Quick Preparation of Moisture-Saturated Carbon Fiber-Reinforced Plastics and Their Accelerated Ageing Tests Using Heat and Moisture
Abstract
:1. Introduction
2. Materials and Methods
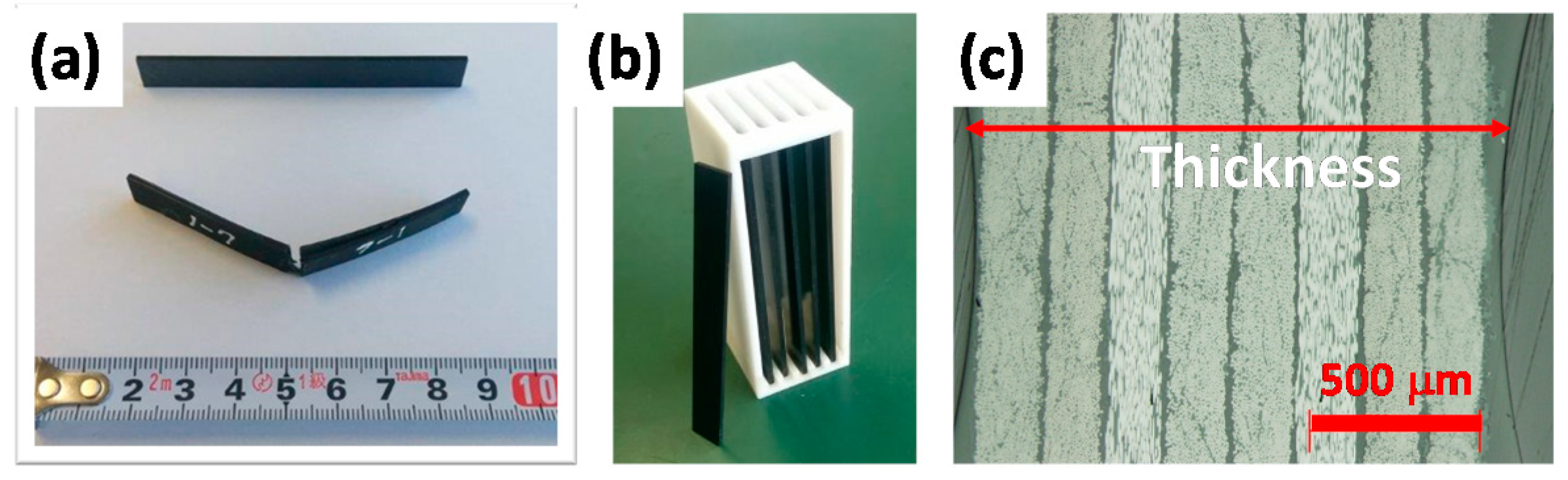
2.1. CFRP Sample
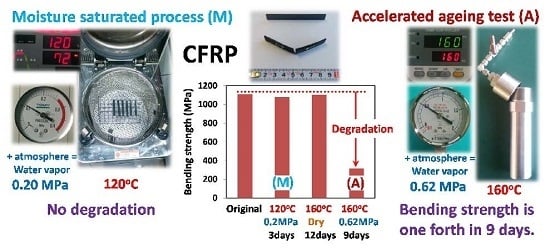
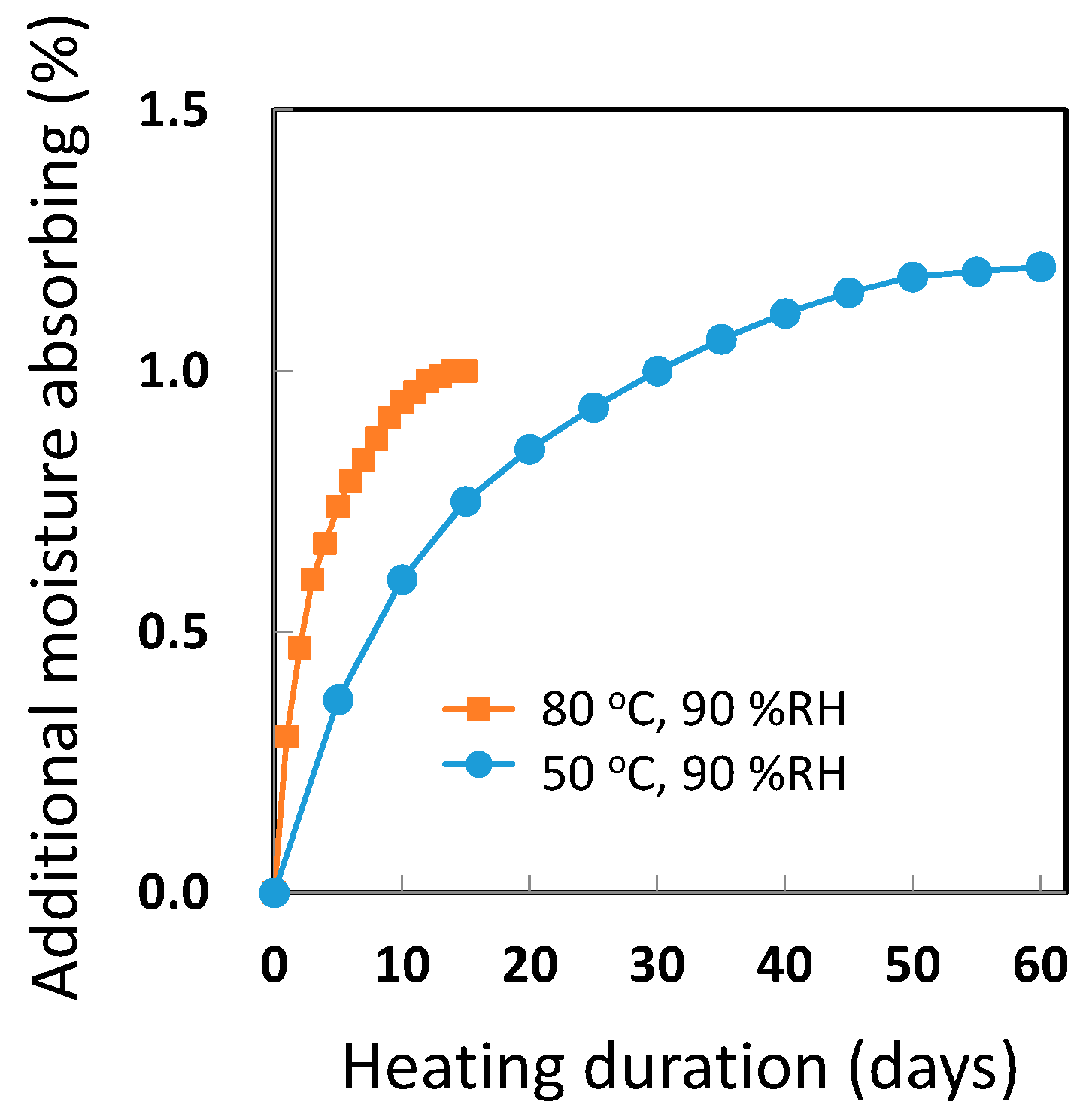
2.2. Quick Preparation of Moisture-Saturated CFRP
2.3. Stability Test
2.4. Accelerated Ageing Test
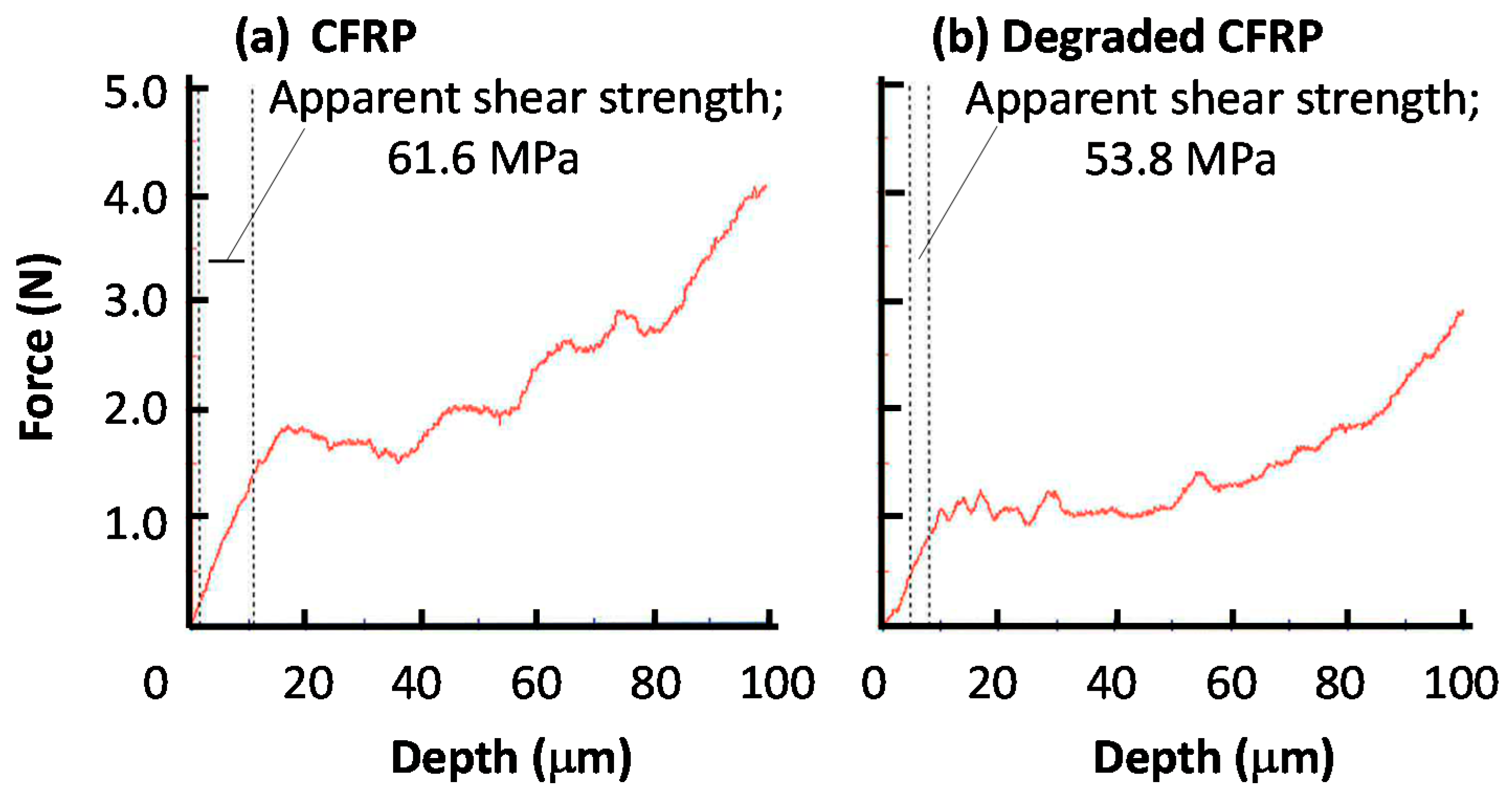
2.5. Mechanical Test
2.6. Dynamic Mechanical Analysis
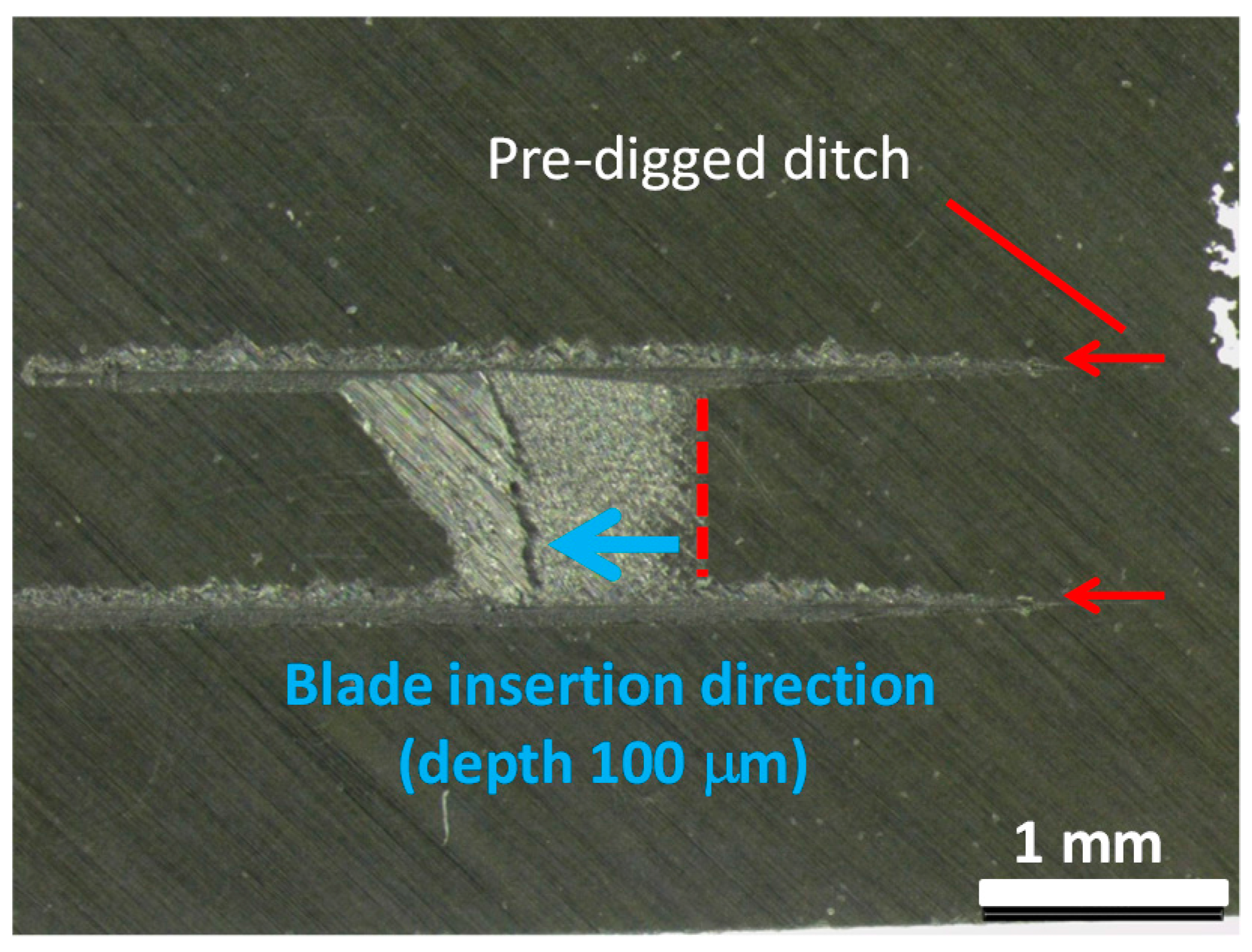
2.7. Optical Microscopy
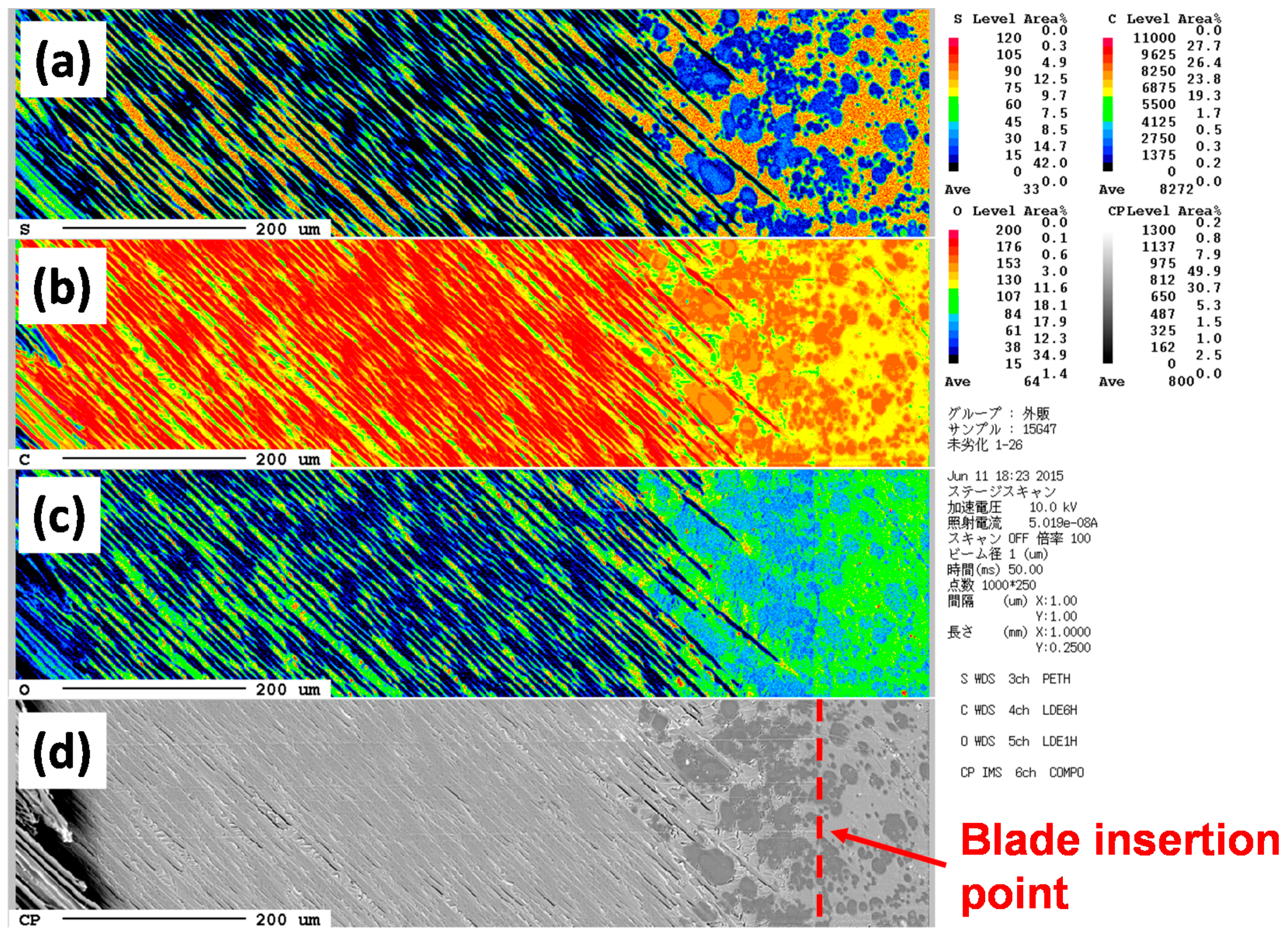
2.8. Electron Probe Micro-Analysis
3. Results and Discussion
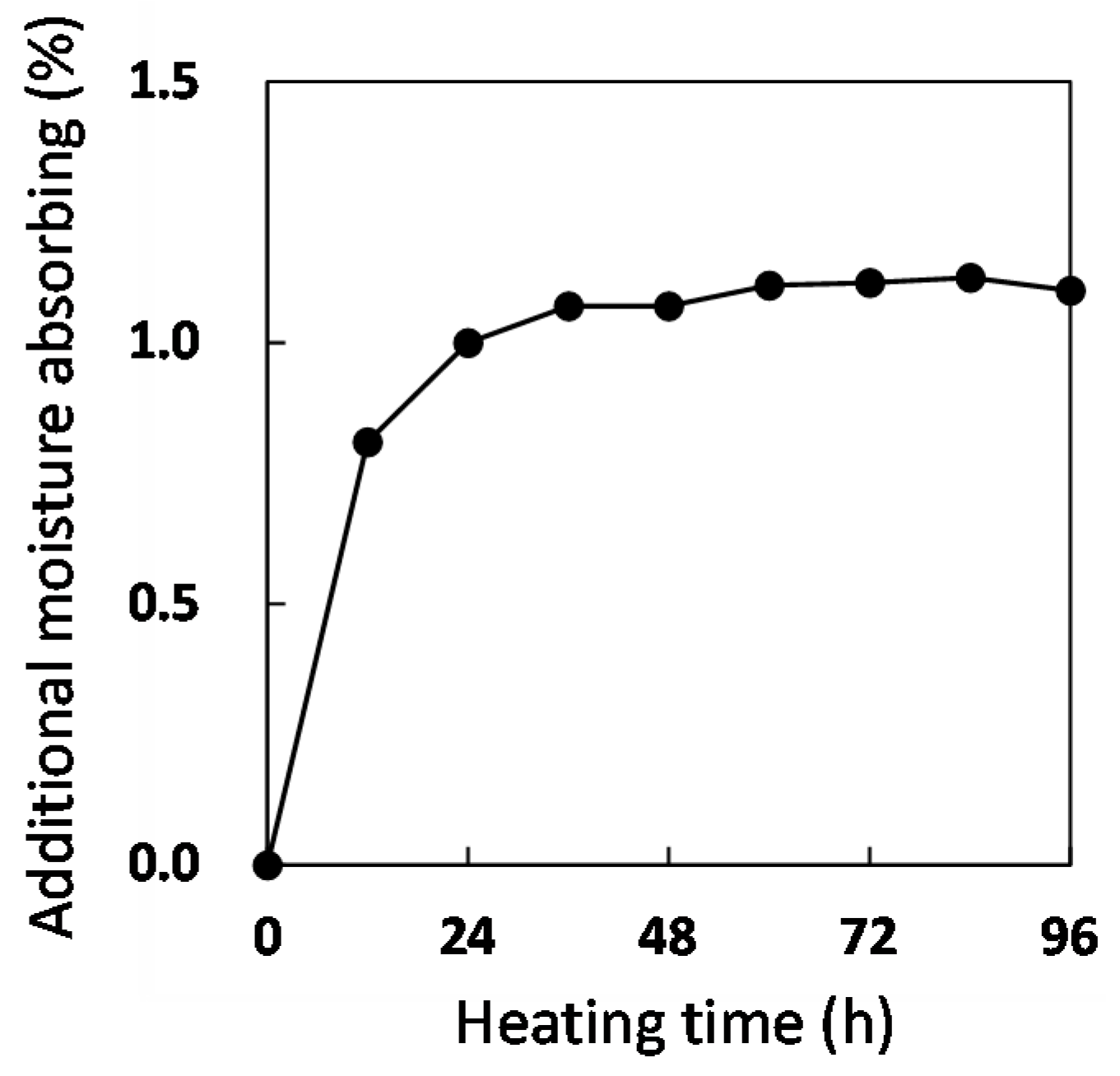
3.1. Quick Preparation of Moisture-Saturated CFRP
3.2. Stability Test
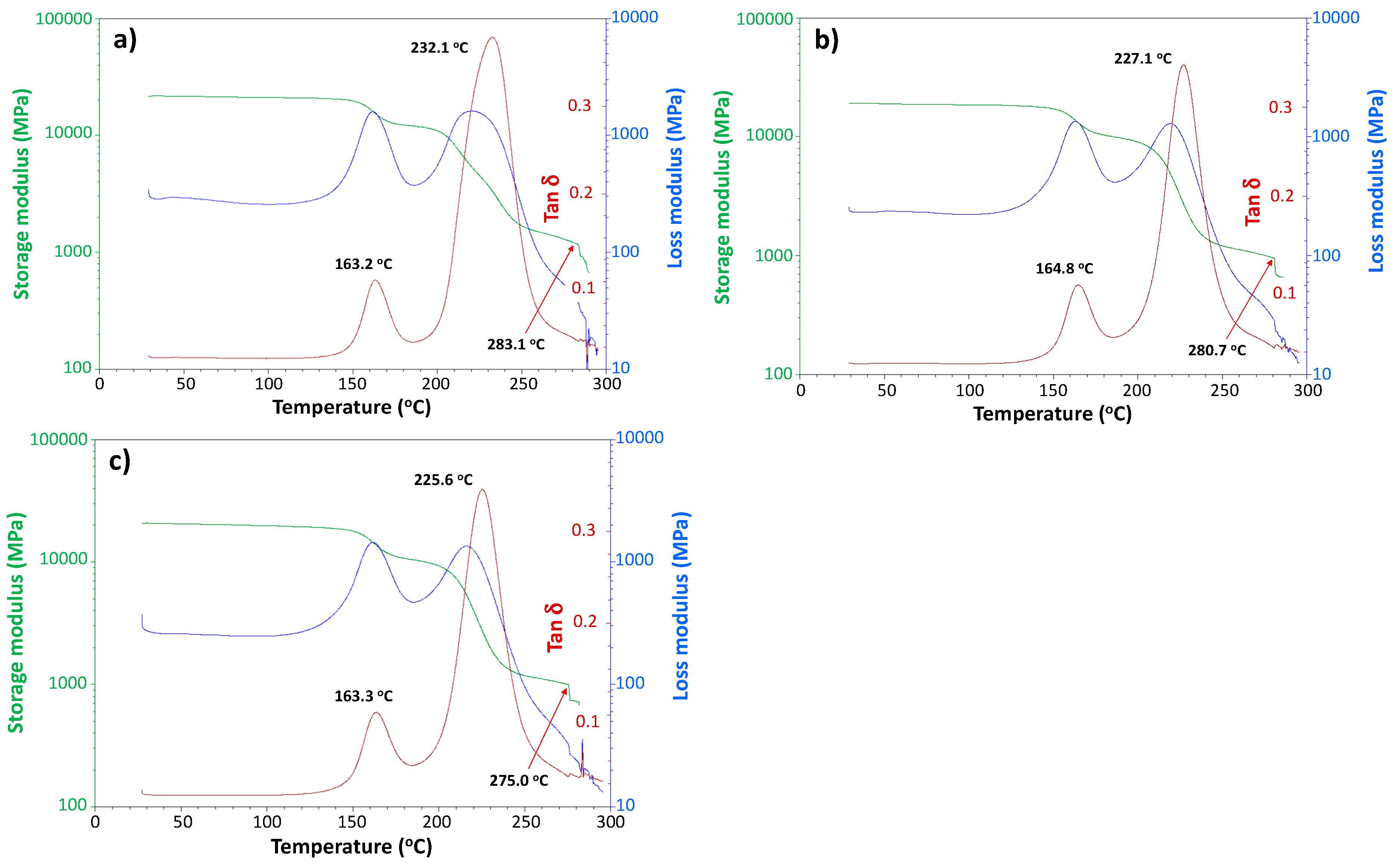
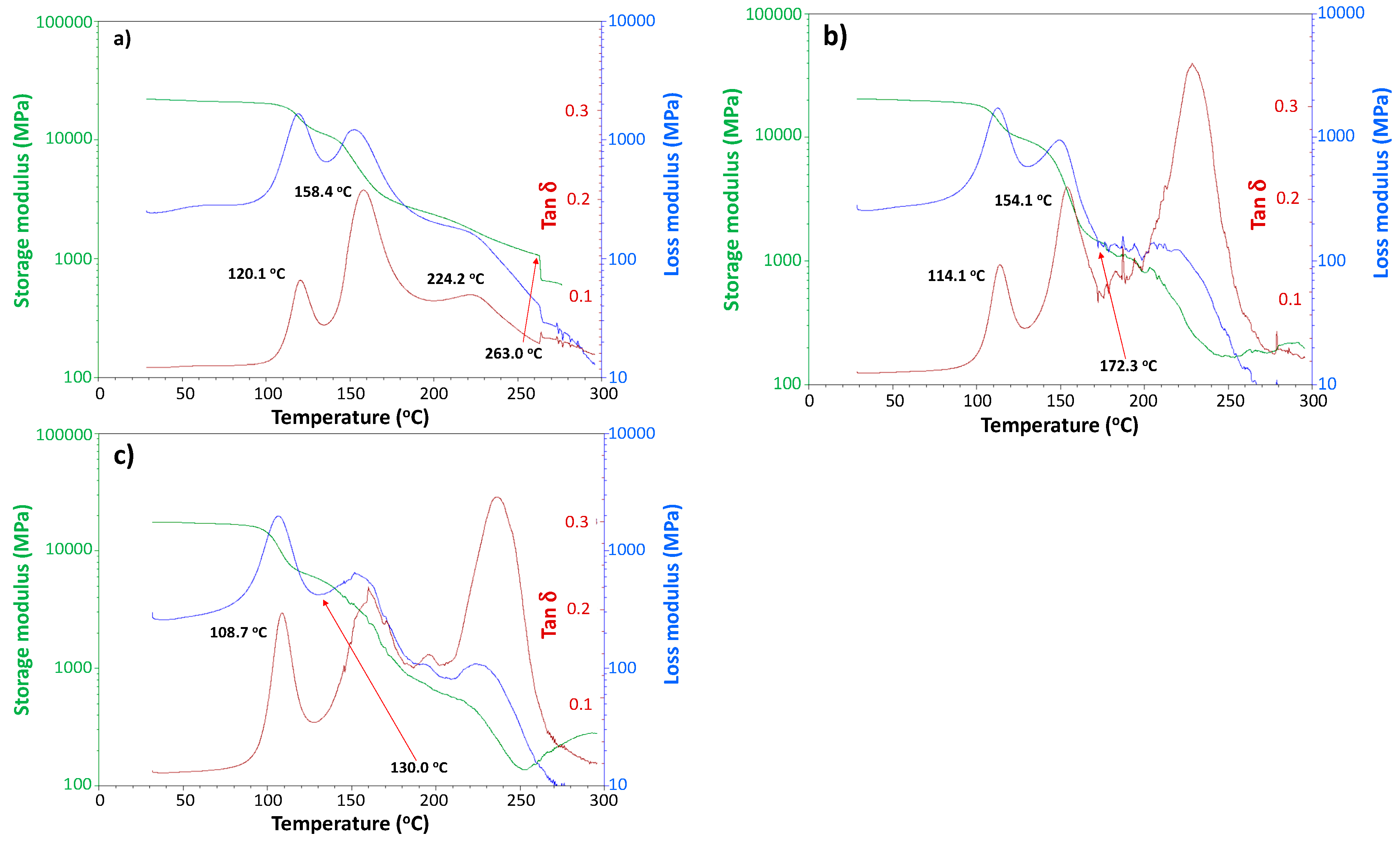
3.3. Viscoelastic Properties of Moisture-Saturated CFRP by DMA
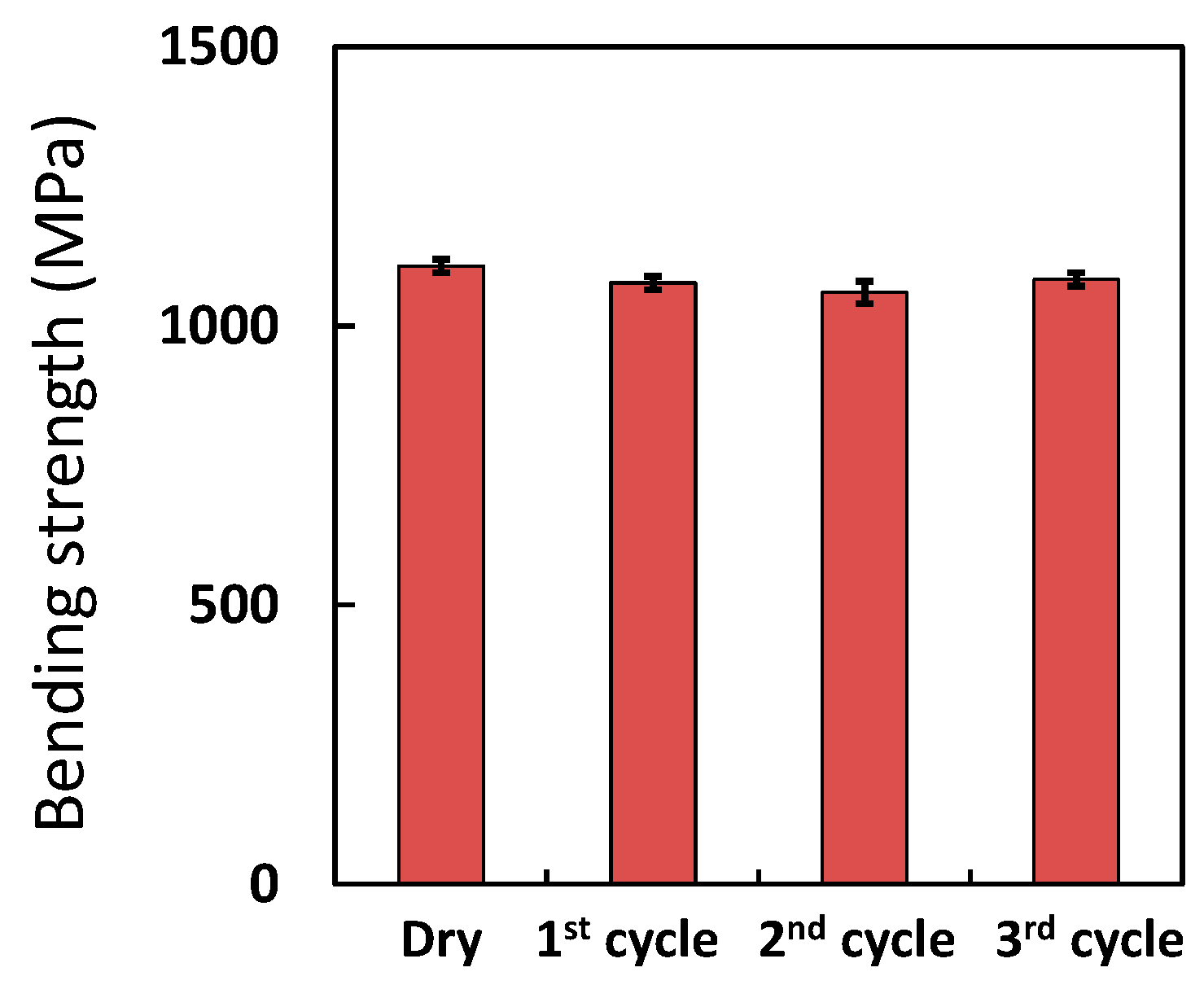
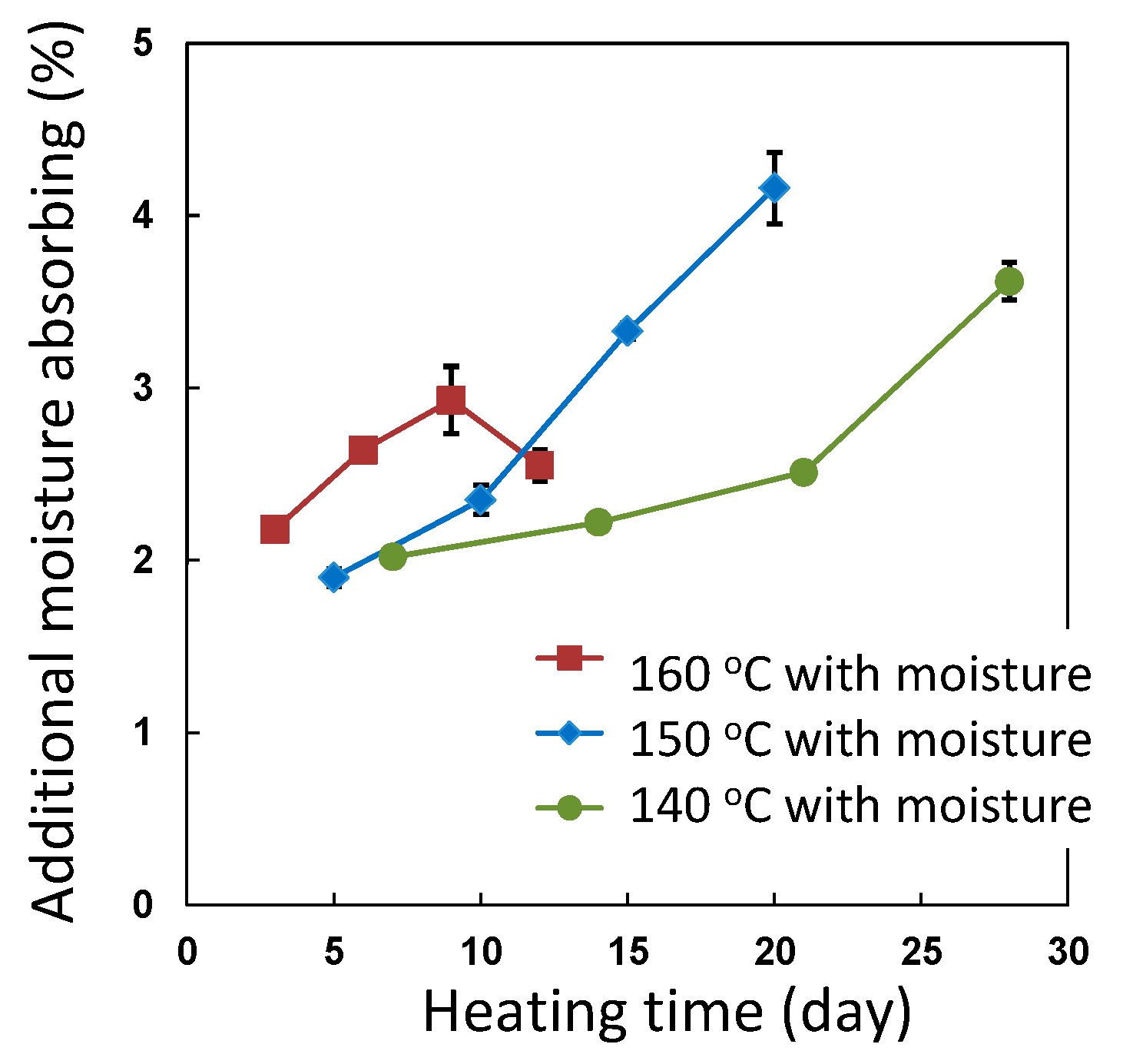
3.4. Accelerated Ageing Test
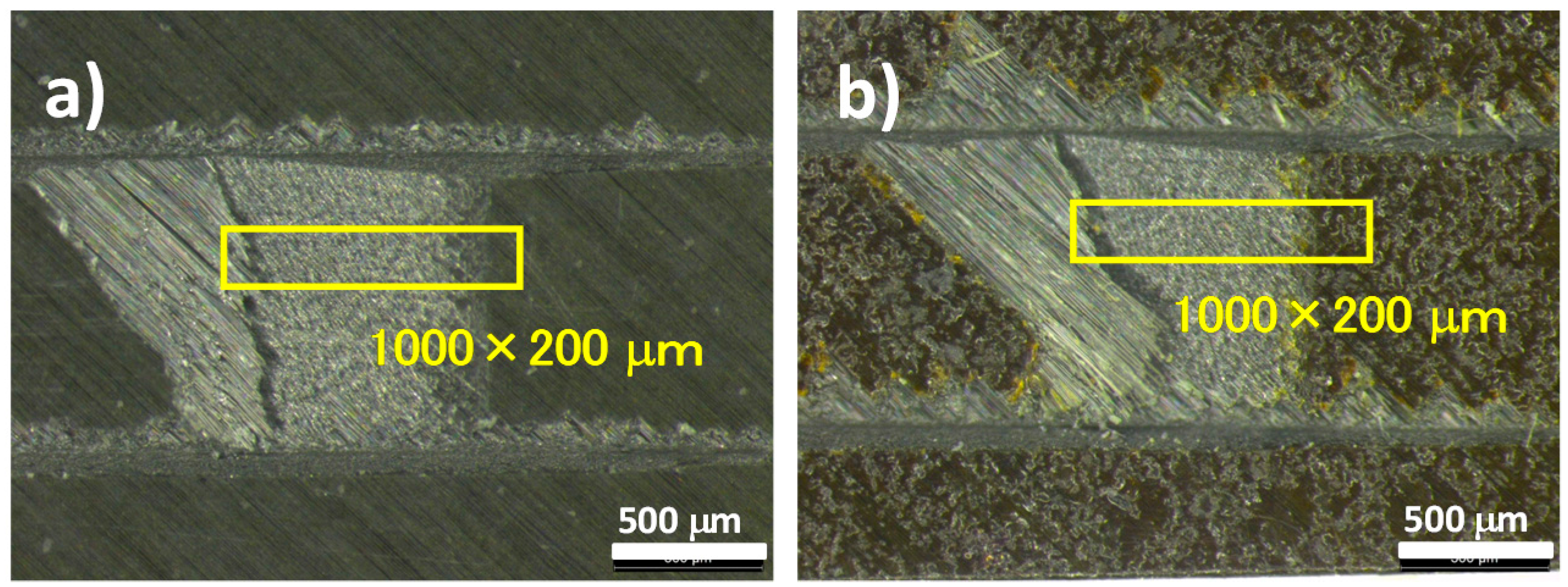
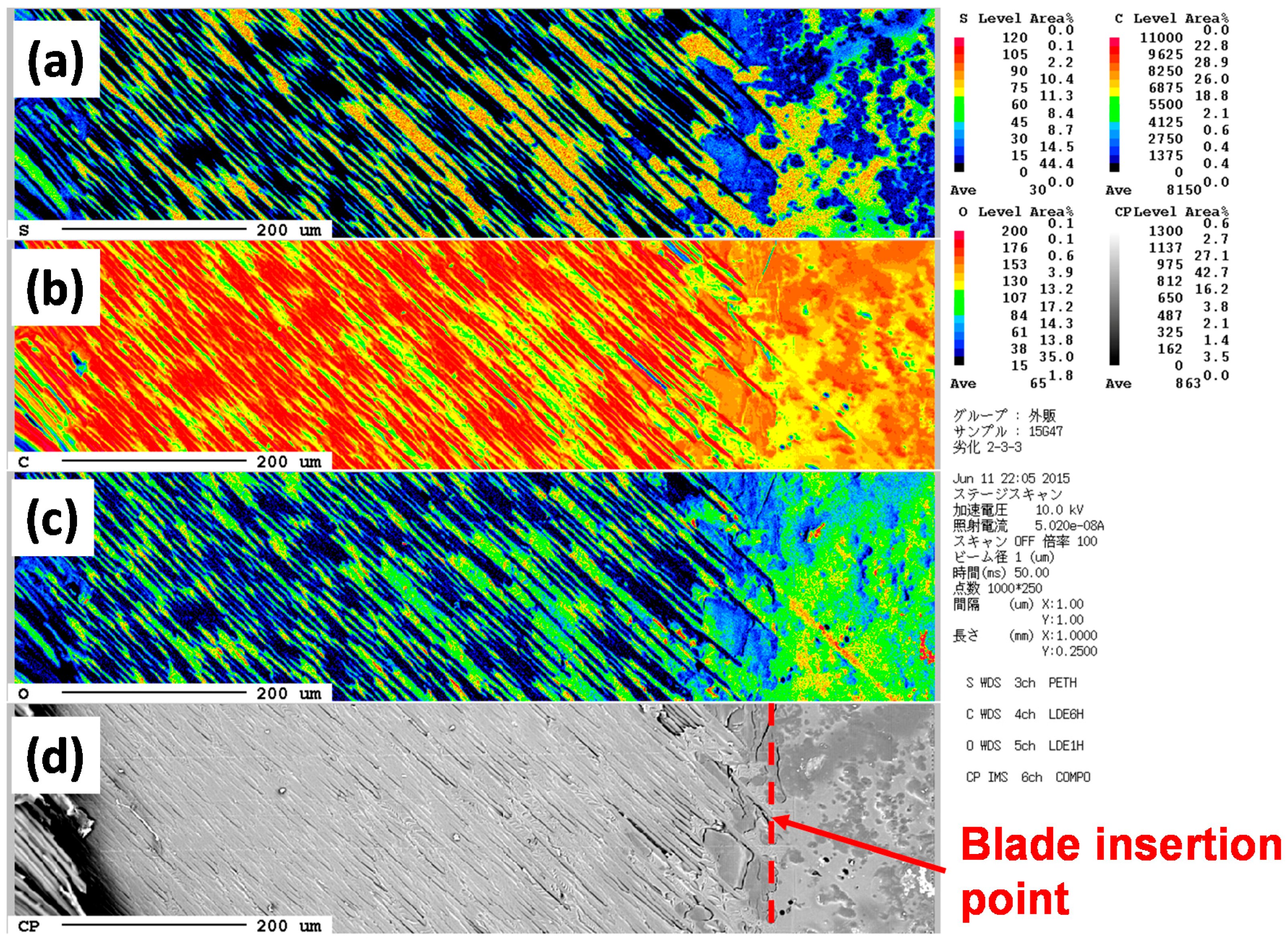
3.5. Analysis for Degradation by Optical Microscopy and Scanning Electron Microscopy (SEM) with Electron Probe Micro-Analyzer
4. Conclusion
Author Contributions
Conflicts of Interest
References and Notes
- Pola, J. Composite materials in the Airbus A380-From history to future. Available online: http://www.iccm-central.org/Proceedings/ICCM13proceedings/SITE/PAPERS/paper-1695.pdf (accessed on 17 June 2016).
- Hale, J. Boeing 787 from the ground up. Aero 2006, 20, 17–23. [Google Scholar]
- A-VaRTM technology application for Japan’s new regional jet aircraft. Available online: http://www.iccm-central.org/Proceedings/ICCM16proceedings/contents/pdf/MonA/MoAM1-04sp_shonot224571p.pdf (accessed on 17 June 2016).
- Hayashi, T. Car production by CFRP. Automot. Technol. 2012, 5, 40–55. [Google Scholar]
- Toray official web site. Available online: http://www.toray.com/ir/pdf/lib/lib_a136.pdf (accessed on 17 June 2016).
- Hamada, M. Car production by CFRTP. Automot. Technol. 2014, 3, 64–69. [Google Scholar]
- American Society for Testing Materials. Standard test method for moisture absorption properties and equilibrium conditioning of polymer matrix composite materials, ASTM D5229; ASTM International: West Conshohocken, PA, USA, 15 May 2014. [Google Scholar]
- International Organization for Standardization (ISO). Plastics—Determination of time-temperature limits after prolonged exposure to heat, ISO 2578; ISO: Geneva, Switzerland, 29 July 1993. [Google Scholar]
- International Organization for Standardization (ISO). Plastics piping and ducting systems–Determination of the long-term hydrostatic strength of thermoplastics materials in pipe form by extrapolation, ISO 9080; ISO: Geneva, Switzerland, 15 October 2012. [Google Scholar]
- International Organization for Standardization (ISO). Rubber, vulcanized or thermoplastic–Estimation of life-time and maximum temperature of use, ISO 11346; ISO: Geneva, Switzerland, 1 December 2014. [Google Scholar]
- Kim, K.Y.; Park, W.L.; Bang, Y.H. A study on durability of carbon fiber reinforced polymers in civil applications. Available online: http://iccm-central.org/Proceedings/ICCM18proceedings/data/3.%20Poster%20Presentation/Aug22%28Monday%29/P1-58~59%20Durability%20and%20Aging/P1-58-IK0515.pdf (accessed on 17 June 2016).
- Koyanagi, J.; Nakada, M.; Miyano, Y. Prediction of long-term durability of unidirectional CFRP. J. Reinf. Plast. Compos. 2011, 30, 1305–1313. [Google Scholar] [CrossRef]
- Celina, M.; Gillen, K.T.; Assink, R.A. Accelerated aging and lifetime prediction: Review of non-Arrhenius behavior due to two competing processes. Polym. Degrad. Stab. 2005, 90, 395–404. [Google Scholar] [CrossRef]
- International Electrotechnical Commission. Environmental testing-Part 2: Test methods-Test Cx: Damp heat, steady state (unsaturated pressurized vapour), IEC 60068–2-66; IEC: Geneva, Switzerland, 22 June 1994. [Google Scholar]
- Kumar, B.G.; Singh, R.P.; Nakamura, T. Degradation of carbon fiber-reinforced epoxy composites by ultraviolet radiation and condensation. J. Compos. Mater. 2002, 36, 2713–2721. [Google Scholar] [CrossRef]
- Naruse, T.; Hattori, T.; Miura, H.; Takahashi, K. Evaluation of thermal degradation of unidirectional CFRP rings. Compos. Struct. 2001, 52, 533–538. [Google Scholar] [CrossRef]
- Funabashi, M.; Ninomiya, F.; Oishi, A.; Ouchi, A.; Hagihara, H.; Suda, H.; Kunioka, M. Highly accelerated aging method for poly(ethylene terephthalate) film using xenon lamp with heating system. J. Polym. 2016, 2016. [Google Scholar] [CrossRef]
- Nogueira, P.; Ramirez, C.; Torres, A.; Abad, M.J.; Cano, J.; Lopez, J.; Lopez-Blueno, I.; Barral, L. Effect of water sorption on the structure and mechanical properties of an epoxy resin system. J. Appl. Polym. Sci. 2001, 80, 71–80. [Google Scholar] [CrossRef]
- Dewimille, B.; Bunsell, A.R. Accelerate ageing of a glass fibre-reinforced epoxy resin in water. Composites 1983, 14, 35–40. [Google Scholar] [CrossRef]
- Ciutacu, S.; Budrugeac, P.; Niculae, I. Accelerated thermal aging of glass-reinforced epoxy resin under oxygen pressure. Polym. Degrad. Stab. 1991, 31, 365–372. [Google Scholar] [CrossRef]
- Hagihara, H.; Oishi, A.; Funabashi, M.; Kunioka, M.; Suda, H. Free-volume hole size evaluated by positron annihilation lifetime spectroscopy in the amorphous part of poly(ethylene terephthalate) degraded by a weathering test. Polym. Degrad. Stab. 2014, 110, 389–394. [Google Scholar] [CrossRef]
- Suzuki, T.; Oki, Y.; Numagiri, M.; Miura, T.; Kondo, K.; Shiomi, Y.; Ito, Y. Free-volume characteristics and water absorption of novolac epoxy resins investigated by positron annihilation. Polymer 1996, 37, 3025–3030. [Google Scholar] [CrossRef]
- Murugan, R.; Ramesh, R.; Padmanabhan, K. Investigation on static and dynamic mechanical properties of epoxy based woven fabric glass/carbon hybrid composite laminates. Procedia. Eng. 2014, 97, 459–468. [Google Scholar] [CrossRef]
- Kishima, Y.; Nishiyama, I.; Kuroki, K. Characterization of polymers by surface and interfacial cutting analysis system (SAICAS method). Mater. Life 1992, 4, 86–91. [Google Scholar]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunioka, M.; Shimada, T.; Hagihara, H.; Funabashi, M.; Suda, H.; Horizono, H. Quick Preparation of Moisture-Saturated Carbon Fiber-Reinforced Plastics and Their Accelerated Ageing Tests Using Heat and Moisture. Polymers 2016, 8, 242. https://doi.org/10.3390/polym8060242
Kunioka M, Shimada T, Hagihara H, Funabashi M, Suda H, Horizono H. Quick Preparation of Moisture-Saturated Carbon Fiber-Reinforced Plastics and Their Accelerated Ageing Tests Using Heat and Moisture. Polymers. 2016; 8(6):242. https://doi.org/10.3390/polym8060242
Chicago/Turabian StyleKunioka, Masao, Tomio Shimada, Hideaki Hagihara, Masahiro Funabashi, Hiroyuki Suda, and Hideki Horizono. 2016. "Quick Preparation of Moisture-Saturated Carbon Fiber-Reinforced Plastics and Their Accelerated Ageing Tests Using Heat and Moisture" Polymers 8, no. 6: 242. https://doi.org/10.3390/polym8060242
APA StyleKunioka, M., Shimada, T., Hagihara, H., Funabashi, M., Suda, H., & Horizono, H. (2016). Quick Preparation of Moisture-Saturated Carbon Fiber-Reinforced Plastics and Their Accelerated Ageing Tests Using Heat and Moisture. Polymers, 8(6), 242. https://doi.org/10.3390/polym8060242