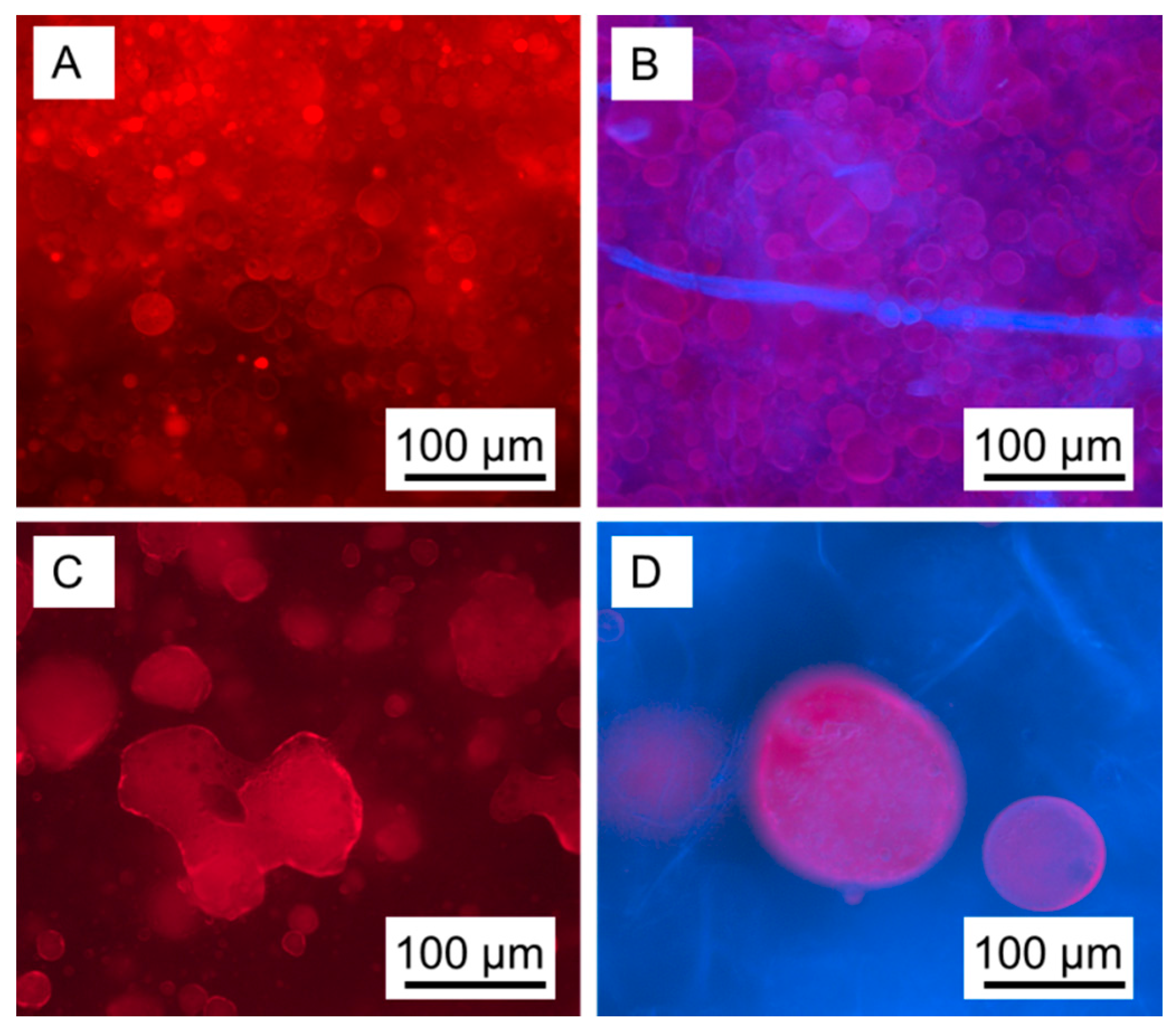
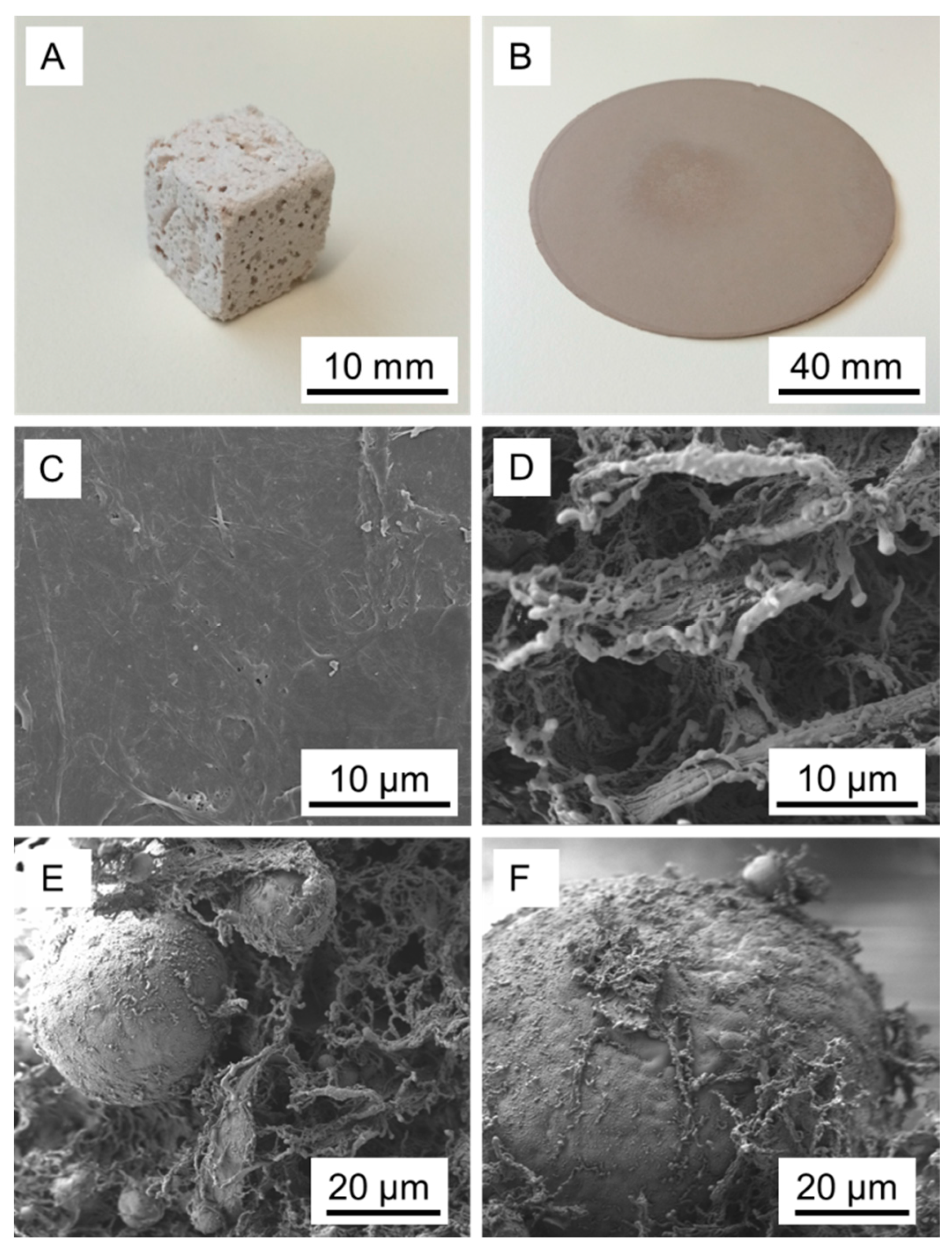
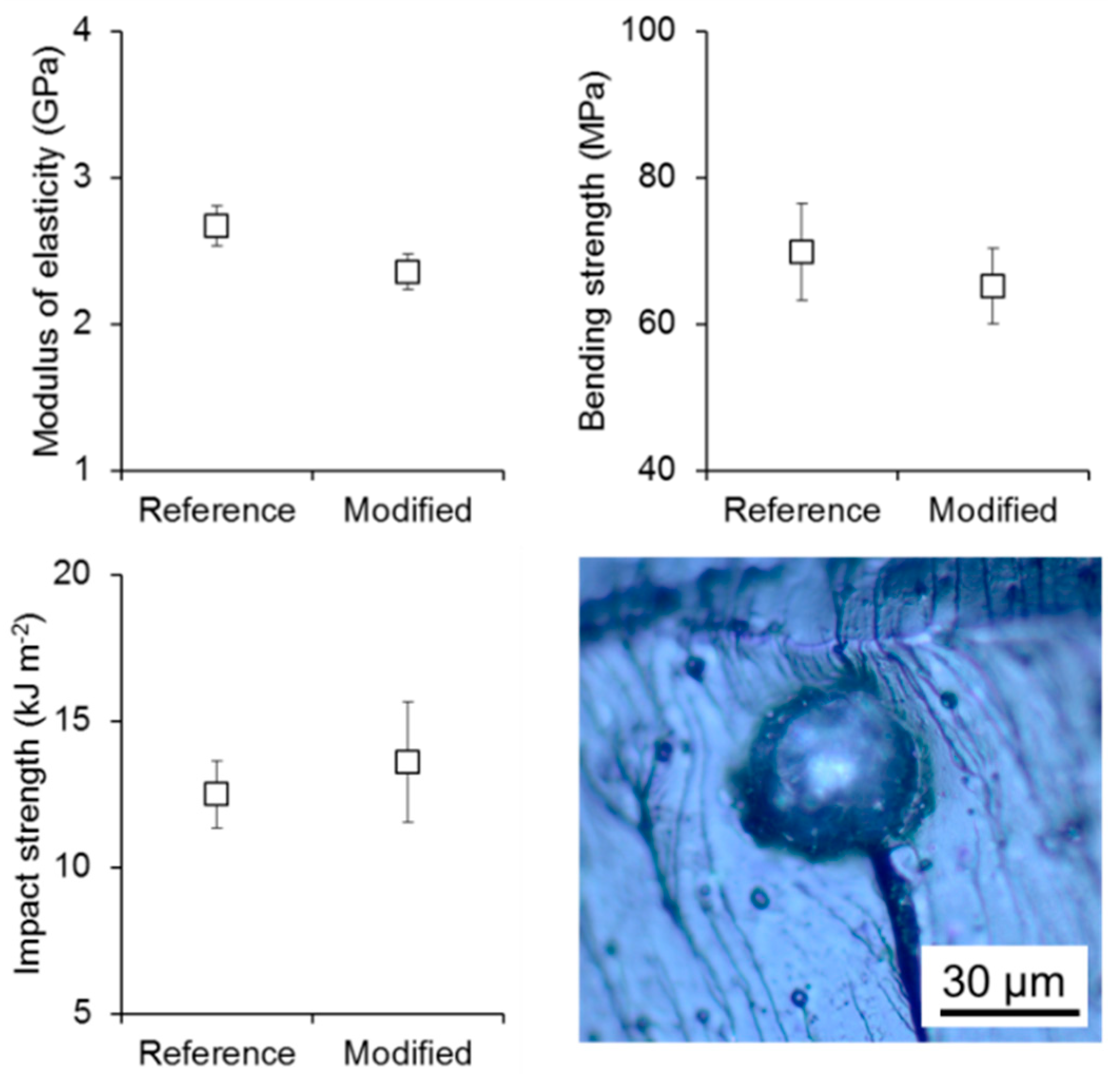
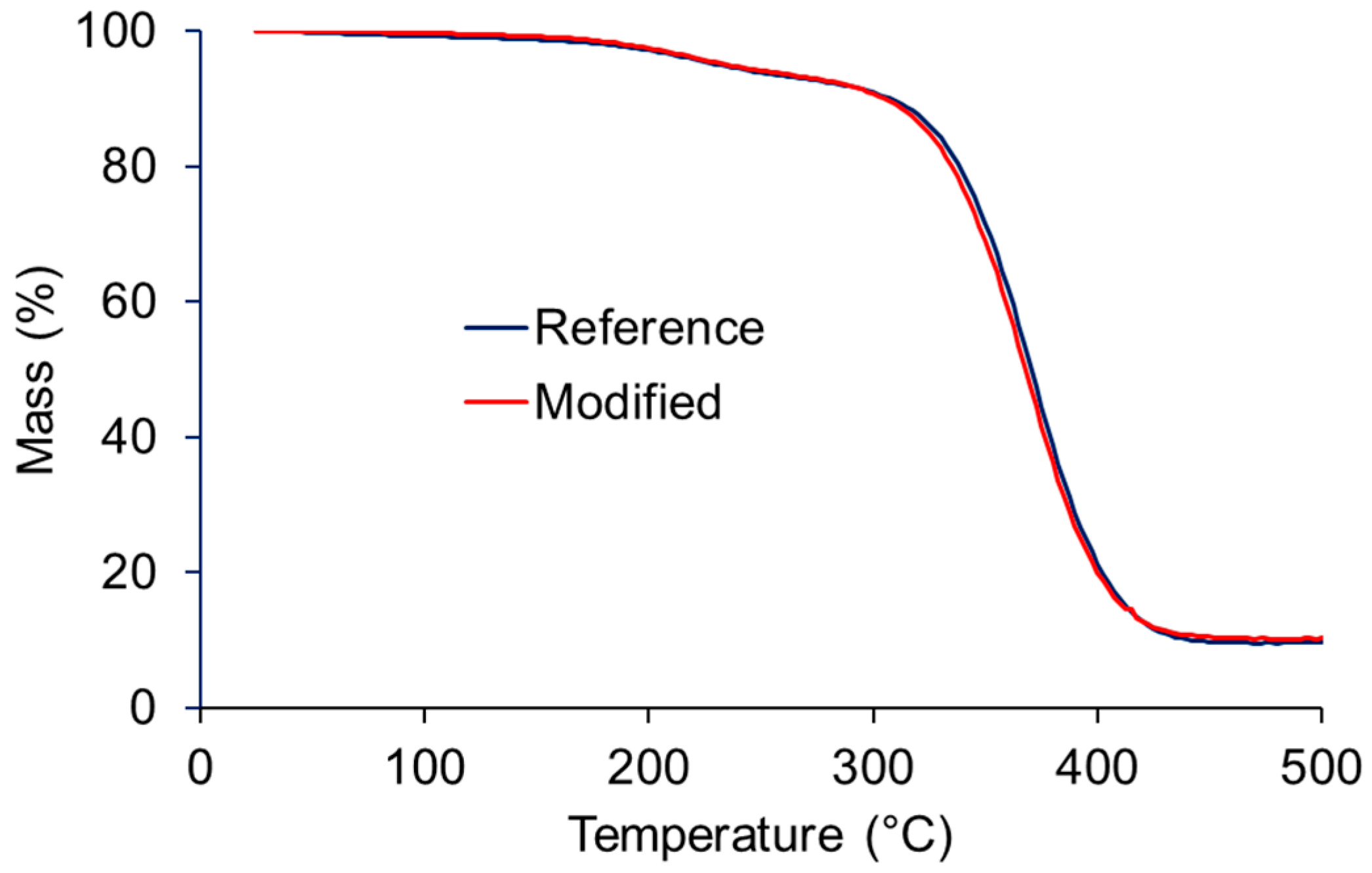
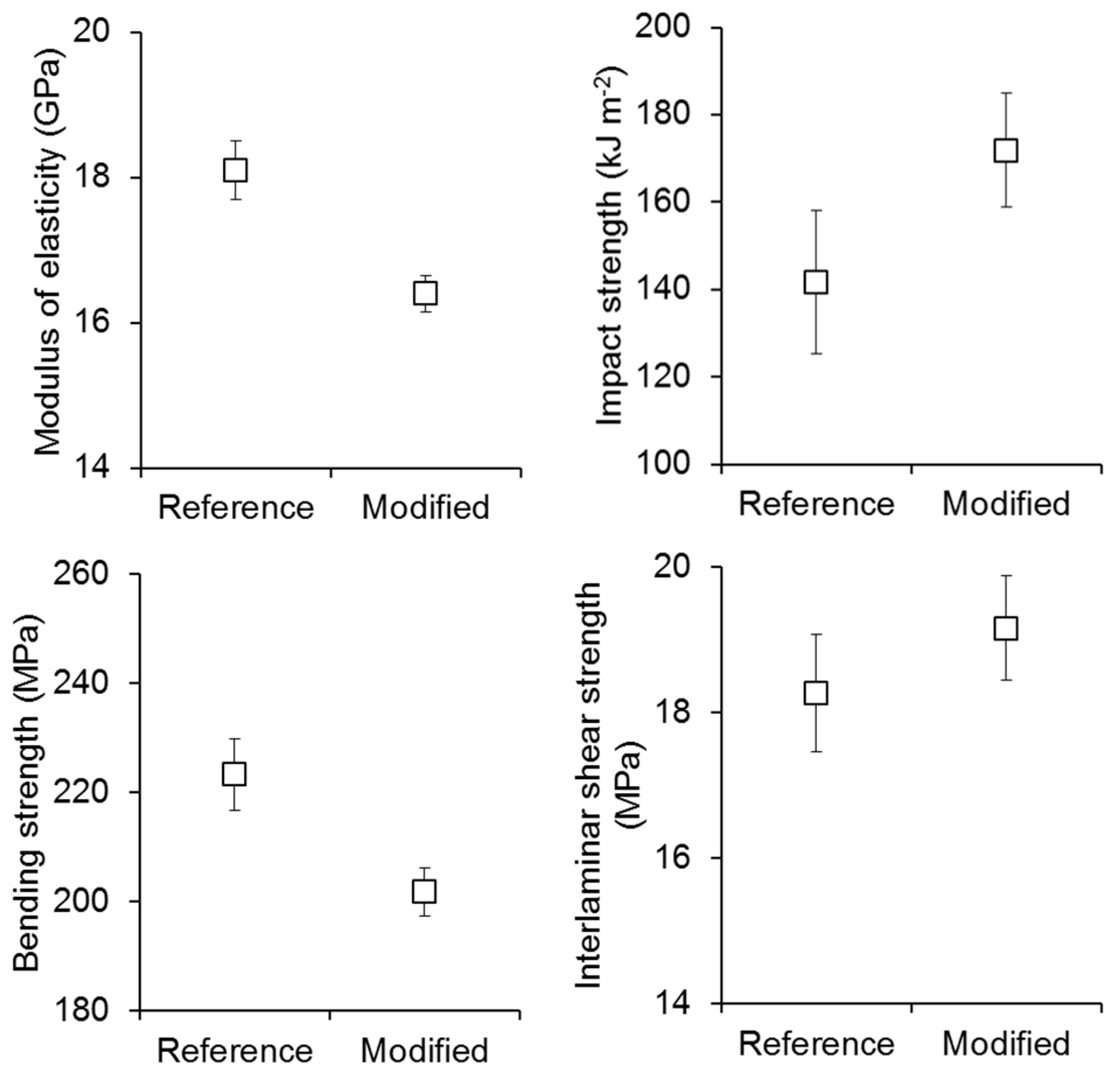
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, K.-Y.; Aitomaki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gardner, D.J.; Han, Y. Drying cellulose nanofibrils: In search of a suitable method. Cellulose 2012, 19, 91–102. [Google Scholar] [CrossRef]
- Henriksson, M.; Fogelstrom, L.; Berglund, L.A.; Johansson, M.; Hult, A. Novel nanocomposite concept based on cross-linking of hyperbranched polymers in reactive cellulose nanopaper templates. Compos. Sci. Technol. 2011, 71, 13–17. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl. Phys. A 2005, 80, 155–159. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of fiber content on the mechanical and thermal expansion properties of biocomposites based on microfibrillated cellulose. Cellulose 2008, 15, 555–559. [Google Scholar] [CrossRef]
- Sabo, R.C.; Elhajjar, R.F.; Clemons, C.M.; Pillai, K.M. Characterization and processing of nanocellulose thermoset composites. In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Pandey, J.K., Takagi, H., Nakagaito, A.N., Kim, H.-J., Eds.; Springer-Verlag: Berlin, Heidelberg, Germany, 2015; Volume C: Polymer Nanocomposites of Cellulose Nanoparticles, pp. 266–295. [Google Scholar]
- Salas, C.; Nypelo, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Ben Mabrouk, A.; Salon, M.C.B.; Magnin, A.; Belgacem, M.N.; Boufi, S. Cellulose-based nanocomposites prepared via mini-emulsion polymerization: Understanding the chemistry of the nanocellulose/matrix interface. Colloids Surf. A 2014, 448, 1–8. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Sain, S.; Bose, M.; Ray, D.; Mukhopadhyay, A.; Sengupta, S.; Kar, T.; Ennis, C.J.; Rahman, P.; Misra, M. A comparative study of polymethylmethacrylate/cellulose nanocomposites prepared by in situ polymerization and ex situ dispersion techniques. J. Reinf. Plastics Compos. 2013, 32, 147–159. [Google Scholar] [CrossRef]
- Banerjee, M.; Sain, S.; Mukhopadhyay, A.; Sengupta, S.; Kar, T.; Ray, D. Surface treatment of cellulose fibers with methylmethacrylate for enhanced properties of in situ polymerized PMMA/cellulose composites. J. Appl. Polym. Sci. 2014, 131, 9. [Google Scholar] [CrossRef]
- Blaker, J.J.; Lee, K.Y.; Li, X.X.; Menner, A.; Bismarck, A. Renewable nanocomposite polymer foams synthesized from pickering emulsion templates. Green Chem. 2009, 11, 1321–1326. [Google Scholar] [CrossRef]
- Nypelo, T.; Rodriguez-Abreu, C.; Kolen’ko, Y.V.; Rivas, J.; Rojas, O.J. Microbeads and hollow microcapsules obtained by self-assembly of pickering magneto-responsive cellulose nanocrystals. ACS Appl. Mater. Interfaces 2014, 6, 16851–16858. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.A.; Nypelo, T.; Rojas, O.J. Double emulsions for the compatibilization of hydrophilic nanocellulose with non-polar polymers and validation in the synthesis of composite fibers. Soft Matter 2016, 12, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.I.; Yano, H. Doubly curved nanofiber-reinforced optically transparent composites. Sci. Rep. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Herzele, S.; Veigel, S.; Liebner, F.; Zimmermann, T.; Gindl-Altmutter, W. Reinforcement of polycaprolactone with microfibrillated lignocellulose. Ind. Crops Prod. 2016. [Google Scholar] [CrossRef]
- Gindl-Altmutter, W.; Obersriebnig, M.; Veigel, S.; Liebner, F. Compatibility between cellulose and hydrophobic polymer provided by microfibrillated lignocellulose. ChemSusChem 2015, 8, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, X.; Zhang, L. Preparation and characterization of lignin-containing nanofibrillar cellulose. Proced. Environ. Sci. 2012, 16, 125–130. [Google Scholar] [CrossRef]
- Ballner, D.; Herzele, S.; Keckes, J.; Edler, M.; Griesser, T.; Saake, B.; Liebner, F.; Potthast, A.; Paulik, C.; Gindl-Altmutter, W. Lignocellulose nanofiber-reinforced polystyrene produced from composite microspheres obtained in suspension polymerization shows superior mechanical performance. ACS Appl. Mater. Interfaces 2016, 8, 13520–13525. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hsieh, Y.-L. Surface and structure characteristics, self-assembling, and solvent compatibility of holocellulose nanofibrils. ACS Appl. Mater. Interfaces 2015, 7, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindstrom, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Sheltami, R.M.; Ahmad, I.; Abdullah, I.; Dufresne, A. Cellulose nanocrystal: A promising toughening agent for unsaturated polyester nanocomposite. Polymer 2015, 56, 346–357. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Wang, Y.; Liu, L.; Huang, Y. Improvements in interfacial and heat-resistant properties of carbon fiber/methylphenylsilicone resins composites by incorporating silica-coated multi-walled carbon nanotubes. J. Adhes. Sci. Technol. 2016, 30, 117–130. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Liu, L.; Wang, Y.; Xie, F.; Zhong, Z.; Zhao, M.; Jiang, B.; Huang, Y. Interface enhancement of carbon fiber reinforced methylphenylsilicone resin composites modified with silanized carbon nanotubes. Mater. Des. 2016, 89, 1343–1349. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Wang, Y.; Liu, L.; Huang, Y. Interfacial properties and thermo-oxidative stability of carbon fiber reinforced methylphenylsilicone resin composites modified with polyhedral oligomeric silsesquioxanes in the interphase. RSC Adv. 2016, 6, 5032–5039. [Google Scholar] [CrossRef]
- Ulus, H.; Ustun, T.; Sahin, O.S.; Karabulut, S.E.; Eskizeybek, V.; Avci, A. Low-velocity impact behavior of carbon fiber/epoxy multiscale hybrid nanocomposites reinforced with multiwalled carbon nanotubes and boron nitride nanoplates. J. Compos. Mater. 2016, 50, 761–770. [Google Scholar] [CrossRef]
- Arnold, M.; Henne, M.; Bender, K.; Drechsler, K. Polyamide 12 modified with nanoparticles: Effect on impact behaviour and on the electrical conductivity of carbon fibre-reinforced epoxy composites. J. Compos. Mater. 2016, 50, 159–171. [Google Scholar] [CrossRef]
| Type of Material | Glucose | Xylan | Lignin | Crystallinity |
|---|---|---|---|---|
| MFC | 99.9 | 0.0 | 0.1 | 71 |
| MFLC | 62.0 | 14.1 | 14.0 | 70 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Herzele, S.; Mahendran, A.R.; Edler, M.; Griesser, T.; Saake, B.; Li, J.; Gindl-Altmutter, W. Microfibrillated Lignocellulose Enables the Suspension-Polymerisation of Unsaturated Polyester Resin for Novel Composite Applications. Polymers 2016, 8, 255. https://doi.org/10.3390/polym8070255
Yan Y, Herzele S, Mahendran AR, Edler M, Griesser T, Saake B, Li J, Gindl-Altmutter W. Microfibrillated Lignocellulose Enables the Suspension-Polymerisation of Unsaturated Polyester Resin for Novel Composite Applications. Polymers. 2016; 8(7):255. https://doi.org/10.3390/polym8070255
Chicago/Turabian StyleYan, Yutao, Sabine Herzele, Arunjunai Raj Mahendran, Matthias Edler, Thomas Griesser, Bodo Saake, Jianzhang Li, and Wolfgang Gindl-Altmutter. 2016. "Microfibrillated Lignocellulose Enables the Suspension-Polymerisation of Unsaturated Polyester Resin for Novel Composite Applications" Polymers 8, no. 7: 255. https://doi.org/10.3390/polym8070255
APA StyleYan, Y., Herzele, S., Mahendran, A. R., Edler, M., Griesser, T., Saake, B., Li, J., & Gindl-Altmutter, W. (2016). Microfibrillated Lignocellulose Enables the Suspension-Polymerisation of Unsaturated Polyester Resin for Novel Composite Applications. Polymers, 8(7), 255. https://doi.org/10.3390/polym8070255