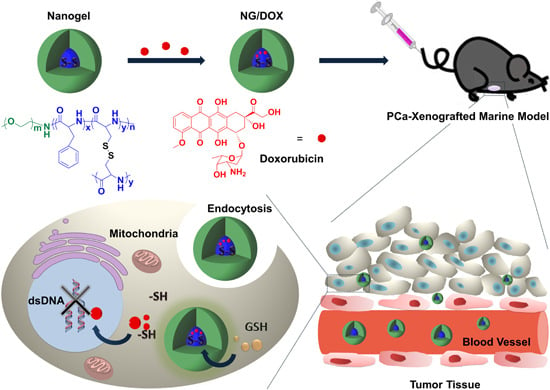
l-Cystine-Crosslinked Polypeptide Nanogel as a Reduction-Responsive Excipient for Prostate Cancer Chemotherapy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials

2.2. DOX Encapsulation
2.3. Characterizations
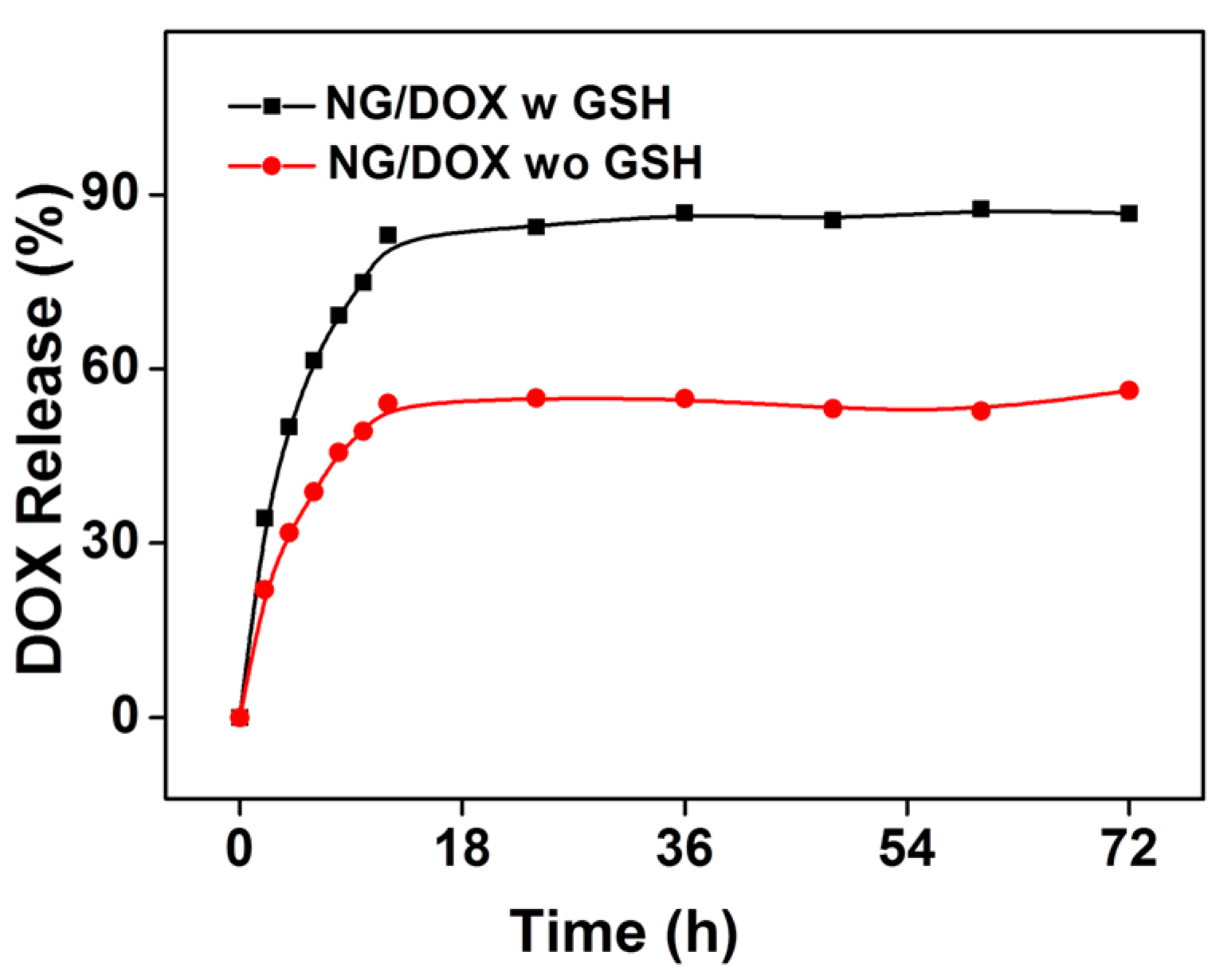
2.4. In Vitro DOX Release
2.5. Cell and Animal Proposals
2.6. In Vitro Cell Viability Assessment of NG/DOX
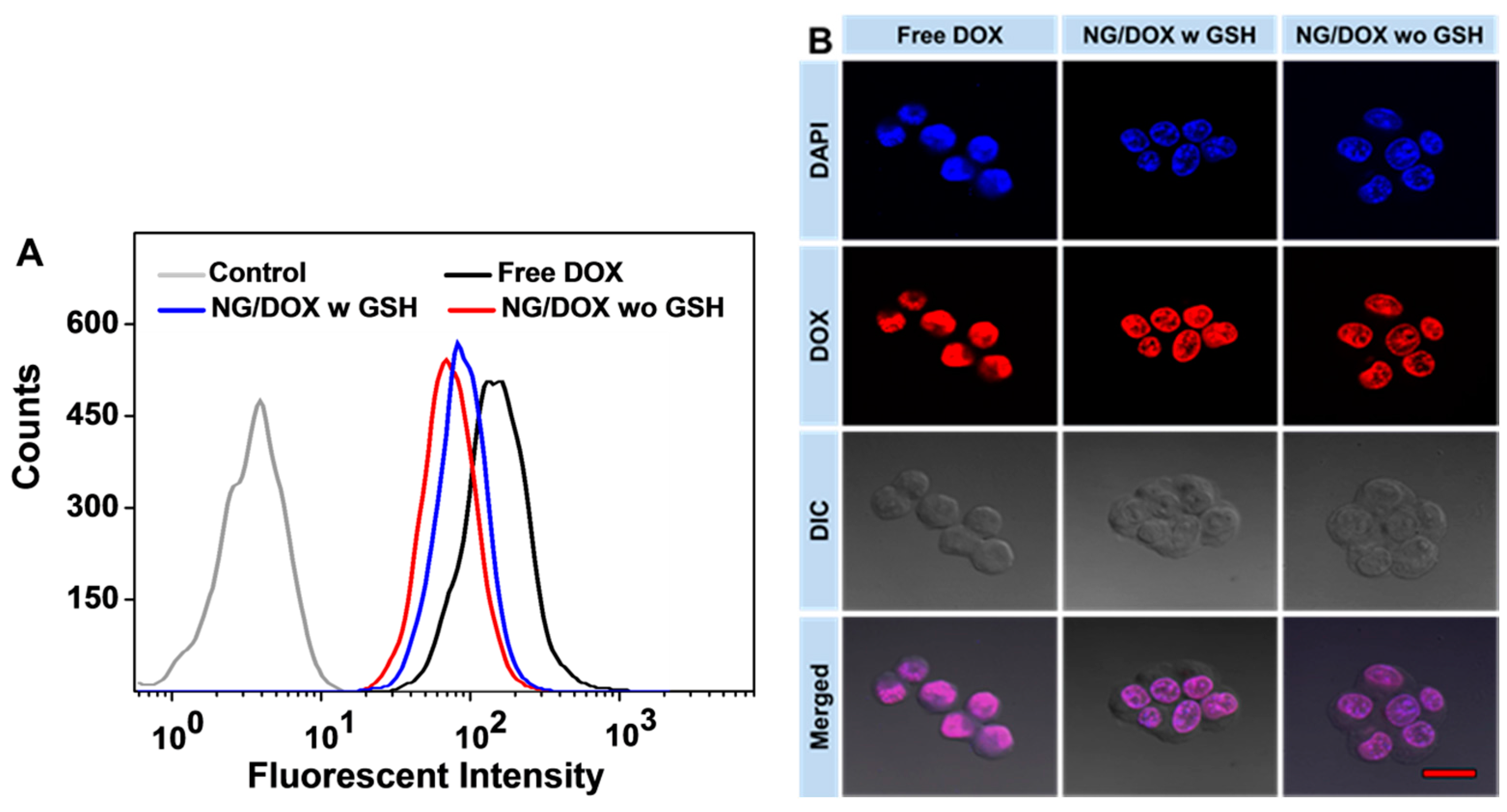
2.7. Intracellular DOX Release
2.8. In Vivo Antitumor Efficacy Assays
2.9. Histopathological Assessments
2.10. Statistical Analyses
3. Results and Discussion
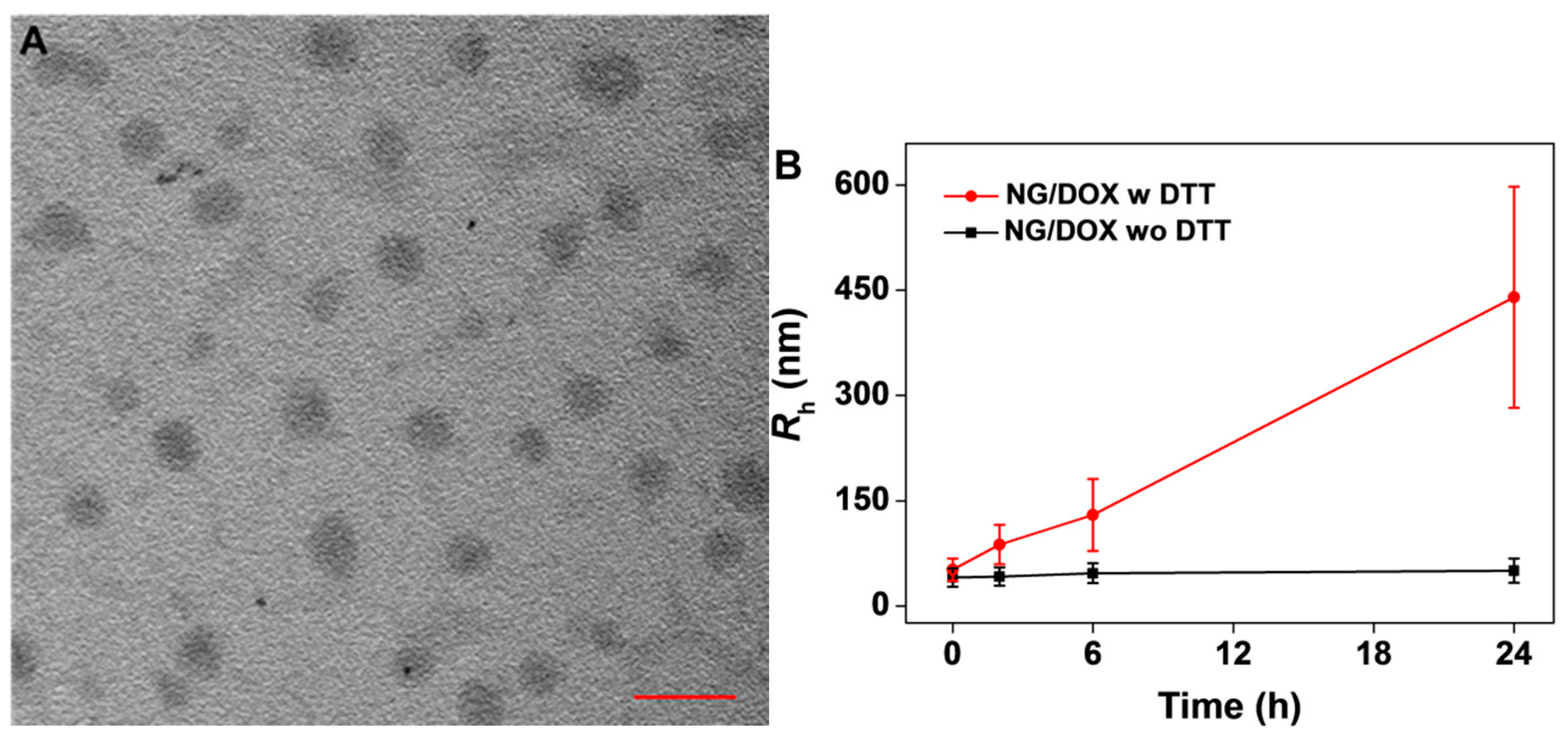
3.1. Preparation and Characterizations of NG/DOX

3.2. Enhanced In Vitro Cellular Proliferation Inhibition and Accelerated Intracellular DOX Release of NG/DOX

3.3. Upregulated In Vivo Efficacy and Security of NG/DOX
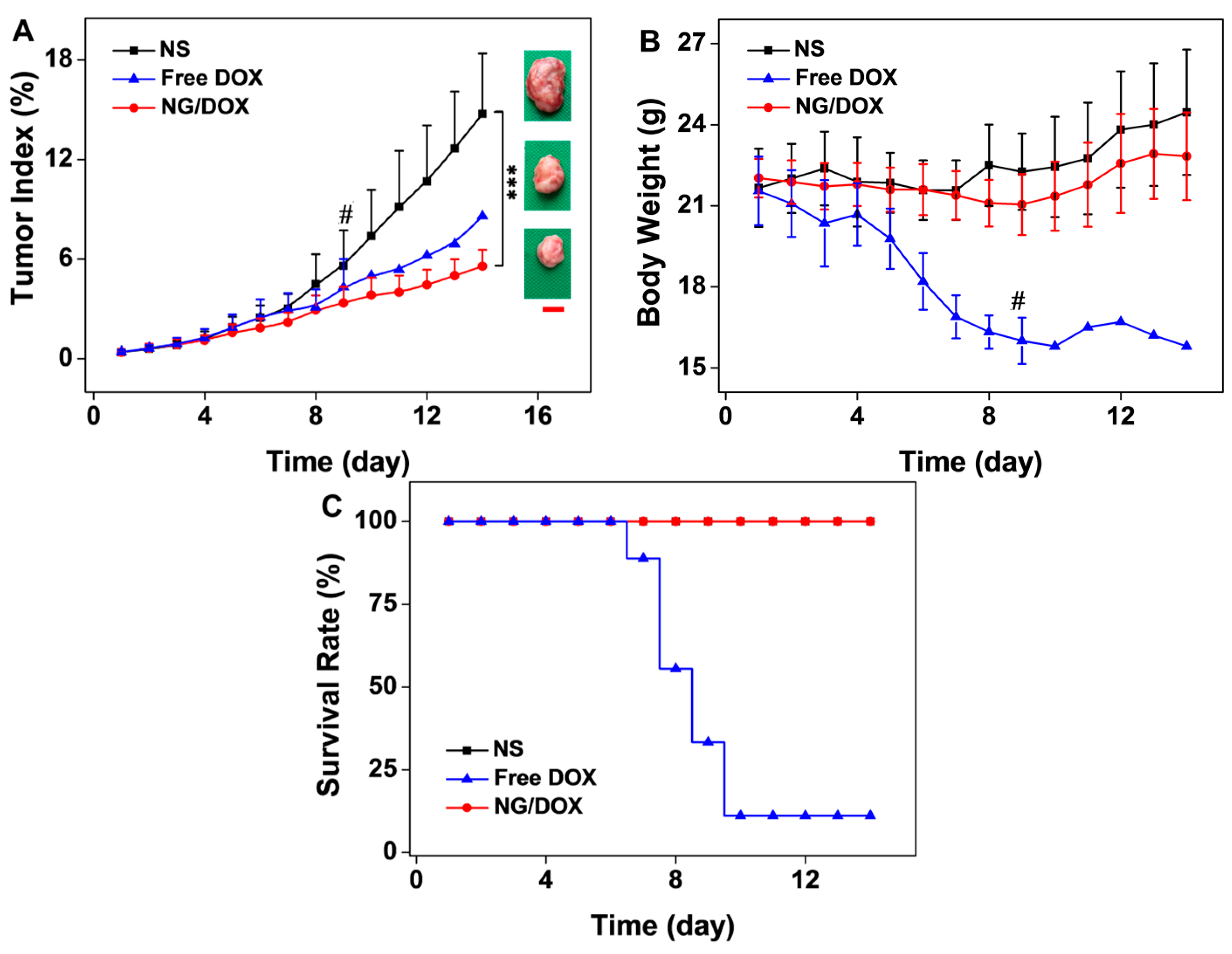

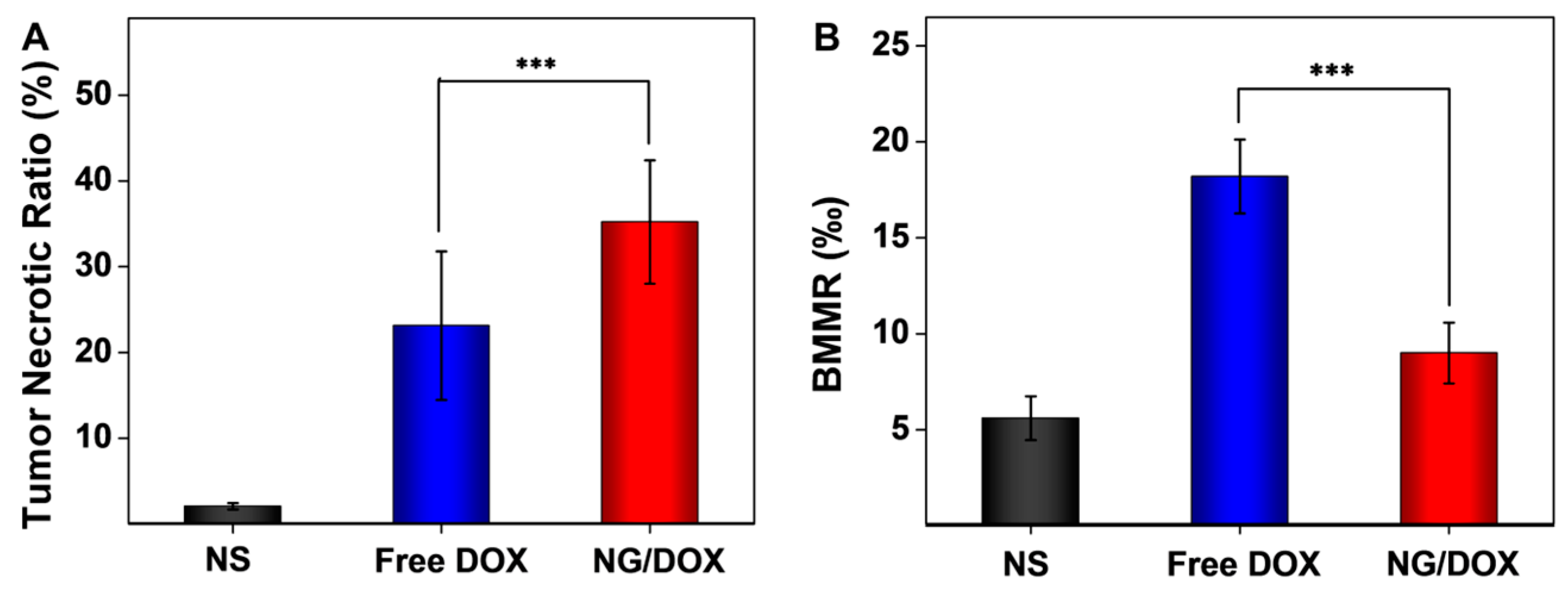
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Peabody, J.; Kapoor, V.; Sammon, J.; Rogers, C.; Stricker, H.; Lane, Z.; Gupta, N.; Bhandari, M.; Menon, M. Oncologic outcomes at 10 years following robotic radical prostatectomy. Eur. Urol. 2015, 67, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.E.; Alsadius, D.; Pettersson, N.; Tucker, S.L.; Wilderang, U.; Johansson, K.A.; Steineck, G. Patient-reported sexual toxicity after radiation therapy in long-term prostate cancer survivors. Br. J. Cancer 2015, 113, 802–808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katzenwadel, A.; Wolf, P. Androgen deprivation of prostate cancer: Leading to a therapeutic dead end. Cancer Lett. 2015, 367, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xiao, C.; Li, Y.; Cheng, Y.; Wang, N.; He, C.; Zhuang, X.; Zhu, X.; Chen, X. Efficacious hepatoma-targeted nanomedicine self-assembled from galactopeptide and doxorubicin driven by two-stage physical interactions. J. Control. Release 2013, 169, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ding, J.; Xiao, C.; Li, L.; Zhuang, X.; Chen, X. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials 2015, 54, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Ding, J.; Lu, S.; Wang, X.; Wang, C.; Chen, X. Cholesterol-enhanced polylactide-based stereocomplex micelle for effective delivery of doxorubicin. Materials 2015, 8, 216–230. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Guo, H.; Ding, J.; Chen, J.; Guan, J.; Wang, C. Selective intracellular drug delivery from pH-responsive polyion complex micelle for enhanced malignancy suppression in vivo. Colloids Surf. B Biointerfaces 2015, 135, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, K.; Xu, W.; Ding, J.; Wang, X.; Liu, T.; Wang, C.; Chen, X. Stereocomplex micelle from nonlinear enantiomeric copolymers efficiently transports antineoplastic drug. Nanoscale Res. Lett. 2015, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xiao, C.; He, C.; Li, M.; Li, D.; Zhuang, X.; Chen, X. Facile preparation of a cationic poly(amino acid) vesicle for potential drug and gene co-delivery. Nanotechnology 2011, 22, 494012. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xiao, C.; Zhuang, X.; He, C.; Chen, X. Direct formation of cationic polypeptide vesicle as potential carrier for drug and gene. Mater. Lett. 2012, 73, 17–20. [Google Scholar] [CrossRef]
- Ding, J.; Zhuang, X.; Xiao, C.; Cheng, Y.; Zhao, L.; He, C.; Tang, Z.; Chen, X. Preparation of photo-cross-linked pH-responsive polypeptide nanogels as potential carriers for controlled drug delivery. J. Mater. Chem. 2011, 21, 11383–11391. [Google Scholar] [CrossRef]
- Ding, J.; Xu, W.; Zhang, Y.; Sun, D.; Xiao, C.; Liu, D.; Zhu, X.; Chen, X. Self-reinforced endocytoses of smart polypeptide nanogels for “on-demand“ drug delivery. J. Control. Release 2013, 172, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Shi, B.; Xu, W.; Ding, J.; Yang, Y.; Liu, H.; Zhuang, X.; Chen, X. Reduction-responsive polypeptide nanogel delivers antitumor drug for improved efficacy and safety. Acta Biomater. 2015, 27, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Ganju, A.; Yallapu, M.M.; Khan, S.; Behrman, S.W.; Chauhan, S.C.; Jaggi, M. Nanoways to overcome docetaxel resistance in prostate cancer. Drug Resist. Updat. 2014, 17, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xue, X.; He, D.; Hsieh, J.T. A prostate cancer-targeted polyarginine-disulfide linked PEI nanocarrier for delivery of microRNA. Cancer Lett. 2015, 365, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Peppicelli, S.; Bianchini, F.; Calorini, L. Extracellular acidity, a “reappreciated“ trait of tumor environment driving malignancy: Perspectives in diagnosis and therapy. Cancer Metastasis Rev. 2014, 33, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, Y.; Zhao, L.; Zhang, C.; Li, Y.; Li, J.; Li, X.; Liu, Y. Enhanced antitumor efficacy by cyclic RGDyK-conjugated and paclitaxel-loaded pH-responsive polymeric micelles. Acta Biomater. 2015, 23, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, X.; Mao, H.; Wu, W.; Liu, B.; Jiang, X. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat. Commun. 2015, 6, 5834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, P. Reduction-responsive core-shell-corona micelles based on triblock copolymers: Novel synthetic strategy, characterization, and application as a tumor microenvironment-responsive drug delivery system. ACS Appl. Mater. Interfaces 2015, 7, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M. Redox-responsive drug delivery. Antioxid. Redox Signal. 2014, 21, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Holley, A.K.; Zhao, Y.; st Clair, W.H.; st Clair, D.K. Redox-mediated and ionizing-radiation-induced inflammatory mediators in prostate cancer development and treatment. Antioxid. Redox Signal. 2014, 20, 1481–1500. [Google Scholar] [CrossRef] [PubMed]
- Chaiswing, L.; Bourdeau-Heller, J.M.; Zhong, W.; Oberley, T.D. Characterization of redox state of two human prostate carcinoma cell lines with different degrees of aggressiveness. Free Radic. Biol. Med. 2007, 43, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Shi, F.; Xiao, C.; Lin, L.; Chen, L.; He, C.; Zhuang, X.; Chen, X. One-step preparation of reduction-responsive poly(ethylene glycol)-poly(amino acid)s nanogels as efficient intracellular drug delivery platforms. Polym. Chem. 2011, 2, 2857–2864. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Xu, W.; Ding, J.; Shi, B.; Huang, K.; Zhuang, X.; Chen, X. Glutathione-degradable drug-loaded nanogel effectively and securely suppresses hepatoma in mouse model. Int. J. Nanomed. 2015, 10, 6587–6602. [Google Scholar]
- Leonetti, C.; D’Agnano, I.; Lozupone, F.; Valentini, A.; Geiser, T.; Zon, G.; Calabretta, B.; Citro, G.; Gabriella, Z. Antitumor effect of c-myc antisense phosphorothioate oligodeoxynucleotides on human melanoma cells in vitro and in mice. J. Natl. Cancer Inst. 1996, 88, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, C.; Wang, B.; Wang, H.; Chen, X.; Moehwald, H.; Cui, X. Sonochemical fabrication of dual-targeted redox-responsive smart microcarriers. Acs Appl. Mater. Interfaces 2014, 6, 22166–22173. [Google Scholar] [CrossRef] [PubMed]
- Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Polymeric nanomicelles for sustained delivery of anti-cancer drugs. Mutat. Res. 2014, 768, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Z.; Lin, J.; Zhang, Y.; Lv, S.; Song, W.; Huang, Y.; Chen, X. Synergistic antitumor effects of doxorubicin-loaded carboxymethyl cellulose nanoparticle in combination with endostar for effective treatment of non-small-cell lung cancer. Adv. Healthc. Mater. 2014, 3, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Li, D.; Wang, Z.; Xu, W.; Wang, J.; Guo, H.; Wang, C.; Ding, J. l-Cystine-Crosslinked Polypeptide Nanogel as a Reduction-Responsive Excipient for Prostate Cancer Chemotherapy. Polymers 2016, 8, 36. https://doi.org/10.3390/polym8020036
He L, Li D, Wang Z, Xu W, Wang J, Guo H, Wang C, Ding J. l-Cystine-Crosslinked Polypeptide Nanogel as a Reduction-Responsive Excipient for Prostate Cancer Chemotherapy. Polymers. 2016; 8(2):36. https://doi.org/10.3390/polym8020036
Chicago/Turabian StyleHe, Liang, Di Li, Zhongtang Wang, Weiguo Xu, Jixue Wang, Hui Guo, Chunxi Wang, and Jianxun Ding. 2016. "l-Cystine-Crosslinked Polypeptide Nanogel as a Reduction-Responsive Excipient for Prostate Cancer Chemotherapy" Polymers 8, no. 2: 36. https://doi.org/10.3390/polym8020036
APA StyleHe, L., Li, D., Wang, Z., Xu, W., Wang, J., Guo, H., Wang, C., & Ding, J. (2016). l-Cystine-Crosslinked Polypeptide Nanogel as a Reduction-Responsive Excipient for Prostate Cancer Chemotherapy. Polymers, 8(2), 36. https://doi.org/10.3390/polym8020036