Functionalized Polymers for Enhance Oral Bioavailability of Sensitive Molecules
Abstract
:1. Introduction
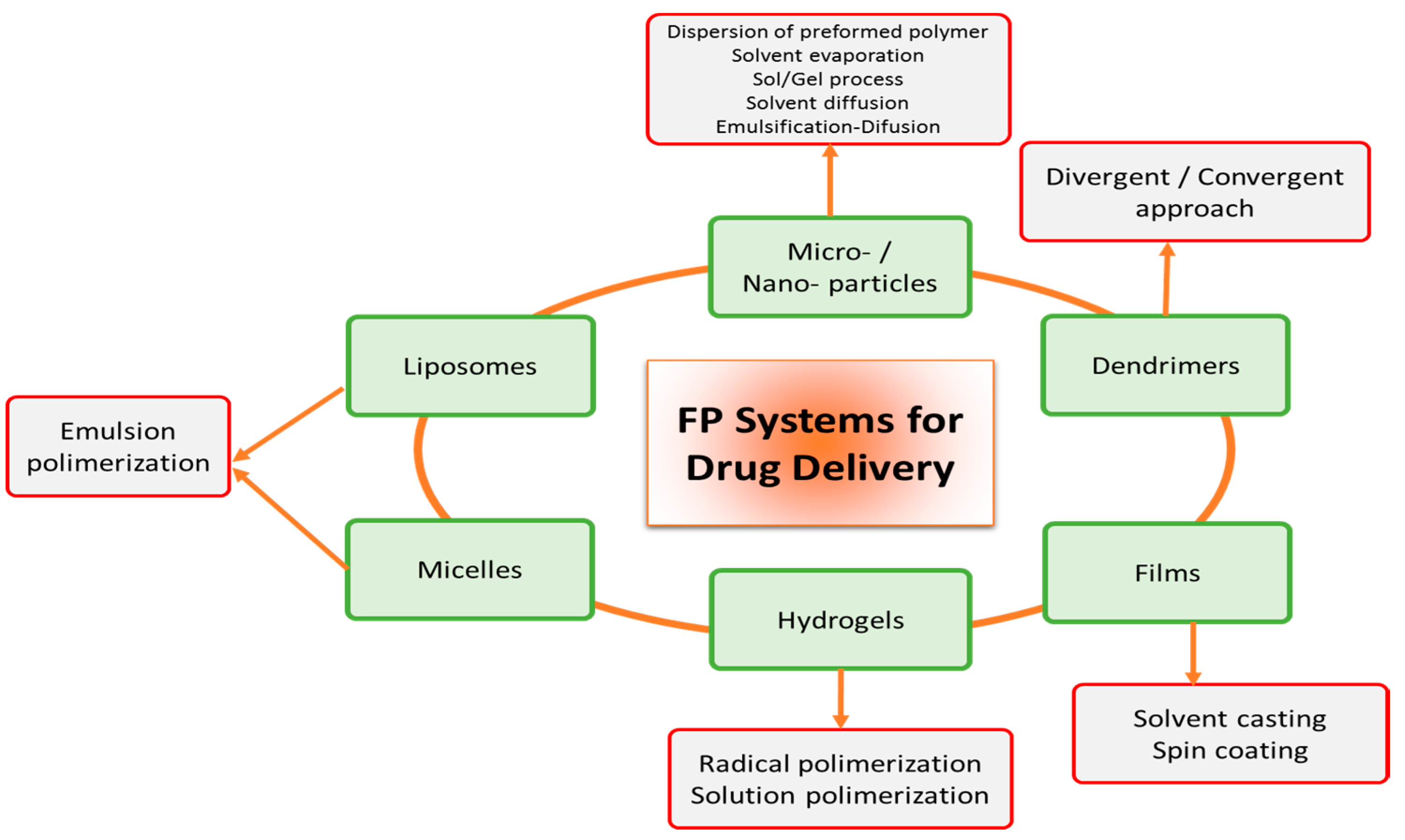
2. Physical Modifications of FP to Enhance Oral Bioavailability of Sensitive Molecules
2.1. FP Emulsion Systems as an Enhancement Alternative of Sensitive Molecules
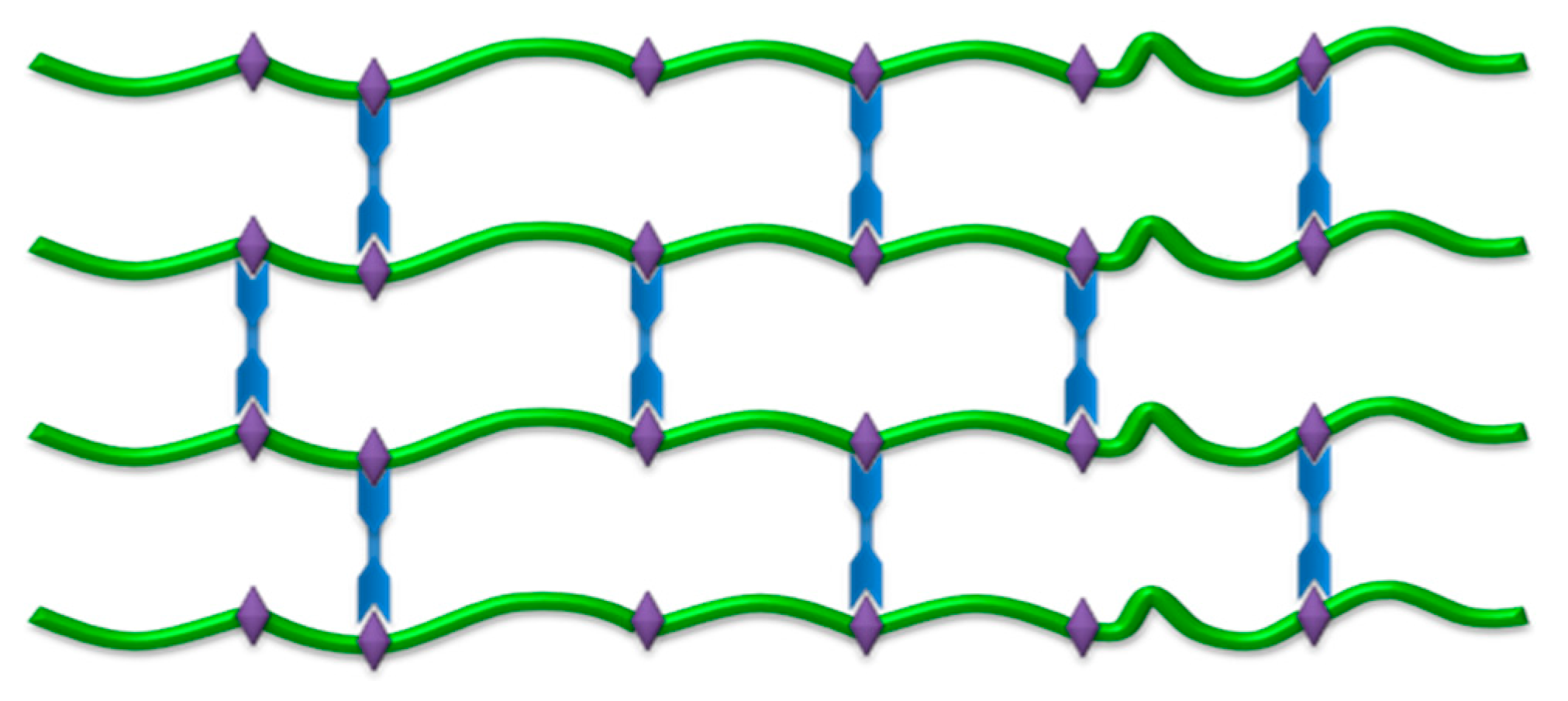
2.2. FP Hydrogel and Film Systems as an Enhancement Alternative of Sensitive Molecules
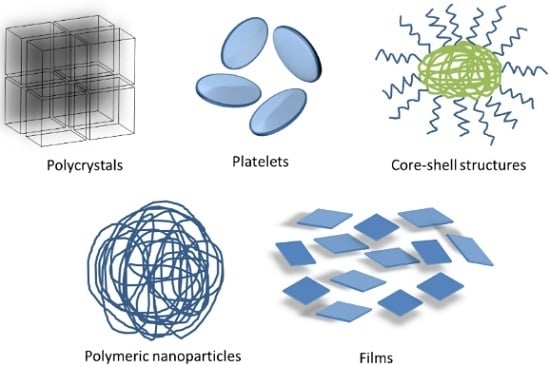
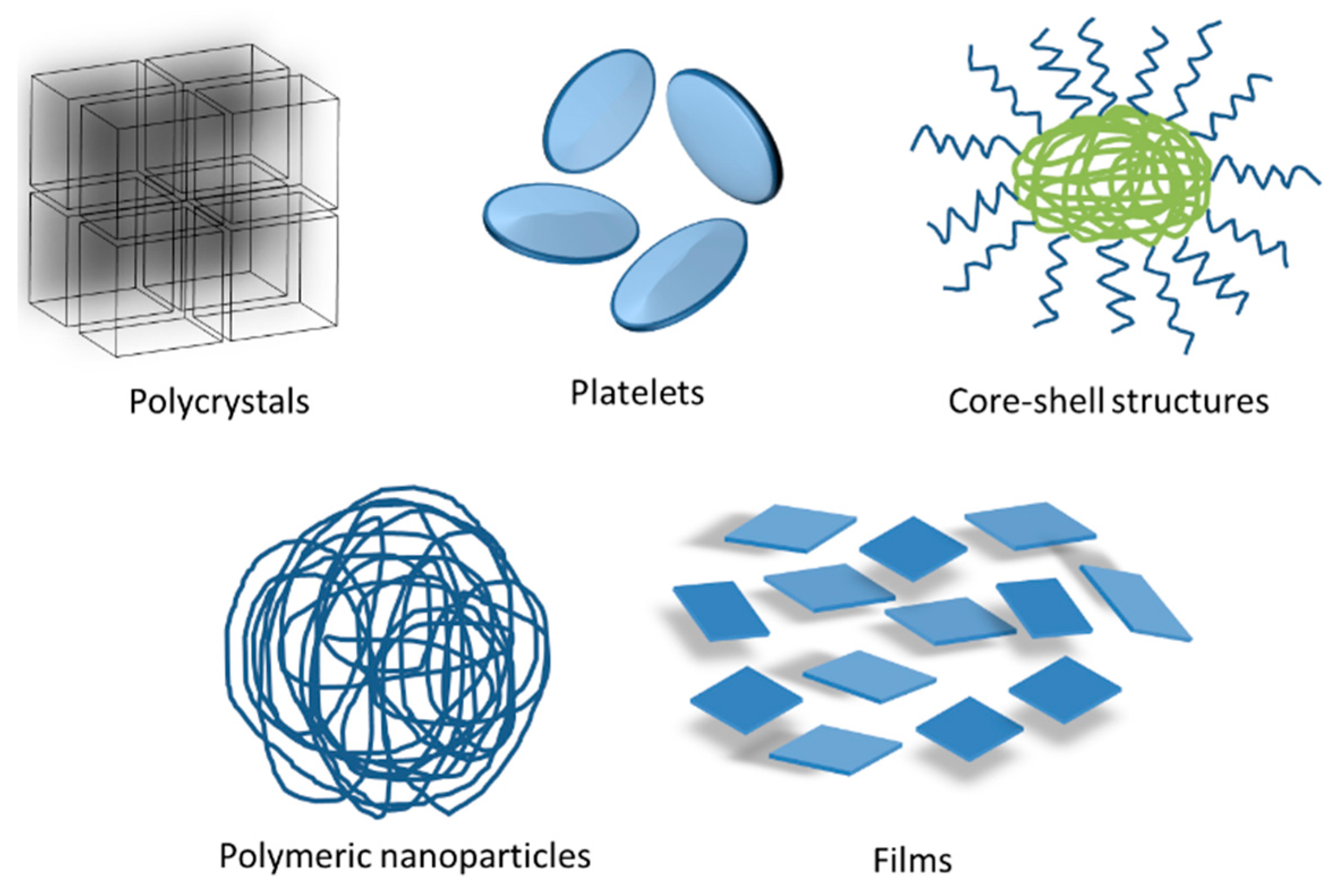
2.3. FP Solid Particles Systems as Enhancement Alternative of Sensitive Molecules
2.4. FP Dendrimers Systems as Enhancement Alternative of Sensitive Molecules
3. Chemical Modification on the Nature of Sensitive Molecules by FP
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sekhon, B.S. Biopharmaceuticals: An overview. Thai J. Pharm. Sci. 2010, 34, 1–19. [Google Scholar]
- Shaji, J.; Patole, V. Protein and peptide drug delivery: Oral approaches. Indian J. Pharm. Sci. 2008, 70, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Know, I.C.; Park, K. Oral protein delivery: Current status and future prospect. React. Funct. Polym. 2011, 71, 280–287. [Google Scholar] [CrossRef]
- Blanchette, J.; Kavidamandan, N.; Peppas, N.A. Principles of transmucosal delivery of therapeutic agents. Biomed. Pharmacol. 2004, 58, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Muro, U.C.; Riera, R.F.; Alvarado, P.Y. Encapsulation of whey proteins. In Whey Proteins Functional Properties Production and Health Benefits; Nova Publishers: New York, NY, USA, 2014; pp. 75–116. [Google Scholar]
- Luo, J.Y.; Zhong, Y.; Cao, J.C.H.; Cui, H.F. Efficacy of oral colon-specific delivery capsule of low-molecular-weight heparin on ulcerative colitis. Biomed. Pharmacother. 2011, 65, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Prajapati, D.S.; Raval, J.A. Fast dissolving films (FDFs) as a newer venture in fast dissolving dosage forms. Int. J. Drug Dev. Res. 2010, 2, 232–246. [Google Scholar]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.P.; Coughlin, J.E.; Padmanabhan, S.; Korba, B.E.; Myong, S. Activation of retinoic acid inducible gene (RIG-I) by nucleotide analogs—A potential novel mechanism for antiviral discovery. In Proceedings of the 23rd International Conference on Antiviral Research, San Francisco, CA, USA, 25–27 April 2010; CA International Society for Antiviral Research: Washington, DC, USA, 2010. [Google Scholar]
- Choonara, B.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. 2014, 32, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Taylor, L.C.; Edgar, K.J. The role of polymers in oral bioavailability enhancement: A review. Polymer 2015, 77, 399–415. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutics drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Mrsny, R.J. Oral peptide delivery: Prioritizing the leading technologies. Ther. Deliv. 2011, 2, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Arrunátegui, L.B.; Silva-Barcellos, N.M.; Bellavinha, K.R.; Ev, L.D.S.; Souza, J.D. Biopharmaceutics classification system: Importance and inclusion in biowaiver guidance. Braz. J. Pharm. Sci. 2015, 51, 143–154. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.; Nam, H.Y.; Lee, S.; Kim, K.; Kwom, I.C. Polymers for bioimaging. Prog. Polym. Sci. 2007, 32, 1031–1053. [Google Scholar] [CrossRef]
- Krug, S.M.; Amasheh, M.; Dittmann, I.; Christoffel, I.; Fromm, M.; Amasheh, S. Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials 2013, 34, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Fonte, P.; Sousa, F.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Oral films as breakthrough tools for oral delivery of proteins/peptides. J. Control. Release 2015, 211, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Meher, J.G.; Singh, Y.; Chaurasia, M.; Reddy, S.; Chourasia, M.K. Targeting of gastrointestinal tract for amended delivery of protein/peptide therapeutics: Strategies and industrial perspectives. J. Control. Release 2014, 196, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2014, 22, 171–282. [Google Scholar] [CrossRef]
- Briones, E.; Colino, C.L.; Lanao, J.M. Study of the factors influencing the encapsulation of zidovudine in rat erythrocytes. Int. J. Pharm. 2010, 401, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Perales, M.; Gruel, J.; Girke, T.; Jönsson, H.; Reddy, G.V. Wuschel protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Gene Dev. 2011, 25, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)–poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.K.; Mohapatra, R.; Sahoo, S.K. Novel functionalized polymers in drug delivery: A brief review. J. Curr. Pharma Res. 2014, 4, 1201–1210. [Google Scholar]
- Sajeesh, S.; Sharma, C.P. Interpolymer complex microparticles based on poly-methacrylic acid–chitosan for oral insulin delivery. J. Appl. Polym. Sci. 2006, 99, 506–512. [Google Scholar]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: A mucoadhesive and pH responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Wang, M.; Xiao, J.; Cheng, Y. Fluorinated poly(propylenimine) dendrimers as gene vectors. Biomaterials 2014, 35, 5407–5413. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Luo, Y. Facile glycosylation of dendrimers for eliciting specific cell-material interactions. Polym. Chem. 2012, 3, 310–313. [Google Scholar] [CrossRef]
- Dutta, T.; Jain, N.K.; McMillan, N.A.; Parekh, H.S. Dendrimer nanocarriers as versatile vectors in gene delivery. Nanomedicine 2010, 6, 25–34. [Google Scholar] [PubMed]
- Thanou, M.M.; Kotze, A.F.; Scharringhausen, T.; Lueben, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Prasad, Y.V.; Puthli, S.P.; Eaimtrakarn, S.; Ishida, M.; Yoshikawa, Y.; Shibata, N.; Takada, K. Enhanced intestinal absorption of vancomycin with Labrasol and d-α-tocopheryl PEG 1000 succinate in rats. Int. J. Pharm. 2003, 250, 181–190. [Google Scholar] [CrossRef]
- Guggi, D.; Kast, C.E.; Bernkop-Schnürch, A. In vivo evaluation of an oral salmon calcitonin-delivery system based on a thiolated chitosan carrier matrix. Pharm. Res. 2003, 20, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.; Yan, G.; Wu, Y.; Li, J.; Fang, X. Effect of self-microemulsifying drug-delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur. J. Pharm. Sci. 2005, 24, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Padula, C.; Nicoli, S.; Colombo, P.; Santi, P. Single-layer transdermal film containing lidocaine: Modulation of drug release. Eur. J. Pharm. Biopharm. 2007, 66, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Hagesaether, E.; Hiorth, M.; Sande, S.A. Mucoadhesion and drug permeability of free mixed films of pectin and chitosan: An in vitro and ex vivo study. Eur. J. Pharm. Biopharm. 2009, 71, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.; Sun, Y.; Tian, Y.; Li, Y.; Li, C.; Mao, S. Exploration of hydrophobic modification degree of chitosan-based nanocomplexes on the oral delivery of enoxaparin. Eur. J. Pharm. Sci. 2013, 50, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.; Shrestha, N.; Shahbazi, M.A.; Liu, D.; Herránz-Blanco, B.; Mäkilä, E.M.; Salonen, J.J.; Hirvonen, J.T.; Granja, P.L.; Sarmento, B.; et al. Microfluidic assembly of a multifunctional tailorable composite system designed for site specific combined oral delivery of peptide-drugs. ACS Nano 2015, 9, 8291–8302. [Google Scholar] [CrossRef] [PubMed]
- Mohri, K.; Morimoto, N.; Maruyama, M.; Nakamoto, N.; Hayashi, E.; Nagata, K.; Miyata, K.; Ochiai, K.; Hiwatari, K.I.; Tsubaki, K.; et al. Potential of d‑octaarginine-linked polymers as an in vitro transfection tool for biomolecules. Bioconjugate Chem. 2015, 26, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Han, L.; Qin, J.; Ru, G.; Li, R.; Wu, L.; Cui, D.; Yang, P.; He, Y.; Wang, J. N-trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl. Mater. Interfaces 2015, 7, 15430–15441. [Google Scholar] [CrossRef] [PubMed]
- Marschütz, M.K.; Bernkop-Schnurch, A. Oral peptide drug delivery: Polymer-inhibitor conjugates protecting insulin from enzymatic degradation in vitro. Biomaterials 2000, 21, 1499–1507. [Google Scholar] [CrossRef]
- Marschütz, M.K.; Bernkop-Schnurch, A. Design and in vivo evaluation of an oral delivery system for insulin. Pharm. Res. 2000, 17, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Pistel, K.F.; Breitenbach, A.; Zange-Volland, R.; Kissel, T. Brush-like branched biodegradable polyesters, part III Protein release from microspheres of poly(vinyl alcohol)-graftpoly(d,l-lactic-co-glycolic acid). J. Control. Release 2001, 73, 7–20. [Google Scholar]
- Dziubla, T.D.; Karim, A.; Muzykantov, V.R. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J. Control. Release 2005, 102, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, G.M.; Prego, C.; Torres, D.; Alonso, M.J. A comparative study of the potential of solid triglyceride nanostructures coated with chitosan or poly (ethylene glycol) as carriers for oral calcitonin delivery. Eur. J. Pharm. Sci. 2005, 25, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, K.B.; Russell-Jones, G.J.; Jain, A.K.; Diwan, P.V.; Jain, S.K. Effective oral delivery of insulin in animal models using vitamin B12-coated dextran nanoparticles. J. Control. Release 2007, 122, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, D.A.; Hocht, C.; Taira, C.; Sosnki, A. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability. Nanomedicine 2010, 5, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.J.; Tetley, L.; Cheng, W.P. The influence of polymer architecture on the protective effect of novel comb shaped amphiphilic poly(allylamine) against in vitro enzymatic degradation of insulin—Towards oral insulin delivery. Int. J. Pharm. 2010, 383, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Jiang, W.; Ren, C.; Li, J.; Xin, J.; Li, K. Effective protection and controlled release of insulin by cationic β-cyclodextrin polymers from alginate/chitosan nanoparticles. Int. J. Pharm. 2010, 393, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Sanguansri, L.; Augustin, M.A. In vitro lipolysis of fish oil microcapsules containing protein and resistant starch. Food Chem. 2011, 124, 1480–1489. [Google Scholar] [CrossRef]
- Balcão, V.M.; Costa, K.I.; Matos, C.M.; Moutinho, M.; Amorim, M.; Pintado, M.E.; Gomes, A.P.; Vila, M.M.; Teixeira, J.A. Nanoencapsulation of bovine lactoferrin for food and biopharmaceutical applications. Food Hydrocoll. 2013, 32, 425–431. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Schwendeman, S.P. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J. Control. Release 2013, 165, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Gradauer, K.; Barthelmes, J.; Vonach, C.; Almer, G.; Mangee, H.; Teubl, B.; Roblegg, E.; Dünnhaupt, S.; Fröhlich, E.; Bernkop-Schnürch, A.; et al. Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats. J. Control. Release 2013, 172, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Marquette, S.; Peerboom, C.; Yates, A.; Denis, L.; Goole, J.; Amighi, K. Encapsulation of immunoglobulin G by solid-in-oil-in-water: Effect of process parameters on microsphere properties. Eur. J. Pharm. Biopharm. 2014, 86, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.J.; Giardiello, M.; McDonald, T.O.; Smith, D.L.; Siccardi, M.; Rannard, S.P.; Owen, A. Augmented inhibition of CYP3A4 in human primary hepatocytes by ritonavir solid drug nanoparticles. Mol. Pharm. 2015, 12, 3556–3568. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Kamm, W.; Breitenbach, A.; Hungerer, K.D.; Hundt, E.; Kissel, T. Tetanus toxoid loaded nanoparticles from sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-co-glycolide): Evaluation of antibody response after oral and nasal application in mice. Pharm. Res. 2001, 18, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Avgoustakis, K.; Beletsi, A.; Panagi, Z.; Klepetsanis, P.; Karydas, A.G.; Ithakissios, D.S. PLGA–mPEG nanoparticles of cisplatin: In vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J. Control. Release 2002, 79, 123–135. [Google Scholar] [CrossRef]
- Amnuaikit, C.; Ikeucki, I.; Ogawara, K.-I.; Higaki, K.; Kimura, T. Skin permeation of propranolol from polymeric film containing terpene enhancers for transdermal use. Int. J. Pharm. 2005, 289, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Prego, C.; Garcia, M.; Torres, D.; Alonso, M.J. Transmucosal macromolecular drug delivery. J. Control. Release 2005, 101, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Shi, K.; Zhang, L.; Tao, A.; Kawashima, Y. Biodegradable nanoparticles loaded with insulin–phospholipid complex for oral delivery: Preparation, in vitro characterization and in vivo evaluation. J. Control. Release 2006, 114, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Hoffart, V.; Lamprecht, A.; Maincent, P.; Lecompte, T.; Vigneron, C.; Ubrich, N. Oral bioavailability of a low molecular weight heparin using a polymeric delivery system. J. Control. Release 2006, 113, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, G.Y.; Kim, Y.S.; Yu, M.; Park, R.W.; Kim, I.S.; Kim, S.Y.; Byun, Y. Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J. Control. Release 2006, 114, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Italia, J.L.; Bhatt, D.K.; Bhardwaj, V.; Tikoo, K.; Ravi-Kumar, M.N.V. PLGA nanoparticles for oral delivery of cyclosporine: Nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral®. J. Control. Release 2007, 119, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Li, Y.P.; Li, Z.L.; Zhou, C.L.; Tam, K.C.; Liu, Z.Y.; Xie, G.X. Vesicles from pluronic/poly(lactic acid) block copolymers as new carriers for oral insulin delivery. J. Control. Release 2007, 120, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.; Dorkoosh, F.A.; Dehpour, A.R.; Moezi, L.; Larijani, B.; Junginger, H.E.; Rafiee-Tehrani, M. Nanoparticles of quaternized chitosan derivatives as a carrier for colon delivery of insulin: Ex vivo and in vivo studies. Int. J. Pharma. 2008, 356, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, E.; Ansorena, E.; Lanciego, J.L.; Aymerich, M.S.; Blanco-Prieto, M.J. Sustained release of bioactive glycosylated glial cell-line derived neurotrophic factor from biodegradable polymeric microspheres. Eur. J. Pharm. Biopharm. 2008, 69, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, S.I.; Mahrous, G.M.; El-Badry, M. Preparation and comparative evaluation of sustained release metoclopramide hydrochloride matrix tablets. Saudi Pharm. J. 2009, 17, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Yin, B.C.; Zhang, W.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloid Surface B 2009, 68, 245–249. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.R.; De Carvalho, J.I.; Trevisan, M.T.; De Paula, R.C.M.; Ricardo, N.; Feitosa, P.A. Chitosan-coated pectin beads: Characterization and in vitro release of mangiferin. Food Hydrocoll. 2009, 23, 2278–2286. [Google Scholar] [CrossRef]
- Wei, W.; Maa, G.H.; Wanga, L.Y.; Wua, J.; Su, Z.G. Hollow quaternized chitosan microspheres increase the therapeutic effect of orally administered insulin. Acta Biomater. 2010, 6, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, D.; Fujimori, T.; Kawaguchi, N.; Tsujimoto, Y.; Nhisimi, M.; Dog, Z.; Katsumi, H.; Sakane, T.; Yamamato, A. Polyamidoamine dendrimers as novel potential absortion enhancers for inproving the small intestinal absortion of poorly absorvable drugs in rat. J. Control. Release 2011, 149, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.C.; Minami, E.; Stayton, P.S.; Murry, C.E. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 2011, 32, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur. J. Pharm. Sci. 2011, 42, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Cetin, M.; Aktas, M.S.; Vural, I.; Ozturk, M. Salmon calcitonin-loaded Eudragit® and Eudragit®-PLGA nanoparticles: In vitro and in vivo evaluation. J. Microencapsul. 2012, 29, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Conn, H.L.; Kelly, H.M.; Murphy, M.J.; Barry, F.P.; O’Brien, F.J.; Duffy, G.P. Development of a thermoresponsive chitosan gel combined with human mesenchymal stem cells and desferrioxamine as a multimodal pro-angiogenic therapeutic for the treatment of critical limb ischaemia. J. Control. Release 2012, 161, 73–80. [Google Scholar]
- Hosseinzadeh, H.; Atyabi, F.; Dinarvanda, R.; Ostad, S.N. Chitosan-pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and in vitro study. Int. J. Nanomedicine 2012, 7, 1851–1863. [Google Scholar] [PubMed]
- Kulkarni, R.V.; Boppana, R.; Mohan, G.K.; Mutalik, S.; Kalyane, N.V. pH-responsive interpenetrating network hydrogel beads of poly(acrylamide)-g-carrageenan and sodium alginate for intestinal targeted drug delivery: Synthesis, in vitro and in vivo evaluation. J. Colloid Interface Sci. 2012, 367, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.K.; Sahu, S. Development of diclofenac sodium loaded magnetic nanocarriers of pectin interacted with chitosan for targeted and sustained drug delivery. Colloid Surface B 2012, 97, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Amato, G.; Cappelli, A.; Paolino, M.; Giuliani, G.; Belmonte, B.; Guarnotta, C.; Pitarresi, G.; Giammona, G. Evaluation of thermoresponsive properties and biocompatibility of polybenzofulvene aggregates for leuprolide delivery. Int. J. Pharm. 2012, 438, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Nath, L.K. Evaluation of acetylated moth bean starch as a carrier for controlled drug. Int. J. Biol. Macromol. 2012, 50, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, J.; Yip, X.; Glynn, F.; Shepherd, R.K.; Caruso, F. Nanoporous peptide particles for encapsulating and releasing neurotrophic factors in an animal model of neurodegeneration. Adv. Mater. 2012, 24, 3362–3366. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.K.; Park, M.S.; Lee, J.H.; Hwang, J.E.; Shim, H.J.; Cho, S.H.; Kim, D.E.; Ko, H.M.; Cho, C.S.; Park, I.K.; et al. Docetaxel-loaded thermoresponsive conjugated linoleic acid-incorporated poloxamer hydrogel for the suppression of peritoneal metastasis of gastric cancer. Biomaterials 2013, 34, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; De Rose, R.; Best, J.P.; Johnston, A.P.R.; Alcantara, S.; Liang, K.; Such, G.K.; Kent, S.J.; Caruso, F. Mechanically tunable, self-adjuvanting nanoengineered polypeptide particles. Adv. Mater. 2013, 25, 3468–3472. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.P.D.; Tomar, L.K.; Tyagi, C.; Choonara, Y.E.; Modi, G.; Du Toit, L.C.; Kumar, P.; Pillay, V. A novel pH-sensitive interferon-β (INF-β) oral delivery system for application in multiple sclerosis. Int. J. Pharm. 2013, 456, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.K.; Mishra, S.; Bajpai, M.; Mishra, A. Enhanced oral bioavailability of efavirenz by solid lipid nanoparticles: In vitro drug release and pharmacokinetics studies. Biomed. Res. Int. 2014, 2014, 363404. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, C.; Song, A.; Chang, D.; Zheng, X.; Gao, Y.; Wang, S. Alginate encapsulated mesoporous silica nanospheres as a sustained drug delivery system for the poorly water-soluble drug indomethacin. Asian J. Pharm. Sci. 2014, 9, 183–190. [Google Scholar] [CrossRef]
- Simón-Yarza, T.; Formiga, F.R.; Tamayo, E.; Pelacho, B.; Prosper, F.; Blanco-Prieto, M.J. PEGylated-PLGA microparticles containing VEGF for long term drug delivery. Int. J. Pharm. 2013, 440, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y.; Hu, D.; Chen, X.; Liu, Y.; Wang, L.; Zhao, Y. One-step synthesis of interpenetrating network hydrogels: Environment sensitivities and drug delivery properties. Saudi J. Biol. Sci. 2016, 23, S22–S31. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Kaur, G. Bioadhesive vaginal drug delivery of nystatin using a derivatized polymer: Development and characterization. Eur. J. Pharm. Biopharm. 2015, 96, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Marizza, P.; Pontoni, L.; Rindzevicius, T.; Alopaeus, J.F.; Su, K.; Zeitler, J.A.; Solinas, D. Supercritical impregnation of polymer matrices spatially confined in microcontainers for oral drug delivery: Effect of temperature, pressure and time. J. Supercrit. Fluid 2016, 107, 145–152. [Google Scholar] [CrossRef]
- Najafi, S.H.M.; Baghaie, M.; Ashori, A. Preparation and characterization of acetylated starch nanoparticles as drug carrier: Ciprofloxacin as a model. Int. J. Biol. Macromol. 2016, 87, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.W.; Hung, C.F.; Fang, J.Y. Current prodrug design for drug discovery. Curr. Pharm. Des. 2009, 19, 2236–2250. [Google Scholar] [CrossRef]
- Han, H.; Amidon, G.L. Targeted prodrug design to optimize drug delivery. AAPS Pharm. Sci. 2010, 2, 48–58. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Rekha, M.R.; Sharma, C.P. Oral delivery of therapeutic protein/peptide for diabetes—Future perspectives. Int. J. Pharm. 2013, 440, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Mashingaidze, F.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Maharaj, V.; Buchmann, E.; Pillay, V. Poly(ethylene glycol) enclatherated pectin-mucin submicron matrices for intravaginal anti-HIV-1 drug delivery. Int. J. Pharm. 2016, 503, 16–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Giuliani, A.; Falciani, C.H.; Runci, Y.; Ricci, C.; Lelli, B.; Malossi, M.; Neri, P.; Rossolini, G.M.; Bracci, L. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob. Agents Chemother. 2005, 49, 26–65. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.Y.; Ross, B.P.; Thomas, B.G.; Toth, I. Polycationic lipophilic-core dendrons as penetration enhancers for the oral administration of low molecular weight heparin. Bioorg. Med. Chem. 2006, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Su, Y.; Zhang, H.; Xu, T.; Cheng, Y. Design of interior-functionalized fully acetylated dendrimers for anticancer drug delivery. Biomaterials 2011, 32, 9950–9959. [Google Scholar] [CrossRef] [PubMed]
- Pinholt, C.; Bukrinsky, J.T.; Hostrup, S.; Frokjaer, S.; Norde, W.; Jorgensen, L. Influence of PEGylation with linear and branched PEG chains on the adsorption of glucagon to hydrophobic surfaces. Eur. J. Pharm. Biopharm. 2011, 77, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mufamadi, M.; Choonara, Y.; Kumar, P.; Modi, G.; Naidoo, D.; Vuuren, S.; Ndesendo, V.; du Toita, L.; Iyuke, S.; Pillay, V. Ligand-functionalized nanoliposomes for targeted delivery of galantamine. Int. J. Pharm. 2013, 448, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Zahariev, S.; Pelillo, C.; Milan, A.; Gennaro, R.; Scocchi, M. PEGylation of the peptide BAc7 (1–35) reduces renal clearance while retaining antibacterial activity and bacterial cell penetration capacity. Eur. J. Med. Chem. 2015, 95, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kurinomaru, T.; Shiraki, K. Noncovalent PEGylation of l-asparaginase using pegylated polyelectrolyte. J. Pharm. Sci. 2015, 104, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Martinez, A.; Martin-Fonseca, S.; Garcia-Granados, A.; Ferrer-Martín, R.M.; Lupiañez, J.A.; Parra-Medina, A. Semi-synthesis and antiproliferative evaluation of PEGylated pentacyclic triterpenes. Eur. J. Med. Chem. 2016, 118, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Tsumura, S.; Harada, A.; Kono, K. A collagen-mimic dendrimer capable of controlled release. J. Am. Chem. Soc. 2009, 131, 6052–6053. [Google Scholar] [CrossRef] [PubMed]
- Yamamato, A. Polyamidoamine dendrimers as novel potential absorption enhancers for improving the small intestinal absorption of poorly absorbable drugs in rats. J. Control. Release 2011, 149, 21–28. [Google Scholar]
- Roots analysis Business, Research & Consulting (2016), Oral Proteins and Peptides Market, 2015–2025. Available online: http://www.rootsanalysis.com/reports/view_document/oral-proteins-andpeptides-market-2015-2025/82.html (accessed on 19 March 2016).
- Morishita, M.; Peppas, N. Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today 2006, 11, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Dollo, G.; Le Corre, P.; Guérin, A.; Chevanne, F.; Burgot, J.L.; Leverge, R. Spray-dried redispersible oil-in-water emulsion to improve oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Sci. 2003, 19, 273–280. [Google Scholar] [CrossRef]
- Mahato, R.I.; Narang, A.S.; Thoma, L.; Miller, D.D. Emerging trends in oral delivery of peptide and protein drugs. Crit. Rev. Ther. Drug 2003, 20, 153–214. [Google Scholar] [CrossRef]
- Antunes, F.; Andrade, F.; Ferreira, D.; Nielson, H.M.; Sarmento, B. Models to predict intestinal absorption of therapeutic peptides and proteins. Curr. Drug Metab. 2013, 14, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Kennelly, R.; Bzik, V.A. Evaluation of intestinal absorption enhancement and local mucosal toxicity of two promoters. I. Studies in isolated rat and human colonic mucosae. Eur. J. Pharm. Sci. 2009, 38, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, P.; Badarinath, A.V.; Naveen, N.; Prasad, K.; Reddy, B.R.S.; Hyndhavi, M.; Nirosha, M. A rationalized description on study of intestinal barrier, drug permeability and permeation enhancers. J. Glob. Trends Pharm. Sci. 2011, 2, 431–449. [Google Scholar]

| System | FP | Sensitive molecule | References | Application trials |
|---|---|---|---|---|
| Micelles | N-trimethyl chitosan (TMC 60, TMC 40 substitution grade) | Hydrophilic [14C]-mannitol | [32] | In vitro, Permeability of drug by Transepithelial electrical resistance (TEER) on Caco-2-cell |
| Micelles | Labrasol-d-a-tocopheryl-PEG 1000 succinate (TPGS) | Vancomycin hydrochloride (VCM) | [33] | In vitro, Permeability by plasma drug concentrations in rat ileum |
| Microtablets | Chitosan-4-thiobutylamidine (TBA)-BBI-chitosan elastatinal-glutathione | Salmon calcitonin (cST) | [34] | In vitro, Permeability by the release profile of drug. In vivo, Plasma calcium level in rats |
| Microemulsion | SMEDDS-Labrasol | Mannitol | [35] | In vitro, TEER measurement on Caco-2-cell. |
| Film | PVA-elastoid® E35H-PPR.PVP | Lidocaine Hydrochloride | [36] | In vitro, Transdermal film in vertical Franz-type diffusion cells. |
| Film | Pectin-chitosan | Paracetamol | [37] | In vitro, Permeability by release of drug on fresh porcine small intestine |
| Emulsion | Chitosan-glyceryl monostearate (GM) | Enoxaparin | [38] | In vitro, Permeability by absorption of drug on intestinal mice |
| Nanoparticles | Chitosan-PLA-co-glycolide)-silicon onto of hydroxyl-propylmethyl-cellulose- acetyl-succinate | Peptide Glucagon-like peptide-1 | [39] | In vitro, Permeability by release of drug in simulated fluids, SGF and SIF |
| Emulsion | Poly(N-vinylacetamide-co-acrylic acid)-d-octaarginine, | Protein (pGFP-C1), β-galactosidase and bovine serum albumin (BSA) | [40] | In vitro, Permeation drugs in HeLa-cells |
| Nanoparticles | N-trimethyl-TMC-(PLGA) | Insulin | [41] | In vitro, Permeability by release of drug in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF). In vivo, Intestinal mucoadhesion in male Kumming mice. |
| System | FP | Sensitive molecule | References | Application trials |
|---|---|---|---|---|
| Capsules/tablets | Sodium carboxy-methyl cellulose (Na-CMC)- Bowman–Birk inhibitor conjugate | Insulin | [42] | In vitro, Protective effect of degradation against intestinal proteases |
| Microcapsules/Tablets | Sodium carboxymethylcellulose (Na-CMC)-Bowman-Birk inhibitor coated with a polymethacrylate | Insulin and mannitol | [43] | In vitro, Protective effect against intestinal enzymes and release profile. In vivo, Glucose levels of diabetic in mice. |
| Microspheres | Poly(vinyl alcohol)-graft-poly(lactic-co-glycolic acid), PVA-g-PLGA | Bovine serum albumin, ovalbumin, cytochrome c and FITC-dextran | [44] | In vitro, Protective effect against intestinal enzymes and release profile. |
| Nanoparticles | PNC of diblock PEG-PLGA | Catalase enzyme | [45] | In vitro, Protective effect against proteolysis. |
| Nanoparticles | Triglyceride nanostructures-chitosan-PEG | Salmon calcitonin (cST) | [46] | In vitro, Protective effect by TEER measurement on cell monolayer Caco-2. In vivo, Serum calcium levels in rats |
| Nanoparticles | Vitamin B12-dextran | Insulin | [47] | In vitro, Protective effect against intestinal enzymes. In vivo, Glucose measure in diabetic rats |
| Block copolymer micelles | Poly(ethylene oxide)–poly(propylene oxide) | Efavirenz (EFV) | [48] | In vitro, Protective effect against intestine-mimicking. In vivo, Plasma concentration in different male Wistar rats. |
| Nanocomplexes | PAA-cetyl or cholesteryl choloroformate pendant groups | Bovine Insulin | [49] | In vitro, Degradation of drug by intestinal enzymes on Caco-2 cells |
| Nanospheres | Cationic-β cyclodextrin polymers (CPβCDs) | Insulin | [50] | In vitro, Degradation of drug against intestinal enzymes into SGF and SIF. |
| Nanocapsules | Sodium caseinate and starch | Fish oil powders protein | [51] | In vitro, Degradation of drug by intestinal enzymes. |
| Nanoemulsions | Bovine Lactoferrin stabilized with poloxamers (PEO-PPO-PEO) | Lactoferrin | [52] | In vitro, Protective degradation of drug and antimicrobial trials. |
| Microcapsules | PLGA | Ovalbumin and tetanus toxoid (TT), | [53] | In vitro, Degradation of drug by intestinal enzymes. |
| Emulsion | Chitosan-thioglycolic acid (CS-TGA) | Salmon calcitonin (cST) | [54] | In vitro, Protective degradation of drug into porcine intestine mucus. In vivo, Release of drug in male Sprague-Dawley (SD) rats |
| Microspheres | PLGA | Immuno-globulin G | [55] | In vitro, Preserving the integrity of the encapsulated antibody |
| Nano-particles Ritonavir (RTV) | PEG- PVA- Pluronic® F68, ® F127- PVP K30- HPC and/or HPMC | Cytochrome P4503A4 (CYP3A4) and P-glycoprotein (P-gp) | [56] | In vitro, Protective degradation of drugs into cells, HepG2, Caco-2, THP-1, A-THP-1, and CEM |
| System | FP | Sensible Molecule | References | Application trials and results |
|---|---|---|---|---|
| Nanoparticles | PVAL-graft-PLGA | Tetanus Toxoid (TT) | [57] | In vitro, Release of drug into intraperitoneal mice. |
| Nanoparticles | PLGA-mPEG | Cisplatin | [58] | In vitro, Release of drug in SGF. In vivo, Effect of drug in female BALB/c mice. |
| Film | Ethyl cellulose (EC)-polyvinyl pyrrolidone (PVP)-dibutyl phthalate (DBP) | Propanolol Hydrochloride (PPL) | [59] | In vitro, Release of drug in SGF. |
| Nanoparticles nanocapsules | Chitosan-oil nanodroplets | Salmon calcitonin (cST) | [60] | In vitro, Release of drug in SGF. In vivo, Calcemic levels observed in rats |
| Nanoparticles | PLA-poly(d,l-lactide-co-glycolide acid) (PLGA) | Porcine Insulin | [61] | In vitro, Release of drug in SGF. In vivo, Glucose evaluation in diabetic rats. |
| Nanoparticles | Polyester-polycationic polymethacrylate | Tinzaparin | [62] | In vitro, Release of drug into intestinal rabbit. In vivo, Anticoagulant effect in rabbit |
| Dispersed nanoparticles in a film | DOCA (HD) dispersed in polyurethane | Heparin | [63] | In vitro, Release of drugs into endothelial cell (HUVEC) In vivo, Anti-proliferative effects on old male C3H/HeN mice |
| Nanoparticles | PLGA-NP | Cyclosporine | [64] | In vitro, Release of drug into heparinized blood from SD rats. In vivo, Effect of drug in male SD rats. |
| Vesicles | PLA-b-Pluronic-b-PLA-F127-PLA | Bovine insulin | [65] | In vitro, Release of drug in SGF. In vivo, Hypo-glycemic effect in Kumming diabetic mice. |
| Nanoparticles | Chitosan-triethylchitosan (TEC)-dimethyl-ethylchitosan (DMEC) | Insulin | [66] | In vitro, Release of drug for 5 h in SGF. |
| Microspheres | PLGA RG 503H | Neurotrophic factor (GDNF) glycosylated | [67] | In vitro, Release of drug into cell neurite. |
| Tablets and pellets | HPMC-CMC-EC | Metoclopramide hydrochloride (MCP) | [68] | In vitro, Release of drug in SGF. |
| Microparticle | Chitosan-alginate-pectin | Bovine serum albumin (BSA) | [69] | In vitro, Release of drug in SGF. |
| Microspheres | (DE) pectin-calcium-chitosan PCaC | Mangiferin | [70] | In vitro, Release of drug in both SGF and SIF. |
| Microspheres | Chitosan-glutaraldehyde | Insulin | [71] | In vitro, Release of drug in SGF. In vivo, Glucose level and powerful therapeutic effects in SD rats |
| Dendrimers | Polyamidoamine (PAMAM) | 5(6)-(CF), fluorescein isothiocyanate-dextrans (FDs), calcitonin and insulin | [72] | In vitro, Release of drugs into rat small intestine. |
| Hydrogel | Poly(N-isopropylacrylamide-co-propylacrylicacid-co-butylacrylate) | Fibroblast growth factor (bFGF) | [73] | In vitro, Delivery of drug in SGF. In vivo, Cardio-vascular function after 28 days in old Fischer rats. |
| Nanoparticles | Chitosan-hydroxypropyl methylcellulose phthalate (HPMCP) | Insulin | [74] | In vitro, Release profiles in SGF without enzymes. In vivo, Mucoadhesion studies in male Wistar rats. |
| Nanoparticles | Poly(lactic-co-glycolic acid) | Salmon calcitonin (sCT) | [75] | In vitro, Release of drug into SGF and SIF In vitro, Plasma calcium level in female SD rats. |
| Hydrogel | Chitosan-β–glycerophosphate (β–GP) | Desferroxamine (DFO) | [76] | In vitro, Release of drug into endothelial cell (HUVEC). |
| Nanoparticles | Chitosan | Anticancer Gemcitabine (GC, 2′,2′difluorodeoxycytidine) | [77] | In vitro, Release of drug into HT-29 cell. |
| Hydrogels | Poly(acrylamide)-graft-κ-carrageenan (PAAm-g-CG) and sodium alginate (SA) | Ketoprofen | [78] | In vitro, Release of drug into rat stomach. |
| Spherical magnetic | Pectin-chitosan | Diclofenac sodium (DS) | [79] | In vitro, Magnetically guided targeted drug delivery SGF. |
| Nanoparticles | Polybenzofulvene derivative (poly-6-MOEG-9-BF3k) | Leuprolide | [80] | In vitro, Drug delivery on epithelial cell; 16 HBE). In vivo, Effect of drug on male Winstar rats. |
| Tablets | Acetylated moth bean starch (AMBS) | Lamivudine | [81] | In vitro, Evaluation of controlled release of drug in stomach of white albino rabbits. |
| Nanoporous peptide particles | Polypeptide poly(l-glutamic acid) (PGA) | Brain-derived neurotrophic factor (BDNF) | [82] | In vitro, Release of drug in SGF. |
| Hydrogel | Conjugated linoleic acid coupled with pluronic F-127 (Plu-CLA) | Docetaxel | [83] | In vitro, Release of drug into human gastric cancer cells. In vivo, Anti-tumor effect on peritoneal gastric cancer metastasis in male BALB/c nude mice. |
| Nanoparticles | CpG-loaded onto poly(l-glutamic acid) (PGA) | Oligonucleotid CpG | [84] | In vitro, Release of drug into peripheral blood mononuclear cells (PBMC’s). |
| Microparticles | Trimethyl-chitosan (TMC)-PEG-PEGDMA-MAA | Interferon-β(INF-β) | [85] | In vitro, Drug release in SGF. In vivo, Effect of drug in rabbits. |
| Nanoparticle (SLN) | Glyceryl monostereate-Tween 80 | Efavirenz (EFV) | [86] | In vitro, Release of EFV in SGF. |
| Nanospheres | Alginate-silica | Indomethacin (IND) | [87] | In vitro, Drug delivery in SGF. |
| Particles | PLGA-PEG | Vascular endothelial growth factor (VEGF) | [88] | In vitro, Release of drug in SGF. |
| Hydrogel (IPN) | Poly(aspartic acid); KPAsp Carboxymethyl chitosan | Salicylic acid | [89] | In vitro, Release of drug in SGF. |
| Film | Tamarind Seed Polysaccharide (TSP)-β (1→4)-d-glucan mostly | Nystatin | [90] | In vitro, Release of drug in SGF. |
| Nanoparticles | Poly(vinylpyrrolidone) (PVP) | Ketoprofen | [91] | In vitro, Drug delivery into a PVP matrix of spatially confined microcontainers. |
| Nanoparticles | Acetylated corn starch | Ciprofloxacin (CFx) | [92] | In vitro, Release of drug in SGF. |
| Enhance modification by FP | Biomolecule | Enhancement action | References | Application trials |
|---|---|---|---|---|
| Monomeric and dendrimeric tetrabranched form by residue substitution | Synthetic antibacterial peptide | Stability to blood proteases | [99] | In vitro, Antimicrobial activity against a panel of gram-negative bacteria |
| Substitution of two polycationic lipophilic-core carbohydrate-based dendrons 2a-b and five polycationic lipophilic-core peptide dendrons 3–6, containing aminoacid terminal residues | Heparin (LMWH) | Absorption molecule in intestinal trials | [100] | In vitro, Absorption in simulated intestine. In vivo, Effect of drug in LMHW rats. |
| PAMAM and PPI dendrimers by introducing functional groups | Methotre-xate sodium | Stability of molecule | [101] | In vitro, Release of molecule in simulated gastric fluids. |
| The 3483 Da peptide glucagon PEGylated to amino acid residue Lys12 (gluc-PEG-L) with branched PEG chain of 2200 Da (gluc-PEG-B) | Glucagon | Increase in adsorbing per unit surface area rate | [102] | In vitro, Release of molecule in simulated gastric fluids. |
| Functionalized nanoliposomes with synthetic coupling of the peptide (Lys-Val-Leu-Phe-Leu-Ser) | Ligand-functionalized nanoliposomes | Absorption of molecule | [103] | In vitro, Release in SGF. In vivo, Effect on neuronal cell in rats. |
| PEGylated derivatives of Bac7 (1-35) | Peptide Bac7(1-35) | Protective effect and stabilization | [104] | In vitro, Protective action against S. typhimurium |
| Poly(ethylene glycol)-l-asparaginase (PEG–ASNase), Poly(N,N-dimethylaminoethyl methacrylate) (PAMA), PEG-b-PAMA | l-Asparaginase (l-Asn) | Stabilization of molecule | [105] | In vitro, Protective degradation against intestinal enzymes. |
| 12 triterpenic PEGylated amine derivatives | Oleanolic and maslinic acids | Cytotoxicity of molecule | [106] | In vitro, Apoptotic effects on cancer-cell lines (B16single bondF10, HT29, and Hep G2) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, Y.A.; Urista, C.M.; Martínez, J.I.; Nava, M.D.C.D.; Rodríguez, F.A.R. Functionalized Polymers for Enhance Oral Bioavailability of Sensitive Molecules. Polymers 2016, 8, 214. https://doi.org/10.3390/polym8060214
Pérez YA, Urista CM, Martínez JI, Nava MDCD, Rodríguez FAR. Functionalized Polymers for Enhance Oral Bioavailability of Sensitive Molecules. Polymers. 2016; 8(6):214. https://doi.org/10.3390/polym8060214
Chicago/Turabian StylePérez, Yolanda Alvarado, Claudia Muro Urista, Javier Illescas Martínez, María Del Carmen Díaz Nava, and Francisco A. Riera Rodríguez. 2016. "Functionalized Polymers for Enhance Oral Bioavailability of Sensitive Molecules" Polymers 8, no. 6: 214. https://doi.org/10.3390/polym8060214
APA StylePérez, Y. A., Urista, C. M., Martínez, J. I., Nava, M. D. C. D., & Rodríguez, F. A. R. (2016). Functionalized Polymers for Enhance Oral Bioavailability of Sensitive Molecules. Polymers, 8(6), 214. https://doi.org/10.3390/polym8060214