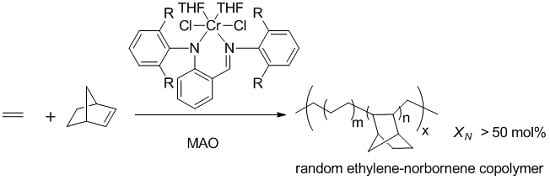
Homo- and Copolymerization of Ethylene and Norbornene with Anilido–Imine Chromium Catalysts
Abstract
:1. Introduction
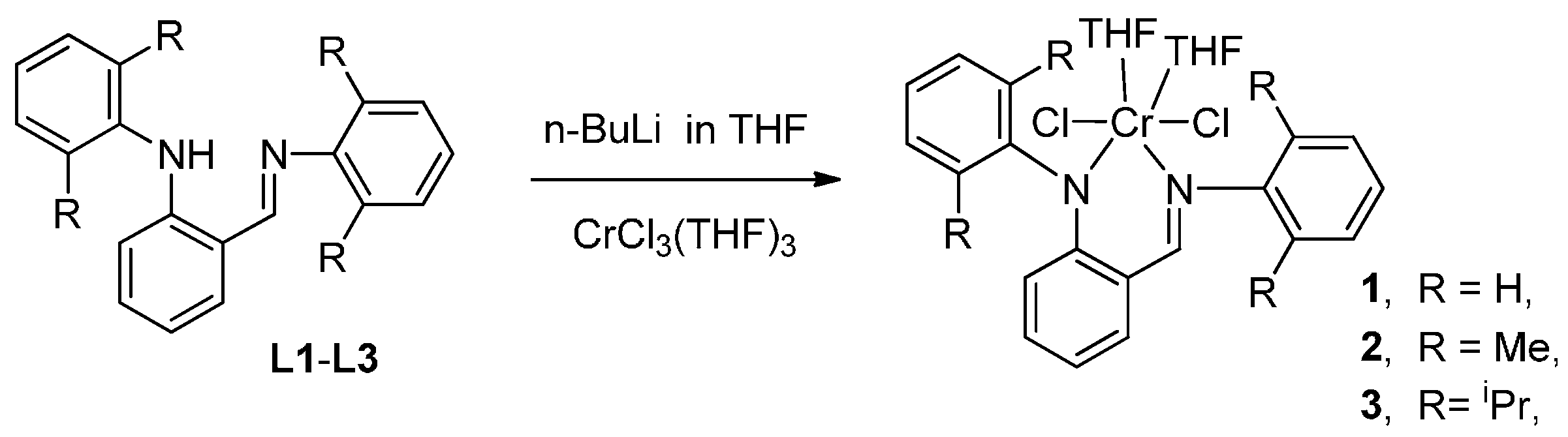
2. Experimental Section
2.1. Materials
2.2. Measurement
2.3. Norbornene Polymerization
2.4. Atmosphere Pressure Polymerization of Ethylene and Copolymerization
2.5. High Pressure Polymerization of Ethylene and Copolymerization
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaminsky, W.; Engehause, R.; Kopf, J. A tailor-made metallocene for the copolymerization of ethene with bulky cycloalkenes. Angew. Chem. Int. Ed. 1995, 34, 2273–2275. [Google Scholar] [CrossRef]
- Rische, T.; Waddon, A.J.; Dickinson, L.C.; MacKnight, W.J. Microstructure and morphology of cycloolefin copolymers. Macromolecules 1998, 31, 1871–1874. [Google Scholar] [CrossRef]
- Kaminsky, W. Olefin polymerization catalyzed by metallocenes. Adv. Catal. 2001, 46, 89–159. [Google Scholar]
- Kaminsky, W.; Beulich, I.; Arndt-Rosenau, M. Copolymerization of ethene with cyclic and other sterically hindered olefins. Macromol. Symp. 2001, 173, 211–226. [Google Scholar] [CrossRef]
- Kaminsky, W.; Bark, A.; Arndt, M. New polymers by homogenous zirconocene/aluminoxane catalysts. Makromol. Chem. Macromol. Symp. 1991, 47, 83–93. [Google Scholar] [CrossRef]
- Kaminsky, W.; Bark, A.; Steiger, R. Stereospecific polymerization by metallocene/aluminoxane catalysts. J. Mol. Catal. 1992, 74, 109–119. [Google Scholar] [CrossRef]
- Kaminsky, W.; Noll, A. Copolymerization of norbornene and ethene with homogenous zirconocenes/methylaluminoxane catalysts. Polym. Bull. 1993, 31, 175–182. [Google Scholar] [CrossRef]
- Ruchats, D.; Fink, G. Ethene-norbornene copolymerization using homogenous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 2. Comparative study of different metallocene-and half-sandwich/methylaluminoxane catalysts and analysis of the copolymers by 13C nuclear magnetic resonance spectroscopy. Macromolecules 1998, 31, 4674–4680. [Google Scholar]
- Ruchats, D.; Fink, G. Ethene-norbornene copolymerization with homogeneous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 3. Copolymerization parameters and copolymerization diagrams. Macromolecules 1998, 31, 4681–4683. [Google Scholar] [CrossRef] [PubMed]
- Ruchats, D.; Fink, G. Ethene-norbornene copolymerization with homogeneous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 4. Development of molecular weights. Macromolecules 1998, 31, 4684–4686. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, C.H.; Sperlich, B.R.; Ruotoistenmaki, J.; Seppala, J.V. Investigation of the microstructure of metallocene-catalyzed norbornene–ethylene copolymers using NMR spectroscopy. J. Polym. Sci A Polym. Chem. 1998, 36, 1633–1638. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kim, Y.H.; Won, Y.C.; Han, J.W.; Suh, W.H.; Lee, I.S.; Chung, Y.K.; Song, K.H. Synthesis of [2,2’-methylenebis (1,3-dimethylcyclopentadienyl)] zirconium dichloride and its reactivity in ethylene-norbornene copolymerization. Organometallics 2002, 21, 1500–1503. [Google Scholar] [CrossRef]
- Ruchats, D.; Fink, G. Ethene–norbornene copolymerizations using two different homogeneous metallocene catalyst systems and investigations of the copolymer microstructure. J. Mol. Catal. A Chem. 2003, 203, 101–111. [Google Scholar]
- Yoshida, Y.; Saito, J.; Mitani, M.; Takagi, Y.; Matsui, S.; Ishii, S.; Nakano, T.; Kashiwa, N.; Fujita, T. Living ethylene/norbornene copolymerisation catalyzed by titanium complexes having two pyrrolide-imine chelate ligands. Chem. Commun. 2002, 1298–1299. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mohri, J.; Ishii, S.; Mitani, M.; Saito, J.; Matsui, S.; Makio, H.; Nakano, T.; Tanaka, H.; Onda, M.; et al. Living copolymerization of ethylene with norbornene catalyzed by bis(pyrrolide-imine) titanium complexes with MAO. J. Am. Chem. Soc. 2004, 126, 12023–12032. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.W.; Sun, W.H.; Zhang, S.; Hao, P.; Shiga, A. Highly active ethylene polymerization and copolymerization with norbornene using bis (imino-indolide) titanium dichloride–MAO system. J. Polym. Sci. A Polym. Chem. 2007, 45, 3415–3430. [Google Scholar] [CrossRef]
- Li, X.F.; Dai, K.; Ye, W.P.; Pan, L.; Li, Y.S. New titanium complexes with two β-enaminoketonato chelate ligands: Syntheses, structures, and olefin polymerization activities. Organometallics 2004, 23, 1223–1230. [Google Scholar] [CrossRef]
- Vijayakrishna, K.; Sundararajan, G. Non-Cp type titanium precatalysts for ethylene/norbornene copolymerization. Polymer 2006, 47, 8289–8296. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.L.; Guo, Y.H.; Gao, Y.; Liu, B.; Ma, Z.; Xia, W.; Shi, L.P.; Tang, Y. Novel titanium catalysts bearing an [O, N, S] rridentate ligand for ethylene homo- and copolymerization. Macromol. Rapid. Commun. 2005, 26, 1609–1614. [Google Scholar] [CrossRef]
- Gao, M.L.; Wang, C.; Sun, X.L.; Qian, C.T.; Ma, Z.; Bu, S.Z.; Tang, Y.; Xie, Z.W. Ethylene-norbornene copolymerization by new titanium complexes bearing tridentate ligands. Sidearm effects on catalytic activity. Macromol. Rapid Commun. 2007, 28, 1511–1516. [Google Scholar] [CrossRef]
- Li, Y.F.; Gao, H.Y.; Wu, Q. Homo- and copolymerization of ethylene and norbornene with bis(β-diketiminato) titanium complexes activated with methylaluminoxane. J. Polym. Sci. A Polym. Chem. 2008, 46, 93–101. [Google Scholar] [CrossRef]
- Hu, H.; Gao, H.Y.; Song, K.M.; Liu, F.S.; Long, J.M.; Zhang, L.; Zhu, F.M.; Wu, Q. Novel bis(benzoin) titanium catalyst for homo-and copolymerization of norbornene with ethylene: Synthesis, characterization and catalytic properties. Polymer 2008, 49, 4552–4558. [Google Scholar] [CrossRef]
- Gao, H.Y.; Hu, H.; Wu, Q. High norbornene incorporation in ethylene-norbornene copolymerization with a bis(α-alkyloxoimine) titanium-MAO catalyst. Sci. China Chem. 2010, 53, 1634–1640. [Google Scholar] [CrossRef]
- Benedikt, G.M.; Elce, E.; Goodall, B.L.; Kalamarides, H.A.; McIntosh, L.H., III; Rhodes, L.F.; Selvy, K.T.; Andes, C.; Oyler, K.; Sen, A. Copolymerization of ethene with norbornene derivatives using neutral nickel catalysts. Macromolecules 2002, 35, 8978–8988. [Google Scholar] [CrossRef]
- Gao, H.Y.; Liu, Y.; Li, G.L.; Xiao, Z.F.; Liang, G.D.; Wu, Q. Catalytic synthesis of polyethylene-block-polynorbornene copolymers using a living polymerization nickel catalyst. Polym. Chem. 2014, 5, 6012–6018. [Google Scholar] [CrossRef]
- Kiesewetter, J.; Kaminsky, W. Ethene/Norbornene copolymerization with palladium (II) α-diimine catalysts: From ligand screening to discrete catalyst species. Chem. Eur. J. 2003, 9, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, J.; Arikan, B.; Kaminsky, W. Copolymerization of ethene with norbornene using palladium (II) α-diimine catalysts: Influence of feed composition, polymerization temperature, and ligand structure on copolymer properties and microstructure. Polymer 2006, 47, 3302–3314. [Google Scholar] [CrossRef]
- Peucker, U.; Heitz, W. Vinylic polymerization by homogeneous chromium (III) catalysts. Macromol. Rapid Commun. 1998, 19, 159–162. [Google Scholar] [CrossRef]
- Peucker, U.; Heitz, W. Vinylic polymerization and copolymerization of norbornene and ethene by homogeneous chromium (III) catalysts. Macromol. Chem. Phys. 2001, 202, 1289–1297. [Google Scholar] [CrossRef]
- Woodman, T.J.; Sarazin, Y.; Garratt, S.; Fink, G.; Bochmann, M. Chromium allyl and alkyl catalysts for the vinyl polymerization of norbornene and ethylene–norbornene copolymerizations. J. Mol. Catal. A Chem. 2005, 235, 88–97. [Google Scholar] [CrossRef]
- Gao, H.; Guo, W.; Bao, F.; Gui, G.; Zhang, J.; Zhu, F.; Wu, Q. Synthesis, molecular structure, and solution-dependent behavior of nickel complexes chelating anilido-imine donors and their catalytic activity toward olefin polymerization. Organometallcs 2004, 23, 6273–6280. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, J.; Chen, Y.; Zhu, F.; Wu, Q. Vinyl-polymerization of norbornene with novel anilido–imino nickel complexes/methylaluminoxane: Abnormal influence of polymerization temperature on molecular weight of polynorbornenes. J. Mol. Catal. A Chem. 2005, 240, 178–185. [Google Scholar] [CrossRef]
- Gao, H.; Chen, Y.; Zhu, F.; Wu, Q. Copolymerization of norbornene and styrene catalyzed by a novel anilido–imino nickel complex/methylaluminoxane system. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5237–5246. [Google Scholar] [CrossRef]
- Gao, H.; Ke, Z.; Pei, L.; Song, K.; Wu, Q. Drastic ligand electronic effect on anilido–imino nickel catalysts toward ethylene polymerization. Polymer 2007, 48, 7249–7254. [Google Scholar] [CrossRef]
- Gao, H.; Pei, L.; Song, K.; Wu, Q. Styrene polymerization with novel anilido–imino nickel complexes/MAO catalytic system: Catalytic behavior, microstructure of polystyrene and polymerization mechanism. Eur. Polym. J. 2007, 43, 908–914. [Google Scholar] [CrossRef]
- Gao, H.; Pei, L.; Li, Y.; Zhang, J.; Wu, Q. Vinyl polymerization of norbornene with nickel catalysts bearing [N, N] six-membered chelate ring: Important influence of ligand structure on activity. J. Mol. Catal. A Chem. 2008, 280, 81–86. [Google Scholar] [CrossRef]
- Hayes, P.G.; Welch, G.C.; Emslie, D.J.; Noack, C.L.; Piers, W.E.; Parvez, M. A new chelating anilido-imine donor related to β-diketiminato ligands for stabilization of organoyttrium cations. Organometallics 2003, 22, 1577–1579. [Google Scholar] [CrossRef]
- Wang, H.Y.; Meng, X.; Jin, G.X. Synthesis, molecular structure and norbornene polymerization behavior of three-coordinate nickel (I) complexes with chelating anilido-imine ligands. Dalton Trans. 2006, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; An, H.; Gao, W.; Mu, Y. Chromium (III) complexes with chelating anilido–imine ligands: Synthesis, structures, and catalytic properties for ethylene polymerization. Eur. J. Inorg. Chem. 2010, 3360–3364. [Google Scholar] [CrossRef]
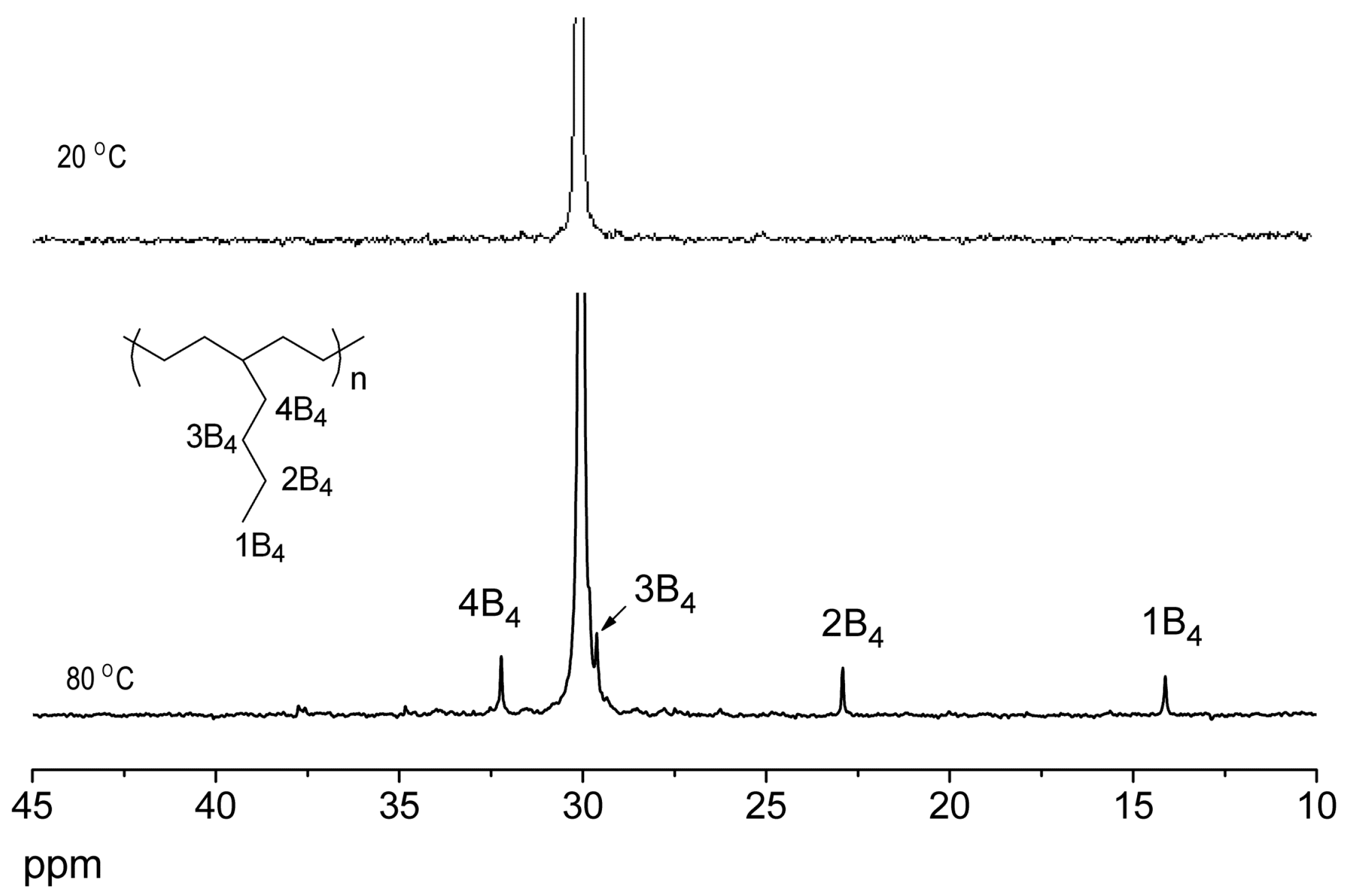
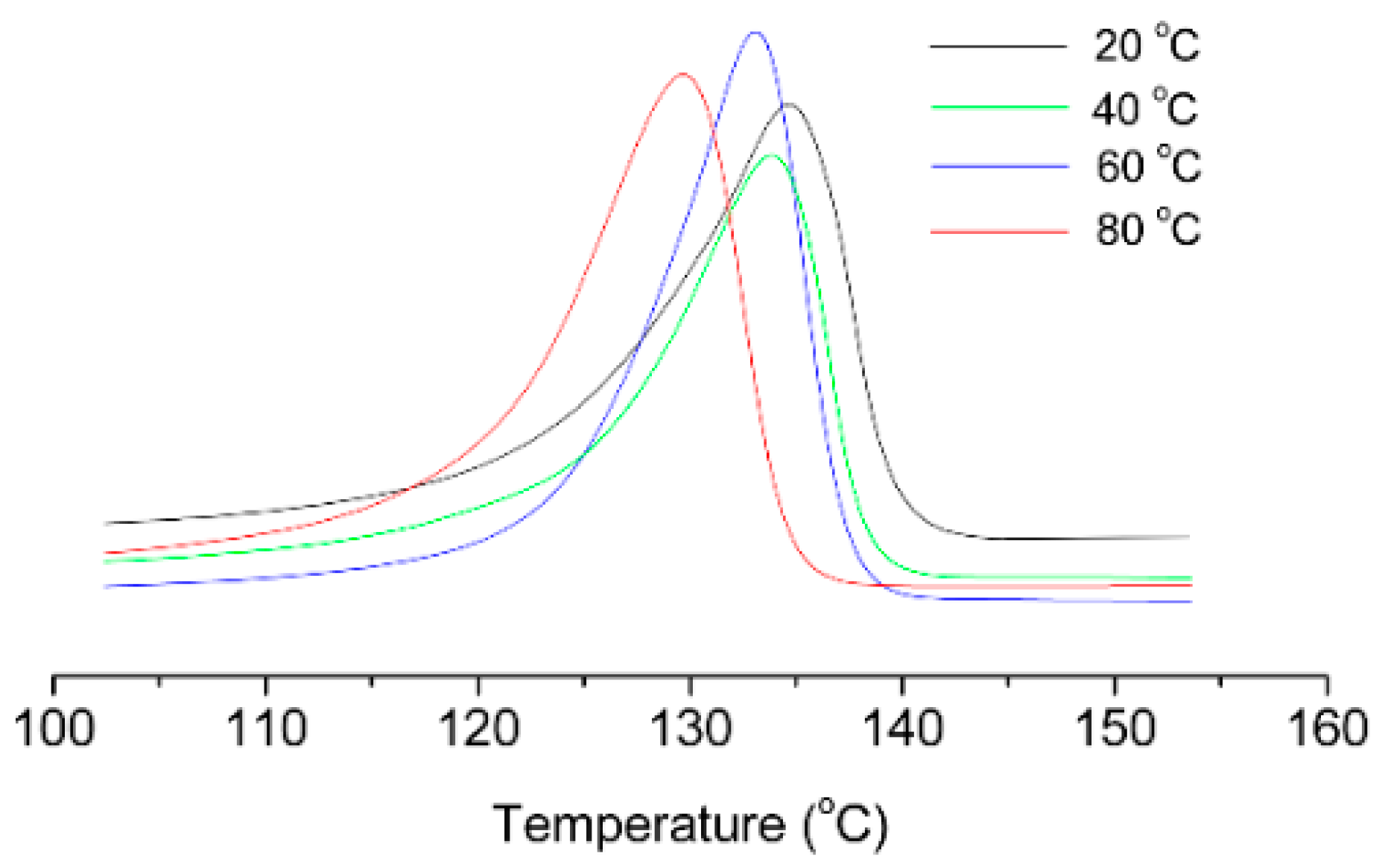
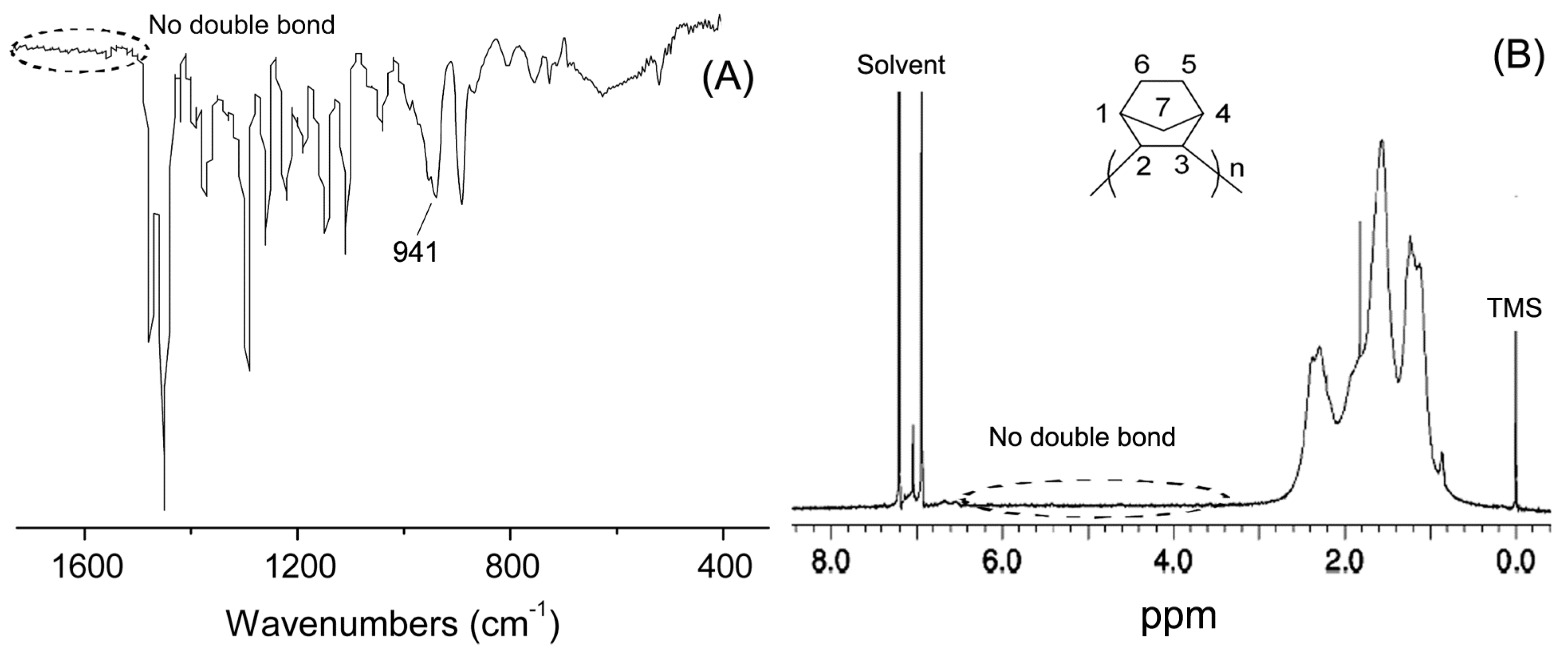

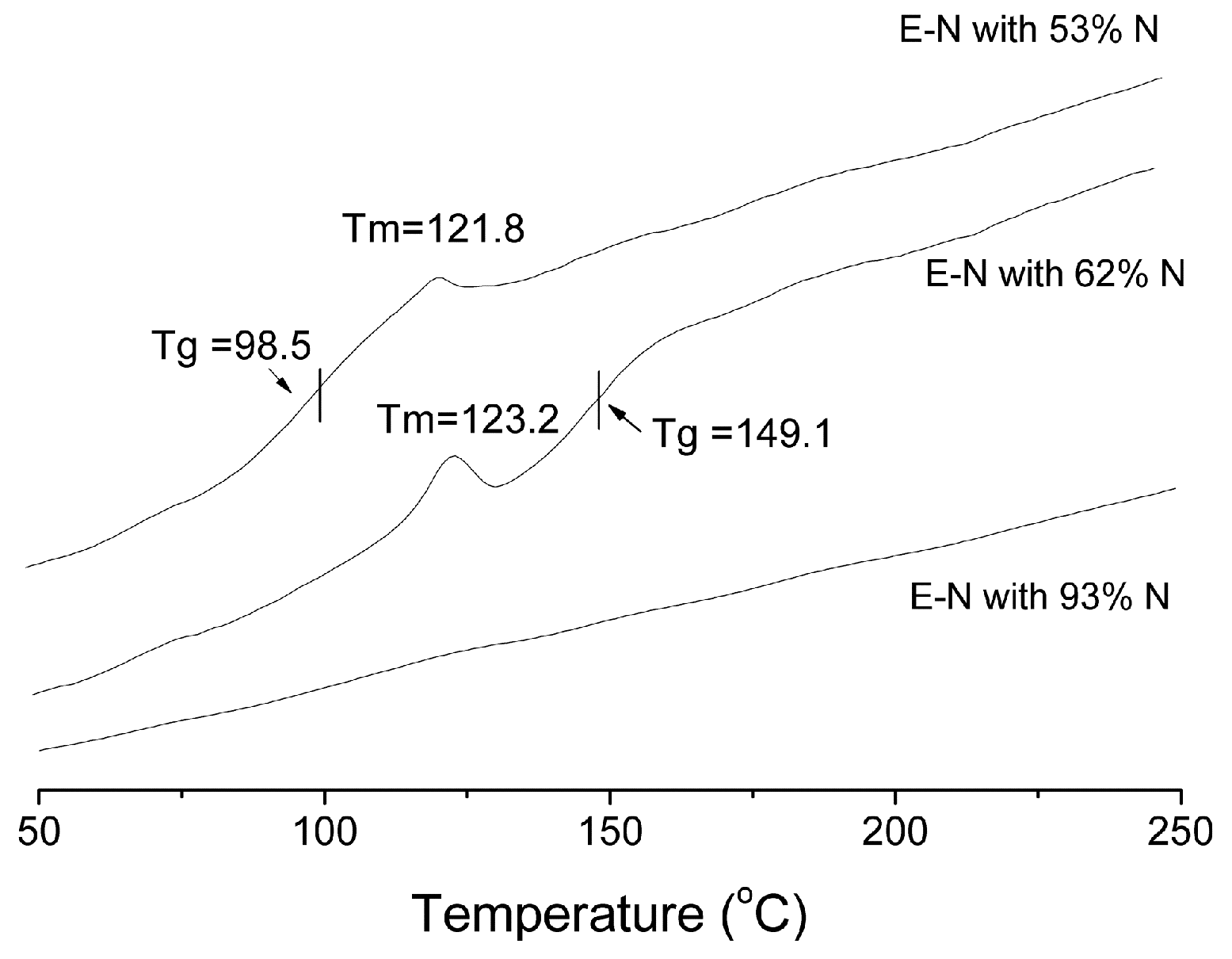
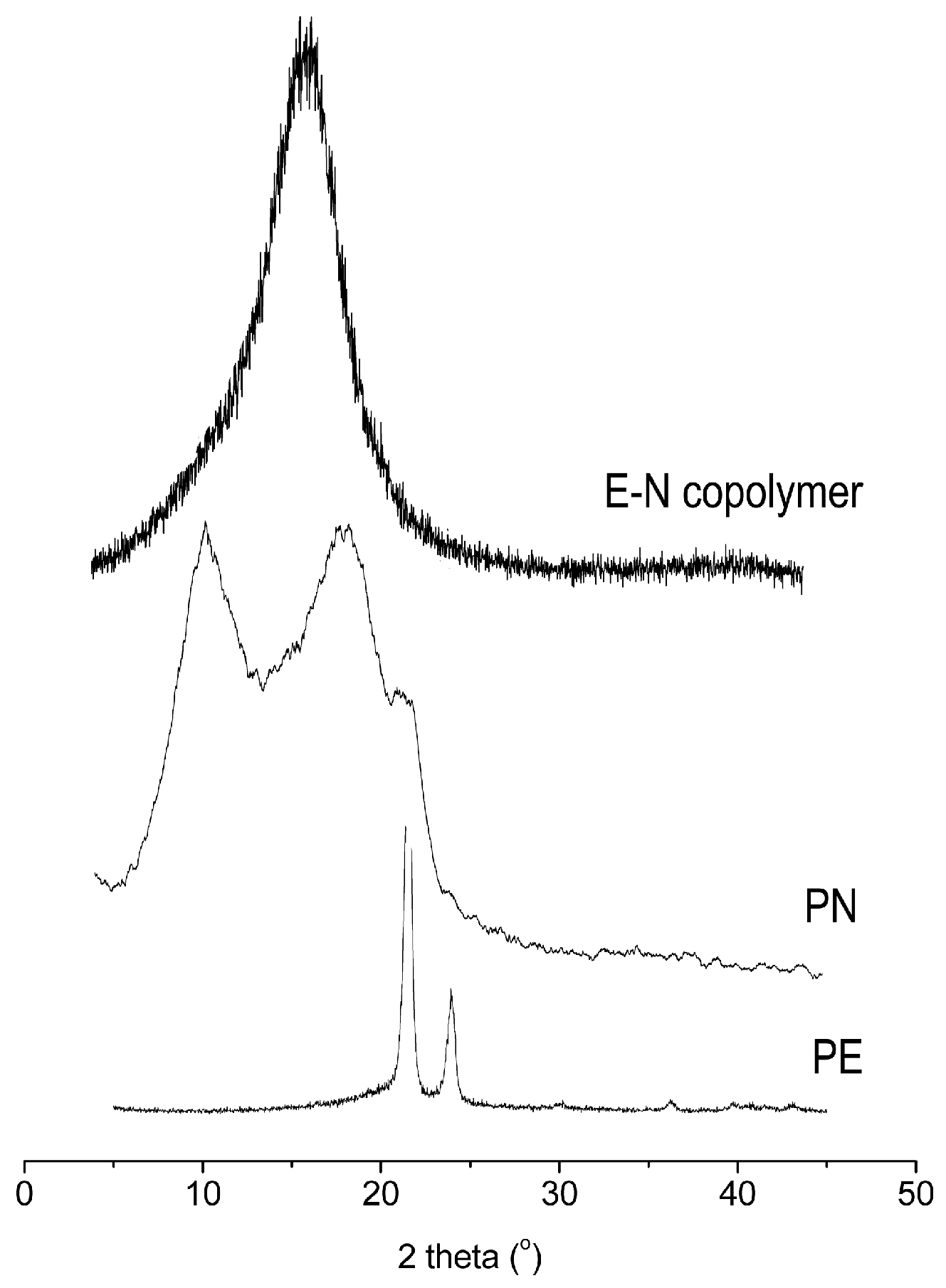
| Entry | Cat. | Tp (°C) | Yield (g) | Activity (kg PE (mol·Cr·h)−1) | Mw a (kg·mol−1) | Mw/Mn a | Tm b (°C) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 20 | 0.34 | 34 | 762 | 3.54 | 134.7 |
| 2 | 1 | 40 | 0.46 | 46 | 667 | 2.13 | 133.8 |
| 3 | 1 | 60 | 0.37 | 37 | 444 | 2.46 | 133.1 |
| 4 | 1 | 80 | 0.22 | 22 | 305 | 2.52 | 129.1 |
| 5 | 2 | 40 | 0.68 | 68 | 845 | 1.95 | 132.9 |
| 6 | 3 | 40 | 1.86 | 186 | 1,020 | 1.88 | 133.6 |
| Entry | Cat. | Tp (°C) | Yield (g) | Activity (kg PN (mol·Cr·h)−1) | Mw a (kg·mol−1) | Mw/Mn a |
|---|---|---|---|---|---|---|
| 1 | 1 | 20 | 1.12 | 11.2 | 25.7 | 1.56 |
| 2 | 1 | 40 | 1.70 | 170 | 17.3 | 1.78 |
| 3 | 1 | 60 | 1.74 | 174 | 13.6 | 1.94 |
| 4 | 1 | 80 | 1.14 | 114 | 7.5 | 1.70 |
| 5 | 2 | 60 | 1.21 | 121 | 10.6 | 1.95 |
| 6 | 3 | 60 | 0.75 | 75 | 8.2 | 1.87 |
| Entry | Cat. | Tp (°C) | PE (atm) | Yield (g) | Activity a | Mw b (kg mol−1) | Mw/Mn b | Incorp.N c mol % |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 20 | 0.5 | 0.95 | 95 | 32.5 | 2.14 | 87 |
| 2 | 2 | 20 | 0.5 | 0.74 | 74 | 18.4 | 2.32 | 84 |
| 3 | 3 | 20 | 0.5 | 0.40 | 40 | 15.3 | 2.47 | 81 |
| 4 | 1 | 60 | 0.5 | 1.18 | 118 | 16.2 | 2.56 | 93 |
| 5 | 1 | 20 | 10 | 1.89 | 189 | 37.1 | 2.94 | 62 |
| 6 | 1 | 40 | 10 | 3.05 | 305 | 37.6 | 2.85 | 71 |
| 7 | 1 | 60 | 10 | 4.08 | 408 | 38.9 | 3.08 | 78 |
| 8 | 1 | 60 | 20 | 4.65 | 465 | 59.4 | 3.21 | 53 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, L.; Tang, Y.; Gao, H. Homo- and Copolymerization of Ethylene and Norbornene with Anilido–Imine Chromium Catalysts. Polymers 2016, 8, 69. https://doi.org/10.3390/polym8030069
Pei L, Tang Y, Gao H. Homo- and Copolymerization of Ethylene and Norbornene with Anilido–Imine Chromium Catalysts. Polymers. 2016; 8(3):69. https://doi.org/10.3390/polym8030069
Chicago/Turabian StylePei, Lixia, Yong Tang, and Haiyang Gao. 2016. "Homo- and Copolymerization of Ethylene and Norbornene with Anilido–Imine Chromium Catalysts" Polymers 8, no. 3: 69. https://doi.org/10.3390/polym8030069
APA StylePei, L., Tang, Y., & Gao, H. (2016). Homo- and Copolymerization of Ethylene and Norbornene with Anilido–Imine Chromium Catalysts. Polymers, 8(3), 69. https://doi.org/10.3390/polym8030069