Phase Separation and Elastic Properties of Poly(Trimethylene Terephthalate)-block-poly(Ethylene Oxide) Copolymers
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of PTT-Block-PEOT Copolymers
2.2. Sample Preparation
2.3. Characterization Methods
3. Results and Discussion
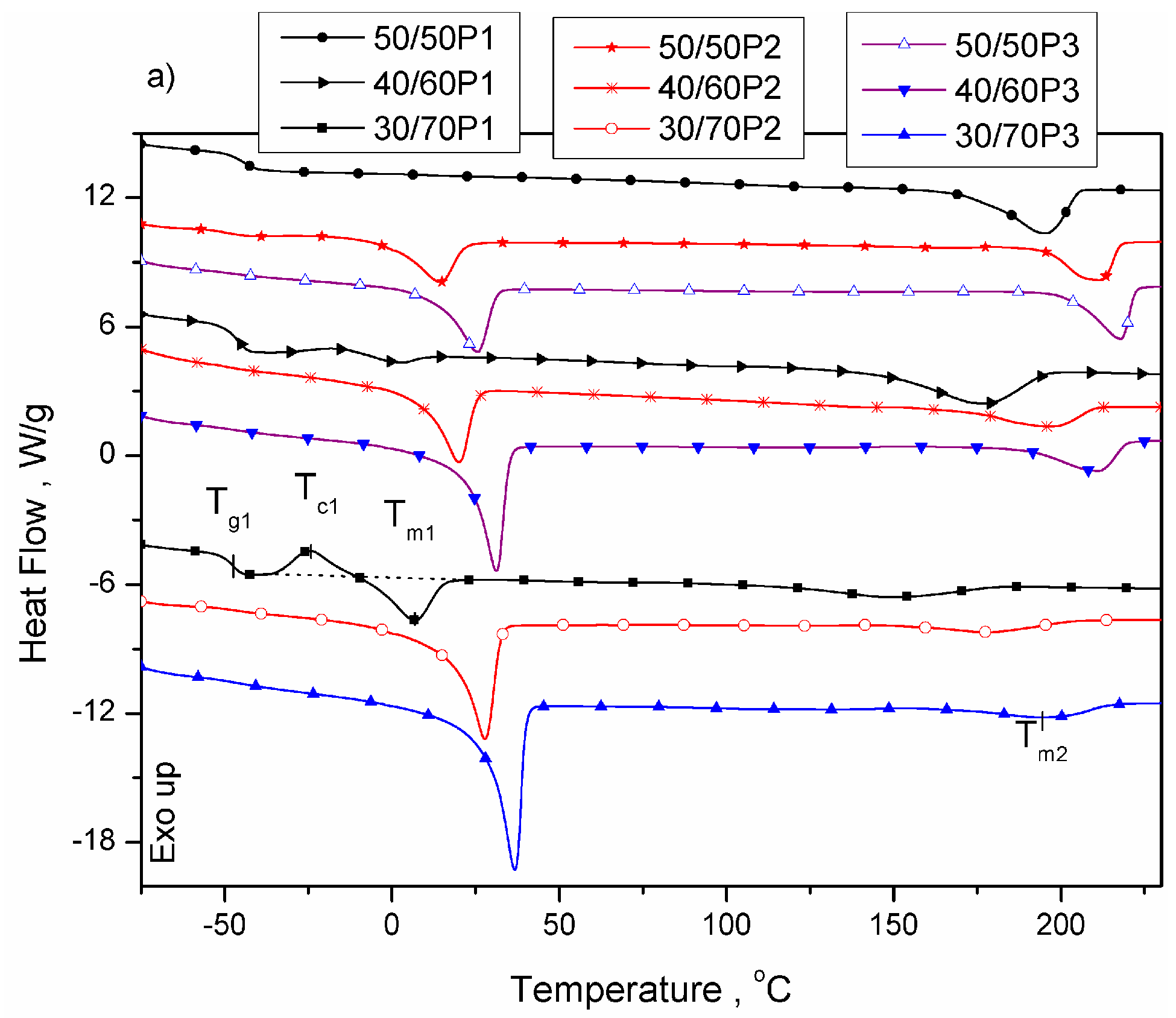
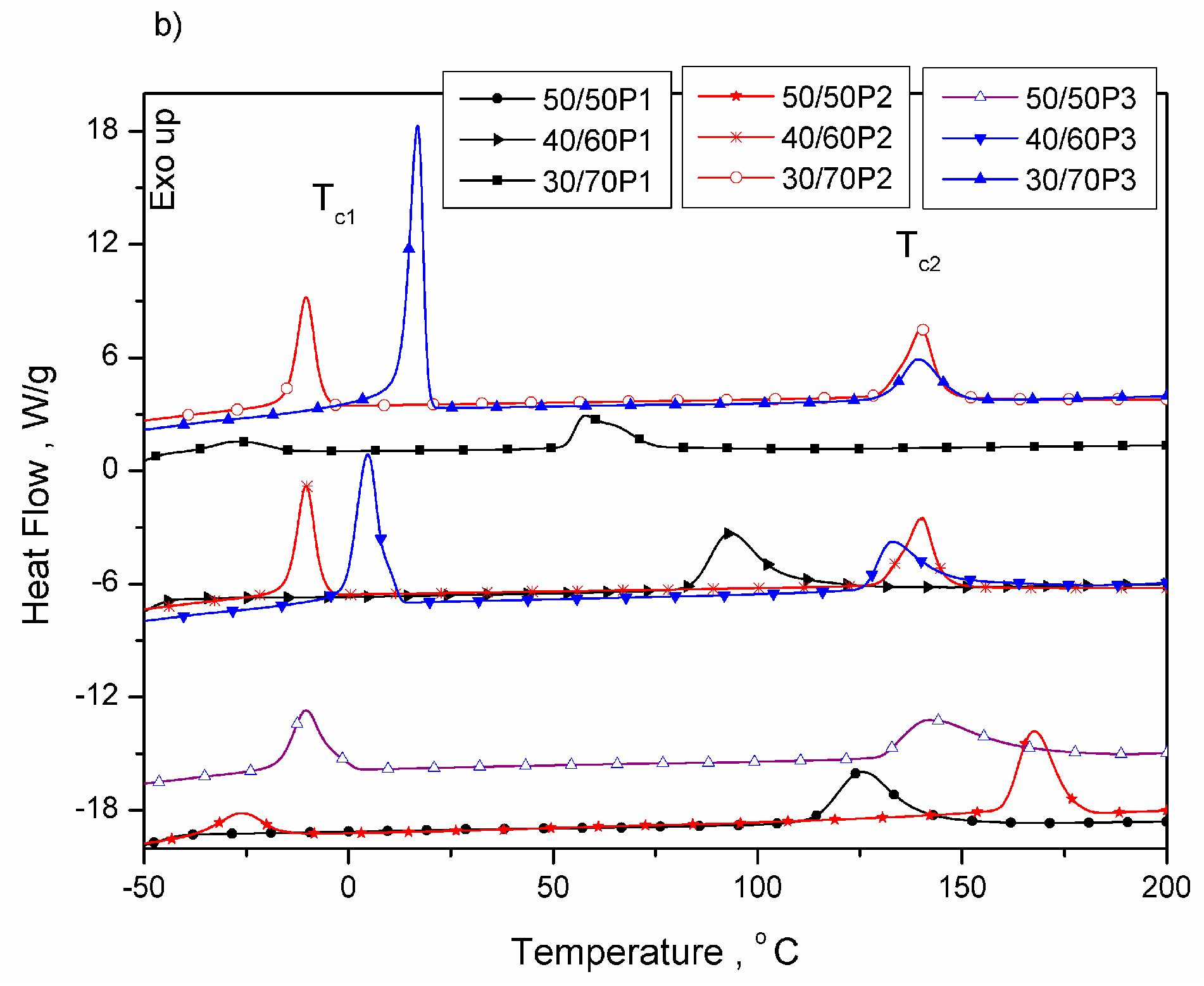
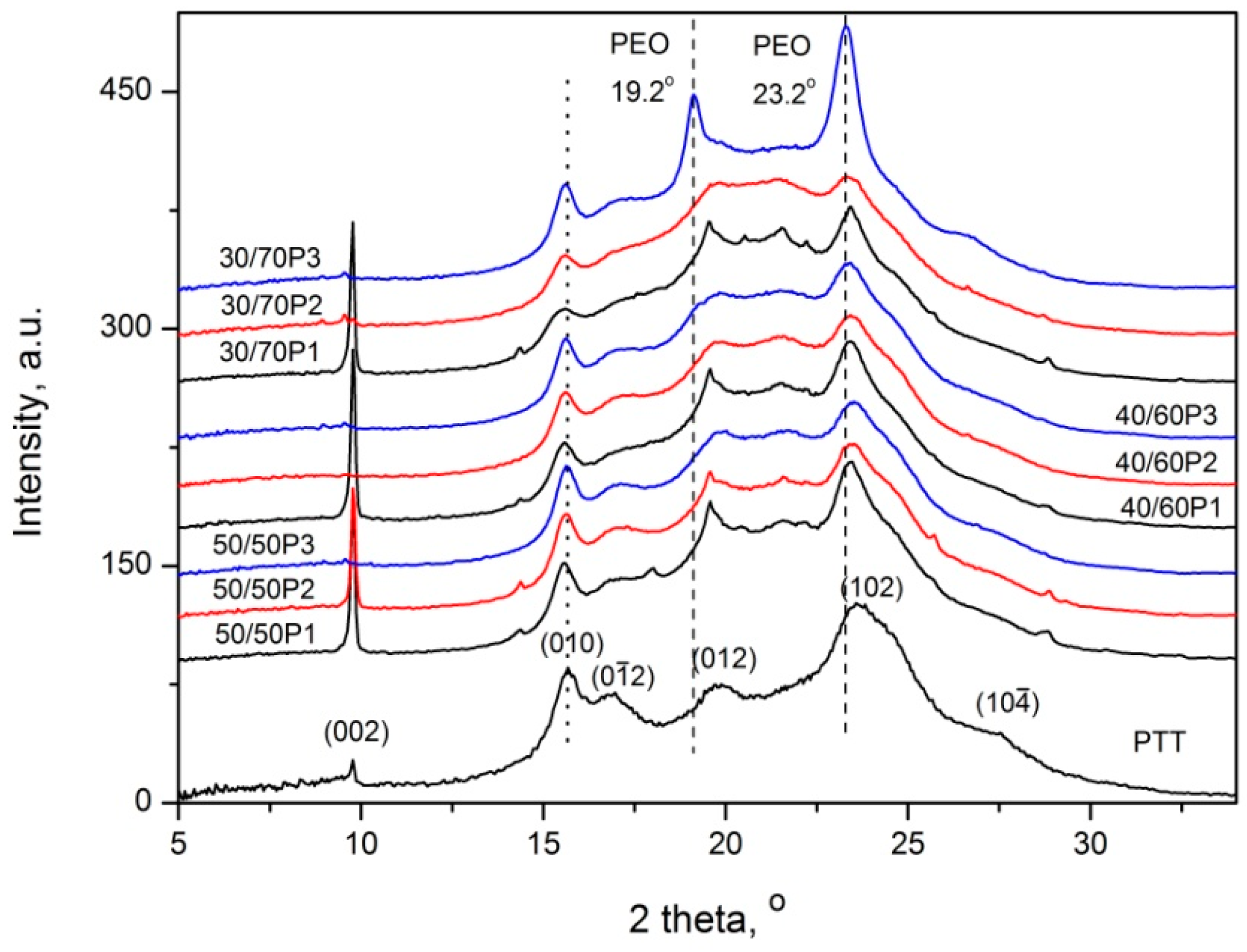
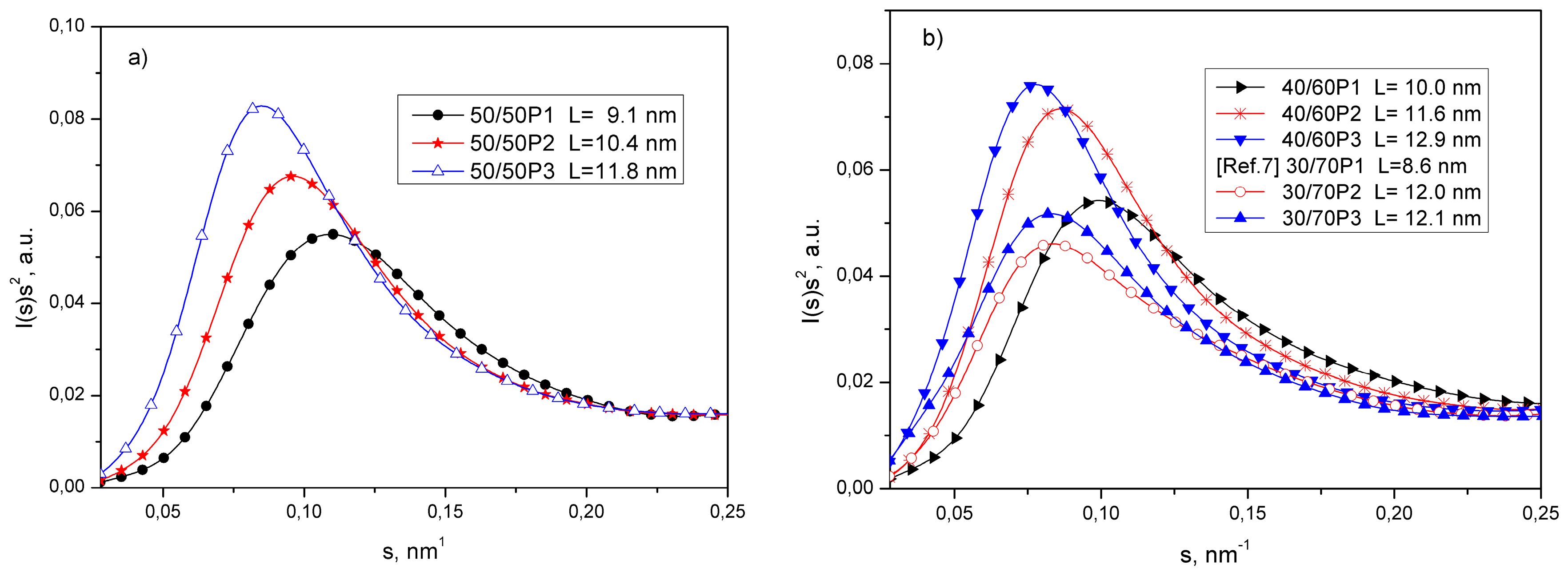
3.1. Phase Separation and Structure of PTT-b-PEOT Copolymers
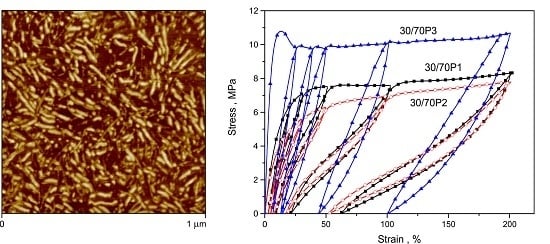
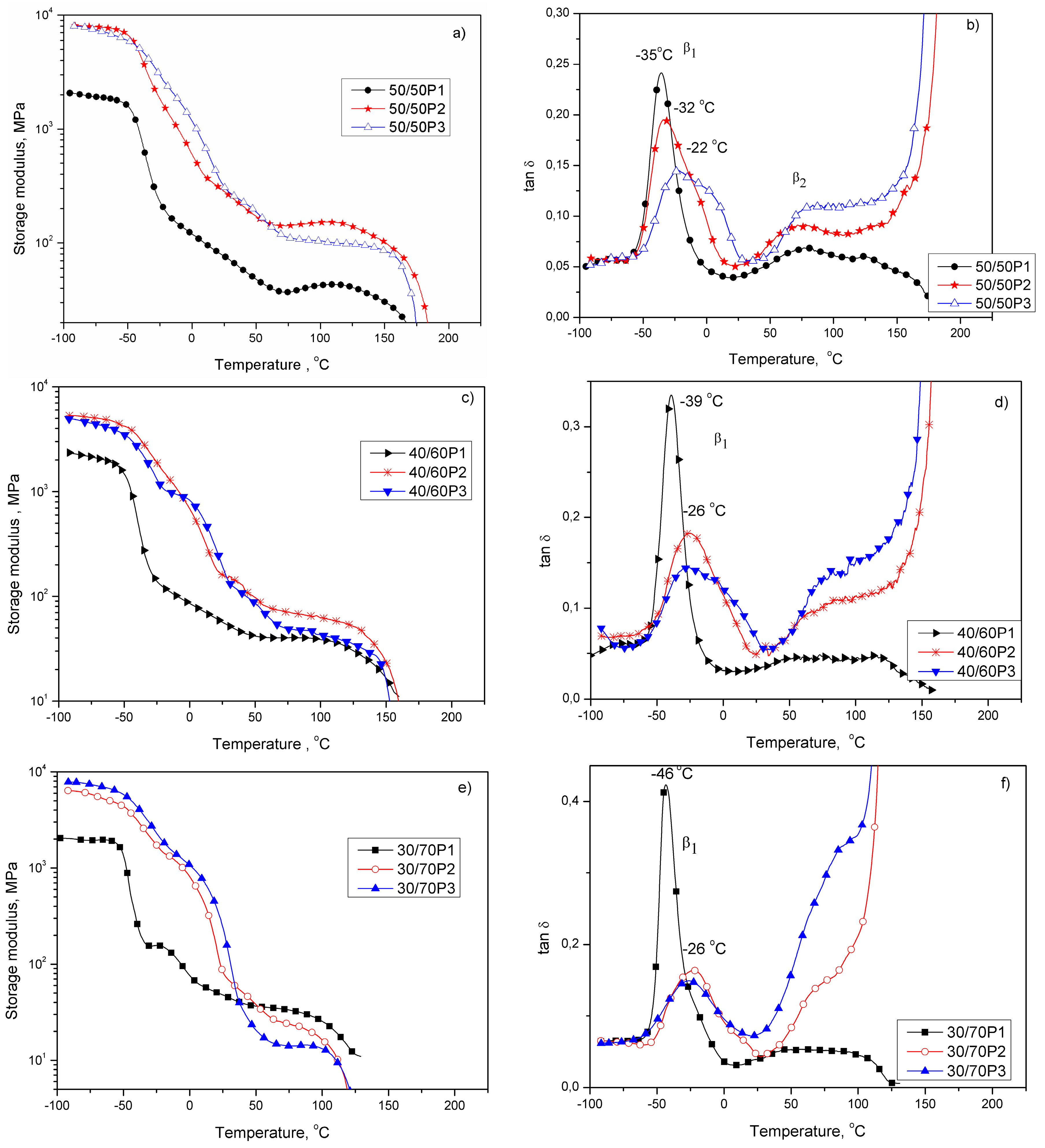
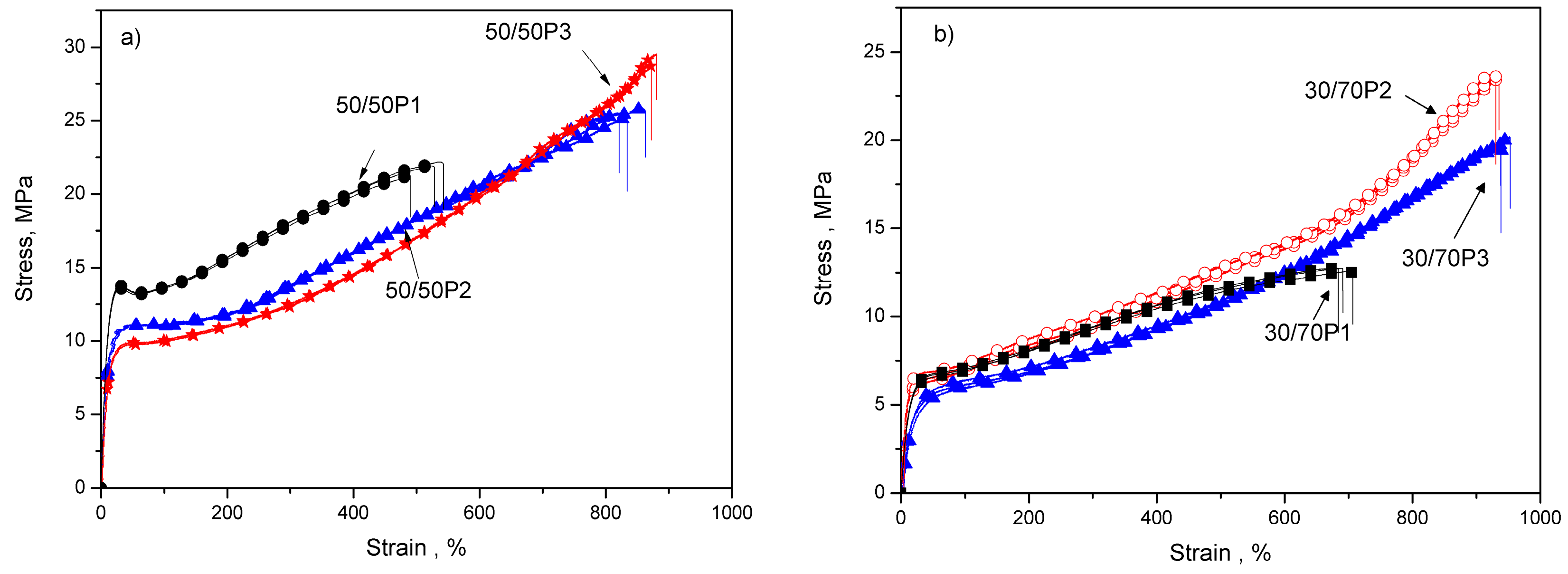
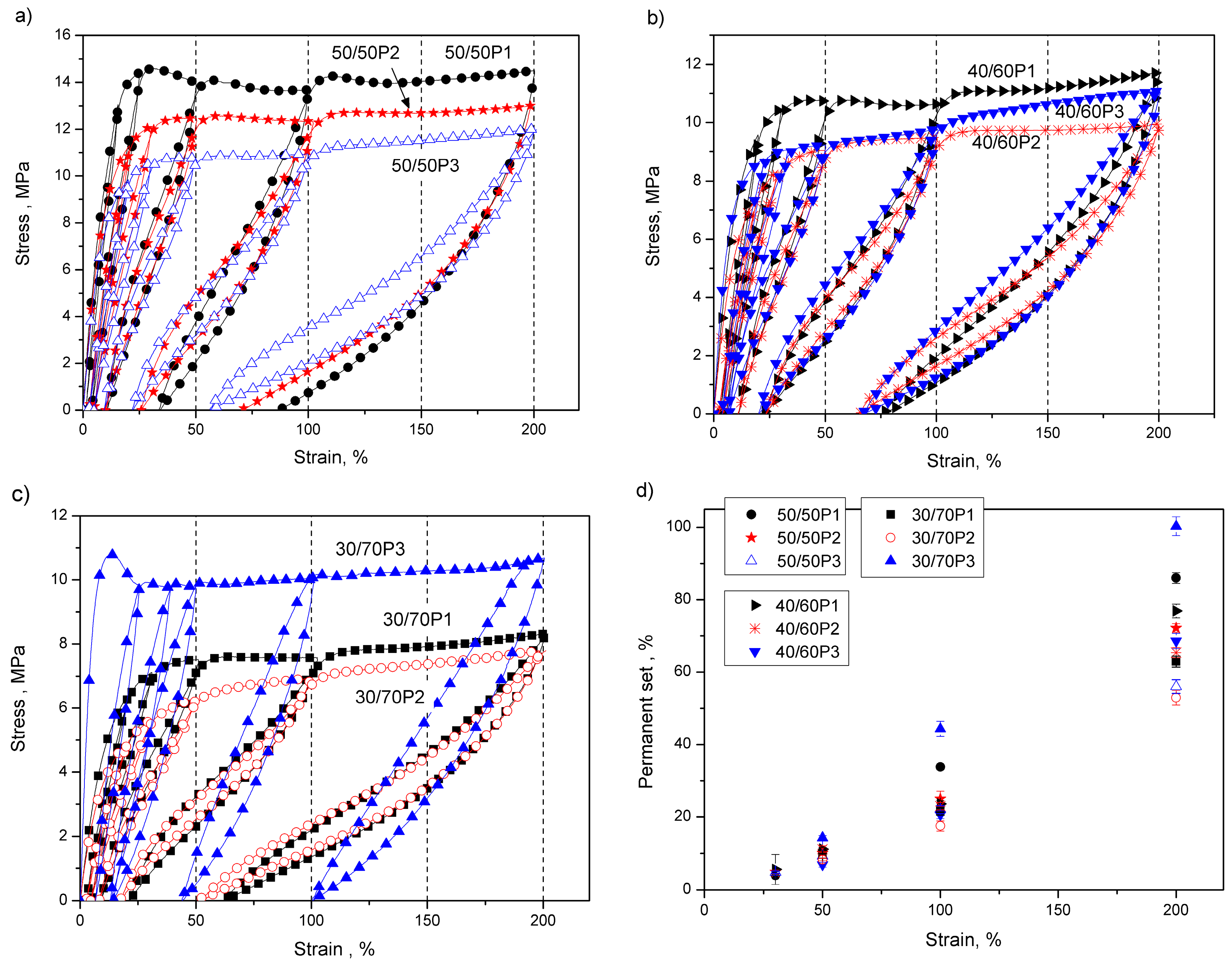
3.2. Elastic Properties of PTT-b-PEOT Copolymers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roslaniec, Z.; Pietkiewicz, D. Synthesis and characteristics of polyester-based thermoplastic elastomers: Chemical aspects. In Handbook of Thermoplastic Polyesters; Fakirov, S., Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2012; Volume 1, pp. 581–622. [Google Scholar]
- Liu, H.; Xu, Y.; Zheng, Z.; Liu, D. 1,3-Propanediol and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Avérous, L. Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 225–233. [Google Scholar]
- Pilla, S. Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 422–424. [Google Scholar]
- Niaounakis, M. Biopolymers: Applications and Trends; Elsevier Science Publishing Company Incorporated: Oxford, UK, 2015; pp. 28–30. [Google Scholar]
- Szymczyk, A.; Nastalczyk, J.; Sablong, R.J.; Roslaniec, Z. The influence of soft segment length on structure and properties of poly(trimethylene terephthalate)-block-poly(tetramethylene oxide) segmented random copolymers. Polym. Adv. Technol. 2011, 22, 72–83. [Google Scholar] [CrossRef]
- Szymczyk, A. Structure and properties of new polyester elastomers composed of poly(trimethylene terephthalate) and poly(ethylene oxide). Eur. Polym. J. 2009, 45, 2653–2664. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Xu, Y.; Wang, P.; Gao, L.; Zhang, W.; Ji, J. Synthesis and characterization of bio-based poly(butylene furandicarboxylate)-b-poly(tetramethylene glycol) copolymers. Polym. Degrad. Stab. 2014, 109, 21–26. [Google Scholar] [CrossRef]
- Szymczyk, A.; Pawelec, I.; Paszkiewicz, S. Novel multiblock copolymers based on biopolyester from reneweable resoures. In Conference Abstract Book of BIT’s, Proceedings of the 2nd Annual World Congress of Smart Materials Theme: Develop New Path of Smartness, Singapore, 4–6 March 2016; p. 568.
- Cohn, D.; Hotovely-Salomon, A. Biodegradable multiblock PEO/PLA thermoplastic elastomers: Molecular design and properties. Polymers 2005, 46, 2068–2075. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Wang, H.; Jin, W.; Li, J. Synthesis of multiblock thermoplastic elastomers based on biodegradable poly(lactic acid) and polycaprolactone. Mater. Sci. Eng. C 2009, 29, 889–893. [Google Scholar] [CrossRef]
- Fabbri, M.; Gigli, M.; Gamberini, R.; Lotti, N.; Gazzano, M.; Rimini, B.; Munari, A. Hydrolysable PBS-based poly(ester urethane)s thermoplastic elastomers. Polym. Degrad. Stab. 2014, 108, 223–231. [Google Scholar] [CrossRef]
- Li, S.-L.; Wu, F.; Wang, Y.-Z.; Zeng, J.-B. Biobased thermoplastic poly(ester urethane) elastomers consisting of poly(butylenes succinate) and poly(propylene succinate). Ind. Eng. Chem. Res. 2015, 54, 6258–6268. [Google Scholar] [CrossRef]
- Webb, A.R.; Yang, J.; Ameer, G.A. Biodegradable polyester elastomers in tissue engineering. Exp. Opin. Biol. Ther. 2004, 4, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J. Biodegradable Elastomers for tissue engineering and cell-biomaterial interactions. Macromol. Biosci. 2011, 11, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Plamper, F.A.; Reinicke, S.; Elomaa, M.; Schmalz, H.; Tenhu, H. Peral necklace architecture: New threaded star-shaped copolymers. Macromolecules 2010, 43, 2190–2203. [Google Scholar] [CrossRef]
- Cohn, D.; Younes, H. PEO/PLA biodegradable block copolymers. J. Biomed. Mater. Res. 1988, 22, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.; Cohn, D. Morphological study of biodegradable PEO/ PLA block copolymers. J. Biomed. Mater. Res. 1987, 21, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.; Younes, H. Compositional and structural analysis of PELA block copolymers degrading under in vitro conditions. Biomaterials 1989, 10, 466–474. [Google Scholar] [CrossRef]
- Cohn, D.; Stern, T.; Gonzalez, M.F.; Epstein, J. Biodegradable poly(ethylene oxide)/poly(ε-caprolactone) multiblock copolymers. J. Biomed. Mater. Res. 2002, 59, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.; Cohn, D.; Hotovely, A.; Pines, E.; Diamond, M.P.; diZerega, G. Evaluation of polyethylene glycol/polylactic acid films in the prevention of adhesions in the rabbit adhesion formation and reformation sidewall models. Fertil. Ster. 1998, 69, 403–408. [Google Scholar] [CrossRef]
- Okuyama, N.; Rodgers, K.; Wang, C.Y.; Girgis, W.; Oz, M.; St. Amand, K.; Pines, E.; de Cherney, A.; Rose, E.; Cohn, D.; et al. Prevention of retrosternal adhesion formation in a rabbit model using bioresorbable films of polyethylene glycol and polylactic acid. J. Surg. Res. 1998, 78, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.J.; Mulder, M.H.V.; Wessling, M. Gas-permeation properties of poly(ethylene oxide) poly(butylene terephthalate) block copolymers. Macromolecules 2004, 37, 4590–4597. [Google Scholar] [CrossRef]
- Yoshino, M.; Ito, K.; Kita, H.; Okamoto, K.-I. Effects of hard-segment polymers on CO2/N2 gas-separation properties of poly(ethylene oxide)-segmented copolymers. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1707–1715. [Google Scholar] [CrossRef]
- Beckman, E.J.; Sarbu, T.; Styranec, T.J. Carbon Dioxide-Philic Compounds and Methods of Synthesis Thereof. US Patent 6 686 438 B1, 29 March 2004. [Google Scholar]
- Lin, H.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57–74. [Google Scholar] [CrossRef]
- Yave, W. Membranes from block copolymers. In Advanced Materials for Membrane Preparation; Buonomenna, M.G., Ed.; Bentham eBooks: Beijing, China, 2012; pp. 148–162. [Google Scholar]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. Tailor-made polymeric membranes based on segmented block copolymers for CO2 separation. Adv. Funct. Matter. 2008, 18, 2815–2823. [Google Scholar] [CrossRef]
- Metz, S.J.; van de Ven, W.J.C.; Mulder, M.H.V.; Wessling, M. Mixed gas water vapor/N2 transport in poly(ethylene oxide) poly(butylene terephthalate) block copolymers. J. Membr. Sci. 2005, 26, 51–61. [Google Scholar] [CrossRef]
- Yave, W.; Szymczyk, A.; Yave, N.; Roslaniec, Z. Design, synthesis, characterization and optimization of PTT-b-PEO copolymers: A new membrane material for CO2 separation. J. Membr. Sci. 2010, 362, 407–416. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Špitalski, Z.; Mosnáček, J.; Kwiatkowski, K.; Rosłaniec, Z. Structure and properties of nanocomposites based on PTT-block-PTMO copolymer and graphene oxide prepared by in situ polymerization. Eur. Polym. J. 2014, 50, 69–77. [Google Scholar] [CrossRef]
- Yao, C.; Yang, G. Crystallization-induced aging in poly(trimethylene terephthalate)/poly(ethylene oxide terephthalate) segmented block copolymers. J. Appl. Polym. Sci. Part B Polym. Phys. 2010, 48, 411–416. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Y.; Yao, C.; Yang, G. Crystallization behaviour and morphology of double crystalline poly(trimethylene terephthalate)/poly(ethylene oxide terephthalate) copolymers. Polym. Int. 2013, 62, 219–227. [Google Scholar] [CrossRef]
- Yao, Y.; Cui, L.; Liu, H.; Huang, Z.; Chen, W. Non-isothermal crystallization behaviors of poly(trimethylene terephthalate isophthalate-co-polyethylene glycol) with low melting point. Adv. Mater. Res. 2011, 332–224, 275–280. [Google Scholar] [CrossRef]
- Szymczyk, A.; Senderek, E.; Nastalczyk, J.; Roslaniec, Z. New multiblock poly(ether–ester)s based on poly(trimetylene terephthalate) as rigid segment. Eur. Polym. J. 2008, 44, 436–443. [Google Scholar] [CrossRef]
- Maclaine, J.Q.G.; Booth, C. Effect of molecular weight on spherulite growth rates of high molecular weight poly(ethylene oxide) fractions. Polymers 1975, 16, 191–195. [Google Scholar] [CrossRef]
- Chen, H.L.; Li, L.J.; Ou-Yang, W.C.; Hwang, J.C.; Wong, W.Y. Spherulitic crystallization behavior of poly (ε-caprolactone) with a wide range of molecular weight. Macromolecules 1997, 30, 1718–1722. [Google Scholar] [CrossRef]
- Chuah, H.H. Synthesis, properties and applications of poly(trimethylene terephthalate). In Modern Polyester: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; John Wiley & Sons: Chichester, UK, 2004; pp. 361–397. [Google Scholar]
- Wang, M.; Zhang, L.; Ma, D. Degree of microphase separation in segmented copolymers based on poly(ethylene oxide) and poly(ethylene terephthalate). Eur. Polym. J. 1999, 35, 1335–1343. [Google Scholar] [CrossRef]
- Liu, C.; Lv, K.; Huang, B.; Hou, C.; Wang, G. Synthesis and characterization of graft copolymers poly(ethylene oxide)-g-[poly(ethylene oxide)-b-poly(ε-caprolactone)] with double crystallizable side chains. RCS Adv. 2013, 3, 17945–17953. [Google Scholar] [CrossRef]
- Balta, F.J.; Calleja, Z. Roslaniec Block Copolymers; Marcel Dekker Inc.: New York, NY, USA, 2000. [Google Scholar]
- Gabrielse, W.; Soliman, M.; Dijkstra, K. Microstructure and phase behavior of block copoly(ether ester) Thermoplastic Elastomers. Macromolecules 2001, 34, 1685–1693. [Google Scholar] [CrossRef]
- Phillips, R.A.; McKenna, J.M.; Cooper, S.L. Glass transition and melting behavior of poly(ether-ester) multiblock copolymers with poly(tetramethylene isophthalate) hard segments. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 791–802. [Google Scholar] [CrossRef]

| Sample | x | wPEOT | Mn × 104 by SEC | Mw/Mn | |
|---|---|---|---|---|---|
| mol | wt % | g/mol | g/mol | ||
| 50/50P1 | 5.48 | 50 | 1130 | 7.38 | 2.15 |
| 40/60P1 | 3.65 | 60 | 1130 | 7.79 | 2.23 |
| 30/70P1 | 2.35 | 70 | 1130 | 8.88 | 2.19 |
| 50/50P2 | 10.34 | 50 | 2130 | 7.69 | 1.69 |
| 40/60P2 | 6.88 | 60 | 2130 | 8.47 | 2.05 |
| 30/70P2 | 4.43 | 70 | 2130 | 9.64 | 1.90 |
| 50/50P3 | 15.20 | 50 | 3130 | 8.41 | 1.68 |
| 40/60P3 | 10.12 | 60 | 3130 | 10.05 | 1.63 |
| 30/70P3 | 6.50 | 70 | 3130 | 11.05 | 1.66 |
| Sample | PEOT Segment | PTT Segment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg1 | Tm1 | ∆Hm1 | Tc1 | ∆TPEO | Tm2 | ∆Hm2 | Tc2 | ∆Hc2 | ∆TPTT | |||
| °C | °C | J/g | °C | °C | % | °C | J/g | °C | J/g | °C | % | |
| 50/50P1 | −45 | - | - | - | - | - | 195 | 27.4 | 125 | 27.5 | 70 | 18.8 |
| 40/60P1 | −47 | - | - | - | - | - | 177 | 20.6 | 93 | 20.6 | 84 | 14.1 |
| 30/70P1 | −48 | 7 | 6.3 | 7 | 0 | 3.2 | 149 | 13.5 | 58 | 13.7 | 91 | 9.2 |
| 50/50P2 | −49 | 15 | 22.9 | -27 | 42 | 11.6 | 212 | 34.0 | 167 | 34.4 | 45 | 23.3 |
| 40/60P2 | −48 | 20 | 30.1 | -10 | 30 | 15.3 | 196 | 21.5 | 140 | 23.1 | 56 | 14.7 |
| 30/70P2 | −49 | 28 | 50.9 | 4 | 24 | 25.8 | 178 | 14.6 | 126 | 14.5 | 52 | 10.0 |
| 50/50P3 | −48 | 26 | 32.5 | 17 | 9 | 16.5 | 218 | 33.8 | 143 | 33.6 | 75 | 23.1 |
| 40/60P3 | −48 | 30 | 45.3 | 4 | 26 | 23.0 | 211 | 22.8 | 133 | 22.6 | 78 | 15.6 |
| 30/70P3 | −48 | 37 | 68.1 | -10 | 47 | 34.6 | 195 | 19.5 | 139 | 19.9 | 56 | 13.6 |
| Sample | E | σb | εb |
|---|---|---|---|
| MPa | MPa | % | |
| 50/50P1 | 134.2 ± 2.3 | 21.70 ± 0.54 | 533 ± 14 |
| 40/60P1 | 87.1 ± 0.9 | 17. 56 ± 0.81 | 582 ± 16 |
| 30/70P1 | 42.5 ± 1.1 | 12.78 ± 1.07 | 685 ± 24 |
| 50/50P2 | 99.2 ± 0.9 | 25.43 ± 0.21 | 841 ± 22 |
| 40/60P2 | 77.4 ± 2.2 | 20.65 ± 0.91 | 940 ± 16 |
| 30/70P2 | 44.5 ± 0.4 | 19.33 ± 0.47 | 948 ± 36 |
| 50/50P3 | 86.5 ± 0.8 | 29.11 ± 1.17 | 877 ± 5 |
| 40/60P3 | 128.3 ± 2.1 | 26.41 ± 0.41 | 938 ± 25 |
| 30/70P3 | 164.2 ± 2.1 | 23.52 ± 0.32 | 921 ± 11 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piesowicz, E.; Paszkiewicz, S.; Szymczyk, A. Phase Separation and Elastic Properties of Poly(Trimethylene Terephthalate)-block-poly(Ethylene Oxide) Copolymers. Polymers 2016, 8, 237. https://doi.org/10.3390/polym8070237
Piesowicz E, Paszkiewicz S, Szymczyk A. Phase Separation and Elastic Properties of Poly(Trimethylene Terephthalate)-block-poly(Ethylene Oxide) Copolymers. Polymers. 2016; 8(7):237. https://doi.org/10.3390/polym8070237
Chicago/Turabian StylePiesowicz, Elżbieta, Sandra Paszkiewicz, and Anna Szymczyk. 2016. "Phase Separation and Elastic Properties of Poly(Trimethylene Terephthalate)-block-poly(Ethylene Oxide) Copolymers" Polymers 8, no. 7: 237. https://doi.org/10.3390/polym8070237
APA StylePiesowicz, E., Paszkiewicz, S., & Szymczyk, A. (2016). Phase Separation and Elastic Properties of Poly(Trimethylene Terephthalate)-block-poly(Ethylene Oxide) Copolymers. Polymers, 8(7), 237. https://doi.org/10.3390/polym8070237