Effect of Saponification Condition on the Morphology and Diameter of the Electrospun Poly(vinyl acetate) Nanofibers for the Fabrication of Poly(vinyl alcohol) Nanofiber Mats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Poly(vinyl acetate) (PVAc) by Suspension Polymerization of vinyl acetate (VAc)
2.3. Electrospinning of PVAc Nanofibers
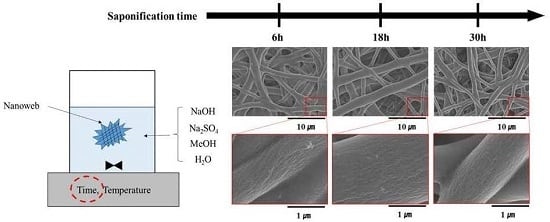
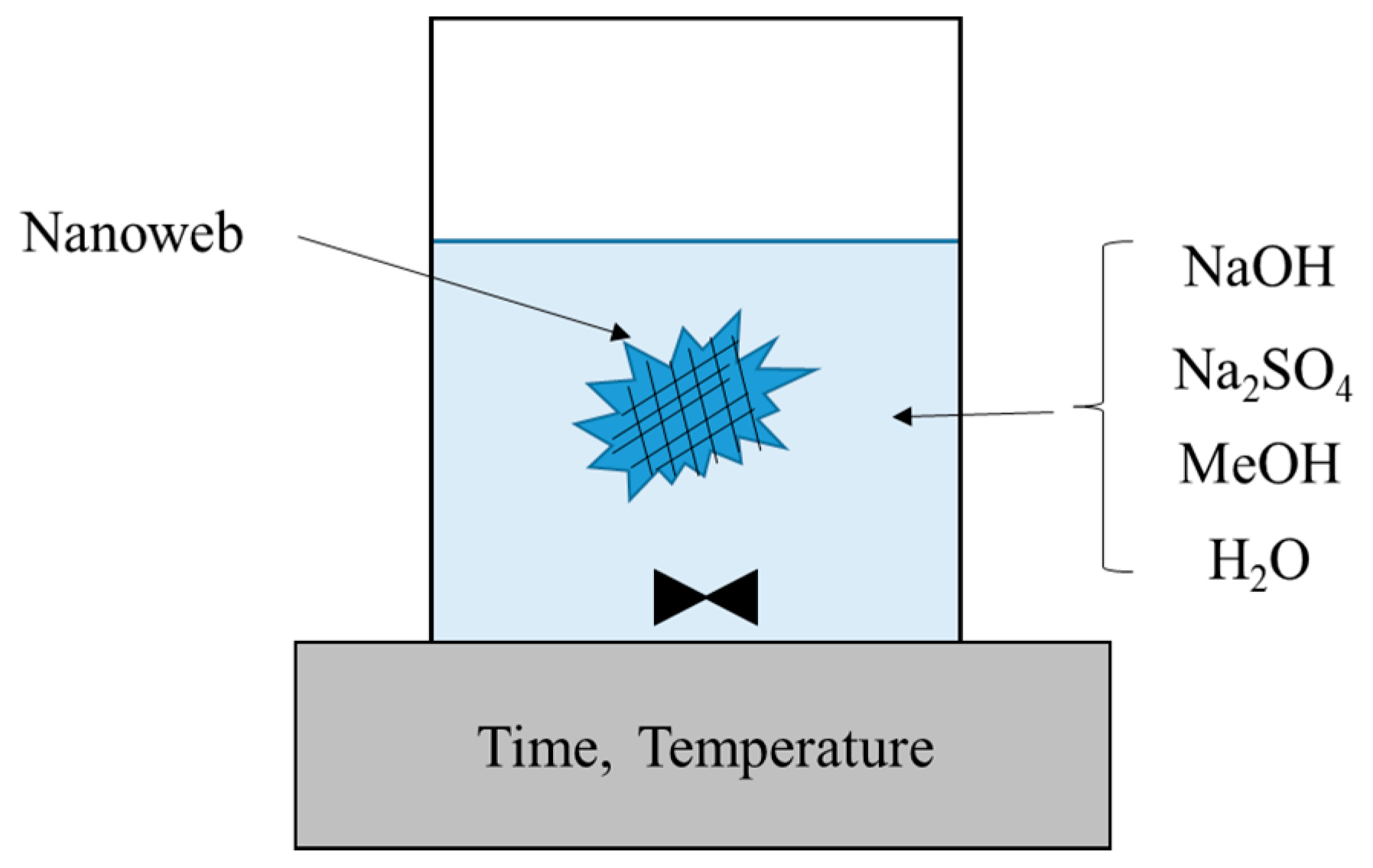
2.4. Heterogeneous Saponification of PVAc Nanofibers
2.5. Characterization
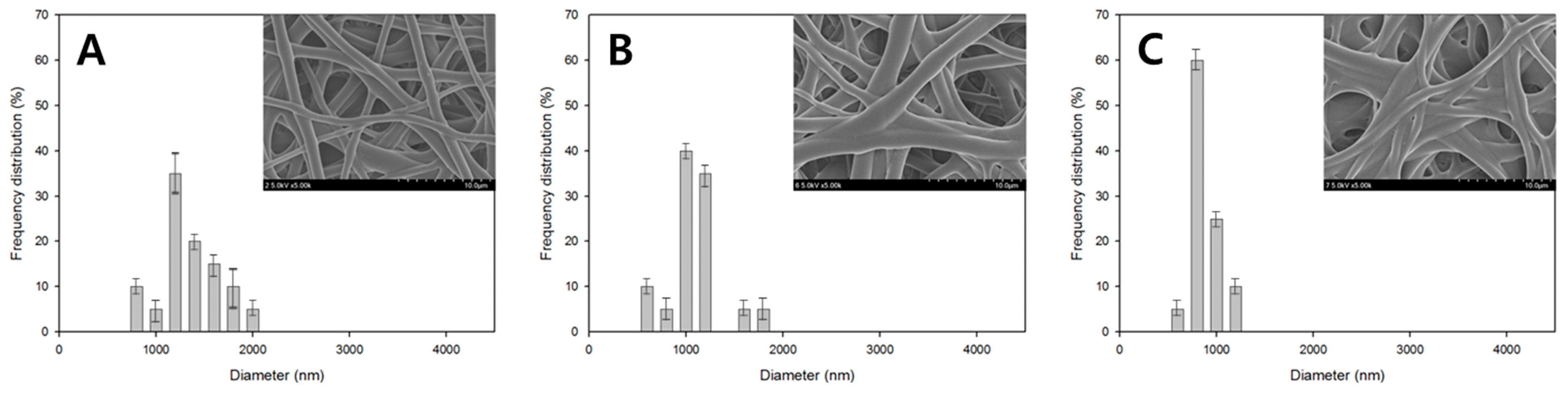
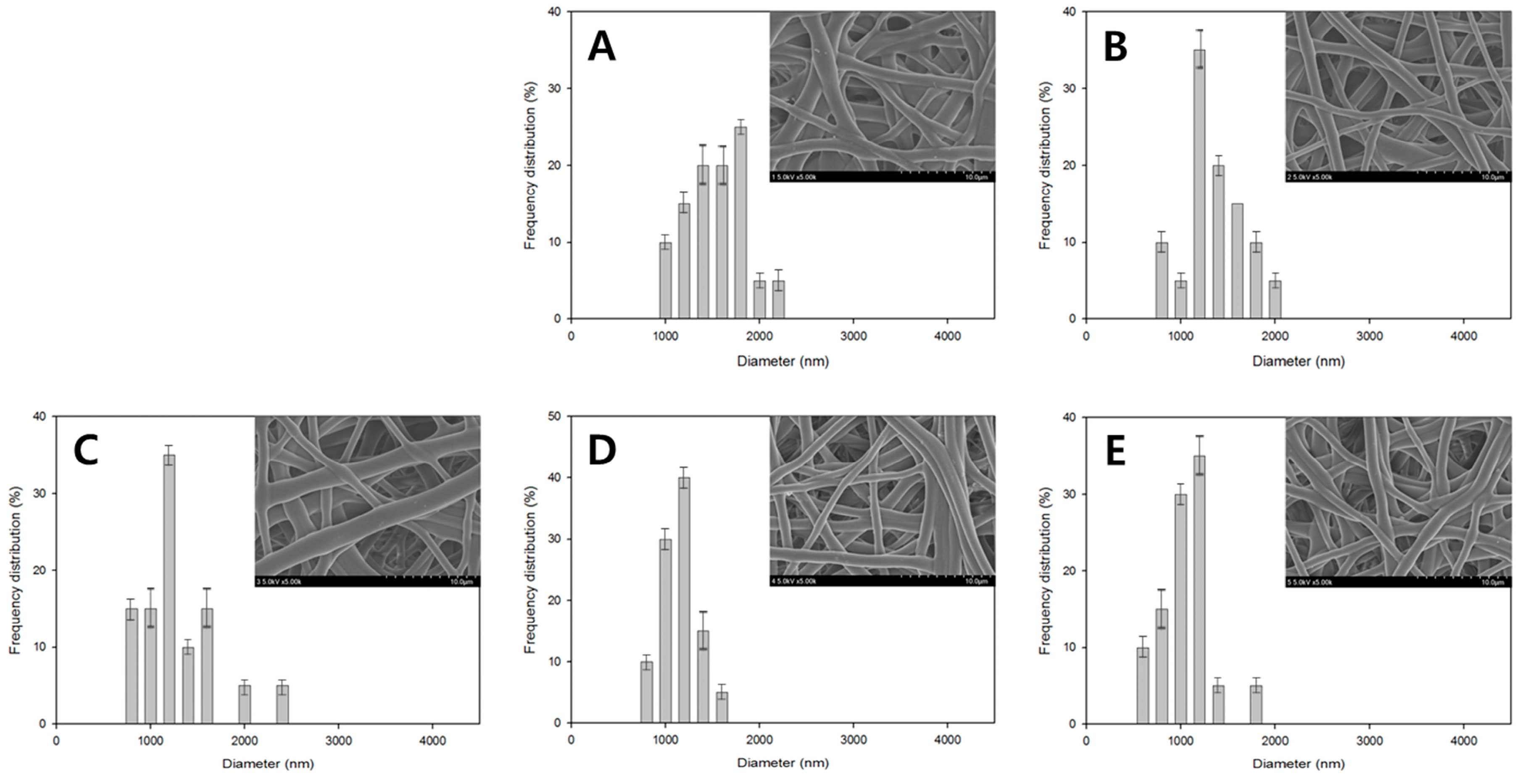
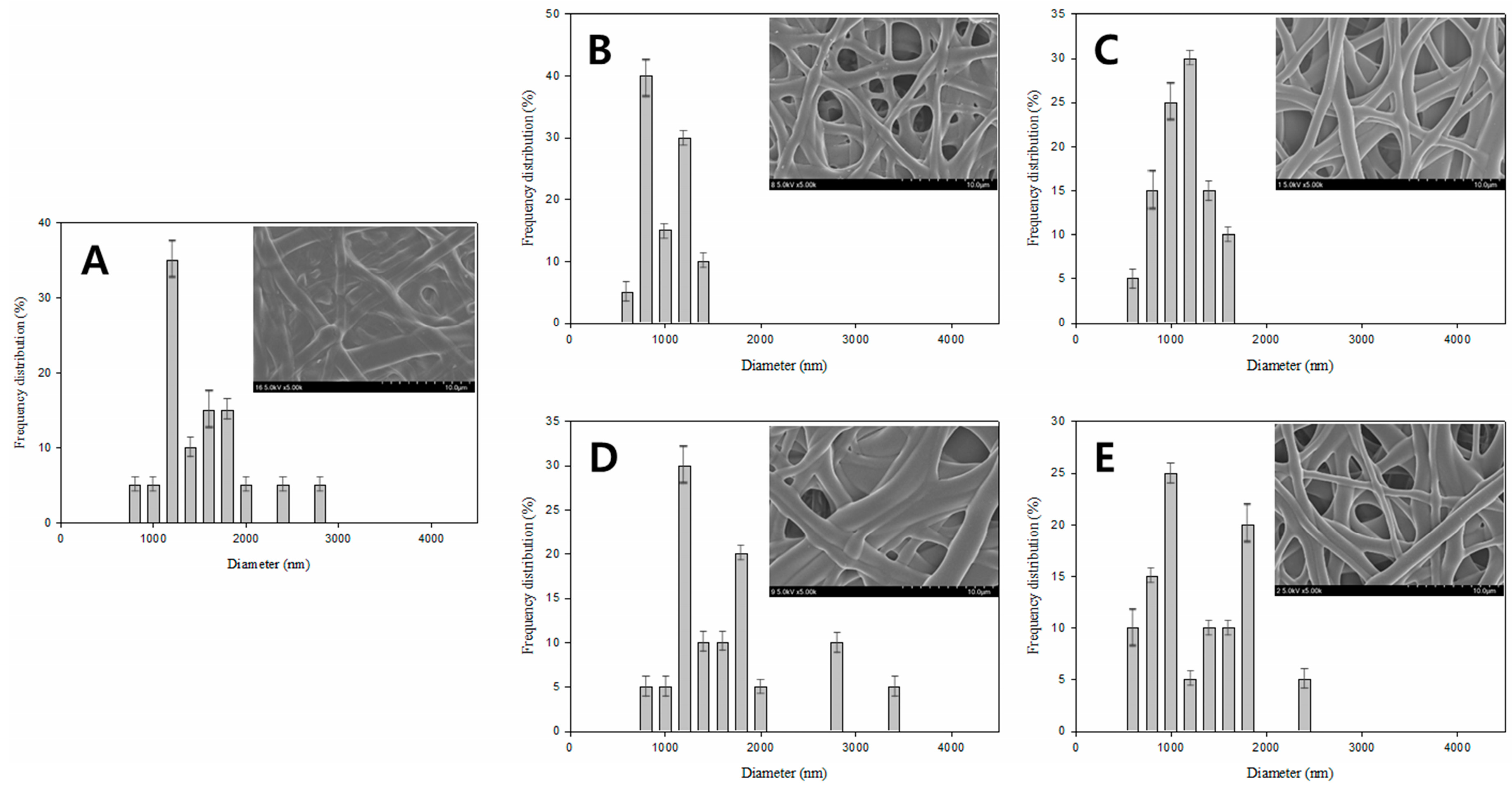
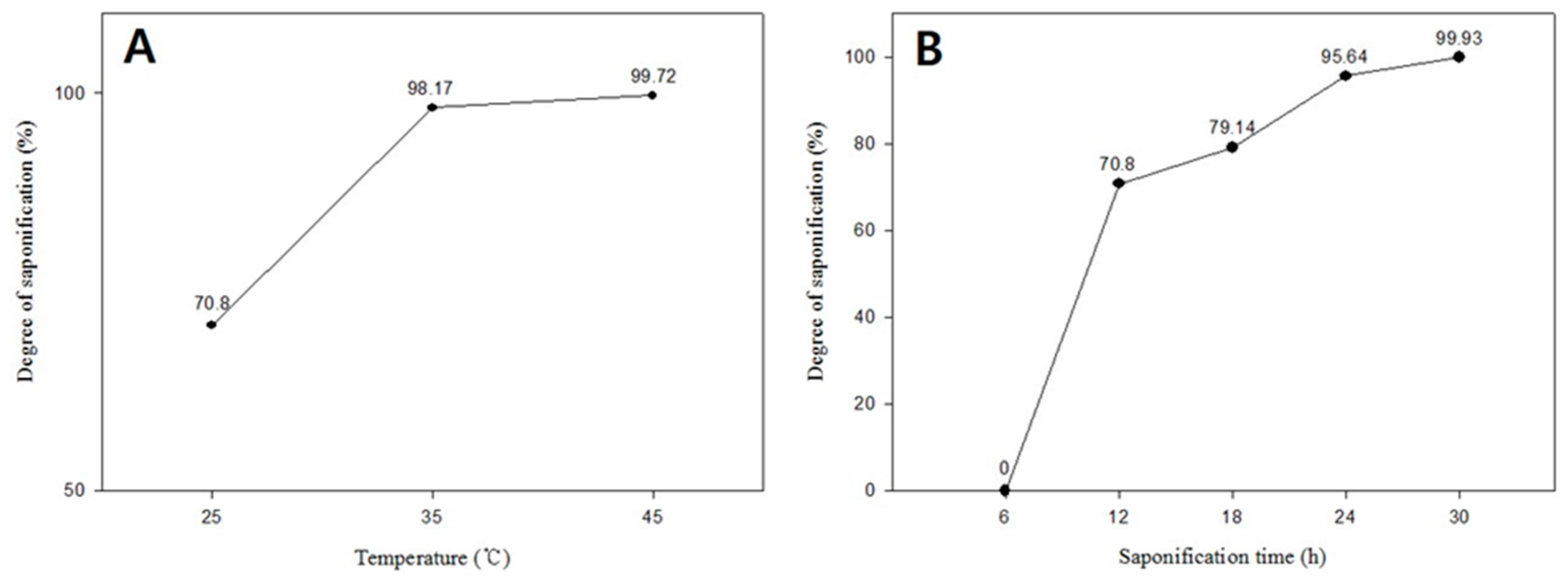
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sakurada, I. Polyvinyl Alcohol Fibers; Dekker: New York, NY, USA, 1985. [Google Scholar]
- Masuda, M. Polyminyl Alcohol-Development; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Lyoo, W.S.; Ha, W.S. Structure and properties of microfibrillar poly(vinyl alcohol) fibres prepared by saponification under shearing force of poly(vinyl pivalate). Polymer 1996, 37, 3121–3129. [Google Scholar] [CrossRef]
- Lyoo, W.S.; Blackwell, J.; Ghim, H.D. Structure of poly(vinyl alcohol) microfibrils produced by saponification of copoly(vinyl pivalate/vinyl acetate). Macromolecules 1998, 31, 4253–4259. [Google Scholar] [CrossRef]
- Lyoo, W.S.; Ha, W.S. In situ fibrillation of poly(vinyl alcohol) during saponification of poly(vinyl ester) (1). Chemorheological and morphological investigations of in situ fibrillation. Polymer 1999, 40, 497–505. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Drug-loaded electrospun mats of poly(vinyl alcohol) fibres and their release characteristics of four model drugs. Nanotechnology 2006, 17, 2317–2329. [Google Scholar] [CrossRef]
- Zeng, J.; Aigner, A.; Czubayko, F.; Kisse, T.; Wendorff, J.H.; Greiner, A. Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules 2005, 6, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Karim, M.R.; Kim, I.K.; Cheong, I.W.; Kim, J.W.; Bae, D.G. Electrospinning fabrication and characterization of poly(vinyl alcohol)/montmorillonite/silver hybrid nanofibers for antibacterial applications. Colloid Polym. Sci. 2010, 288, 115–121. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Binulal, N.S.; Selvamurugan, N.; Nair, S.V.; Menon, D.; Furuike, T. Electrospinning of carboxymethyl chitin/poly(vinyl alcohol) nanofibrous scaffolds for tissue engineering applications. Carbohydr. Polym. 2009, 77, 863–869. [Google Scholar] [CrossRef]
- Jia, Y.T.; Gong, J.; Gu, X.H.; Kim, H.Y.; Dong, J.; Shen, X.Y. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.P.; Kwon, I.C.; Park, K.H.; Noh, S.K.; Han, S.S. Heterogeneous surface saponification of suspension-polymerized monodisperse poly(vinyl acetate) microspheres using various ions. J. Polym. Sci. Polym. Chem. 2006, 44, 3567–3576. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.P.; Lyoo, W.S.; Kwak, J.W.; Noh, S.K.; Park, C.S. Preparation of novel syndiotactic poly(vinyl alcohol) microspheres through the low-temperature suspension copolymerization of vinyl pivalate and vinyl acetate and heterogeneous saponification. J. Appl. Polym. Sci. 2005, 95, 1539–1548. [Google Scholar] [CrossRef]
- Lyoo, W.S.; Lee, H.W. Synthesis of high-molecular-weight poly(vinyl alcohol) with high yield by novel one-batch suspension polymerization of vinyl acetate and saponification. Colloid Polym. Sci. 2002, 280, 835–840. [Google Scholar]
- Lyoo, W.S.; Kwak, J.W.; Choi, K.H.; Noh, S.K. Preparation of high molecular weight poly(vinyl alcohol) with high yield by emulsion polymerization of vinyl acetate using 2,2′-azobis(2-amidinopropane) dihydrochloride. J. Appl. Polym. Sci. 2004, 94, 2356–2362. [Google Scholar] [CrossRef]
- Jung, H.M.; Lee, E.M.; Ji, B.C.; Deng, Y.; Yun, J.D.; Yeum, J.H. Poly(vinyl acetate)/poly(vinyl alcohol)/montmorillonite nanocomposite microspheres prepared by suspension polymerization and saponification. Colloid Polym. Sci. 2007, 285, 705–710. [Google Scholar] [CrossRef]
- Yeum, J.H. Novel Poly(vinyl alcohol)/Clay Nanocomposite Microspheres via suspension polymerization and saponification. Polym. Plast. Technol. Eng. 2011, 50, 1149–1154. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Yang, S.; Du, J.; Wang, S.; Zheng, J. A simple and effective route for the preparation of poly(vinylalcohol) (PVA) nanofibers containing gold nanoparticles by electrospinning method. Solid State Commun. 2007, 141, 292–295. [Google Scholar] [CrossRef]
- Jeong, J.S.; Moon, J.S.; Jeon, S.Y.; Park, J.H.; Alegaonkar, P.S.; Yoo, J.B. Mechanical properties of electrospun PVA/MWNTs composite nanofibers. Thin Solid Films 2007, 515, 5136–5141. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Effect of pH on electrospinning of poly(vinyl alcohol). Mater. Lett. 2005, 59, 1571–1575. [Google Scholar]
- Zhang, X.; Gao, X.; Jiang, L.; Zhang, X.; Qin, J. Nanofiber-modified surface directed cell migration and orientation in microsystem. Biomicrofluidics 2011, 5, 032007/1–032007/10. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhang, H.; Yan, J.; Gong, X. Effect of nanofiber orientation of electrospun nanofibrous scaffolds on cell growth and elastin expression of muscle cells. Collids Surf. B Biointerfaces 2015, 136, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Lee, H.J.; Sabina, Y.; Kim, J.W.; Yeum, J.H. Novel poly(vinyl alcohol) nanofibers prepared by heterogeneous saponification of electrospun poly(vinyl acetate). Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 265–270. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, C.; Yeh, J.; Lin, W. Preparation and properties of poly(vinyl alcohol)-clay nanocomposite materials. Polymer 2003, 44, 3553–3560. [Google Scholar] [CrossRef]
- Baskaran, R.; Selvasekarapandian, S.; Hirankumar, G.; Bhuvaneswari, M.S. Dielectric and conductivity relaxations in PVAc based polymer electrolytes. Ionics 2004, 10, 129–134. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Unidirectional water-penetration composite fibrous film via electrospinning. Soft Matter 2012, 8, 5996–5999. [Google Scholar] [CrossRef]


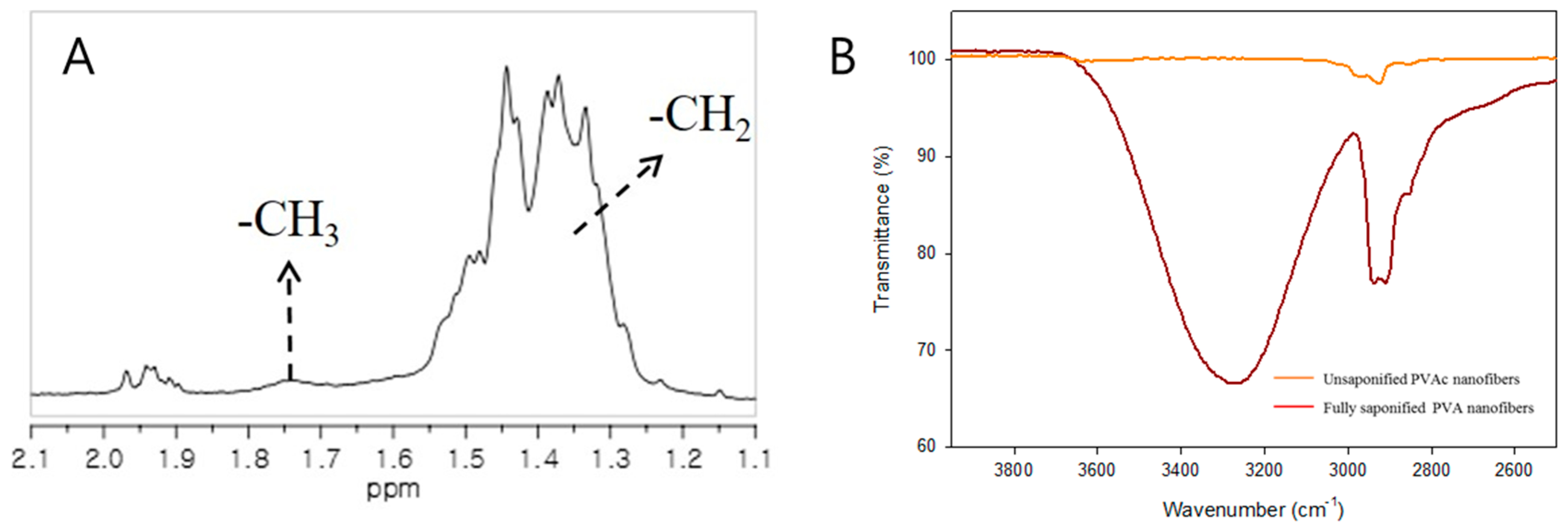
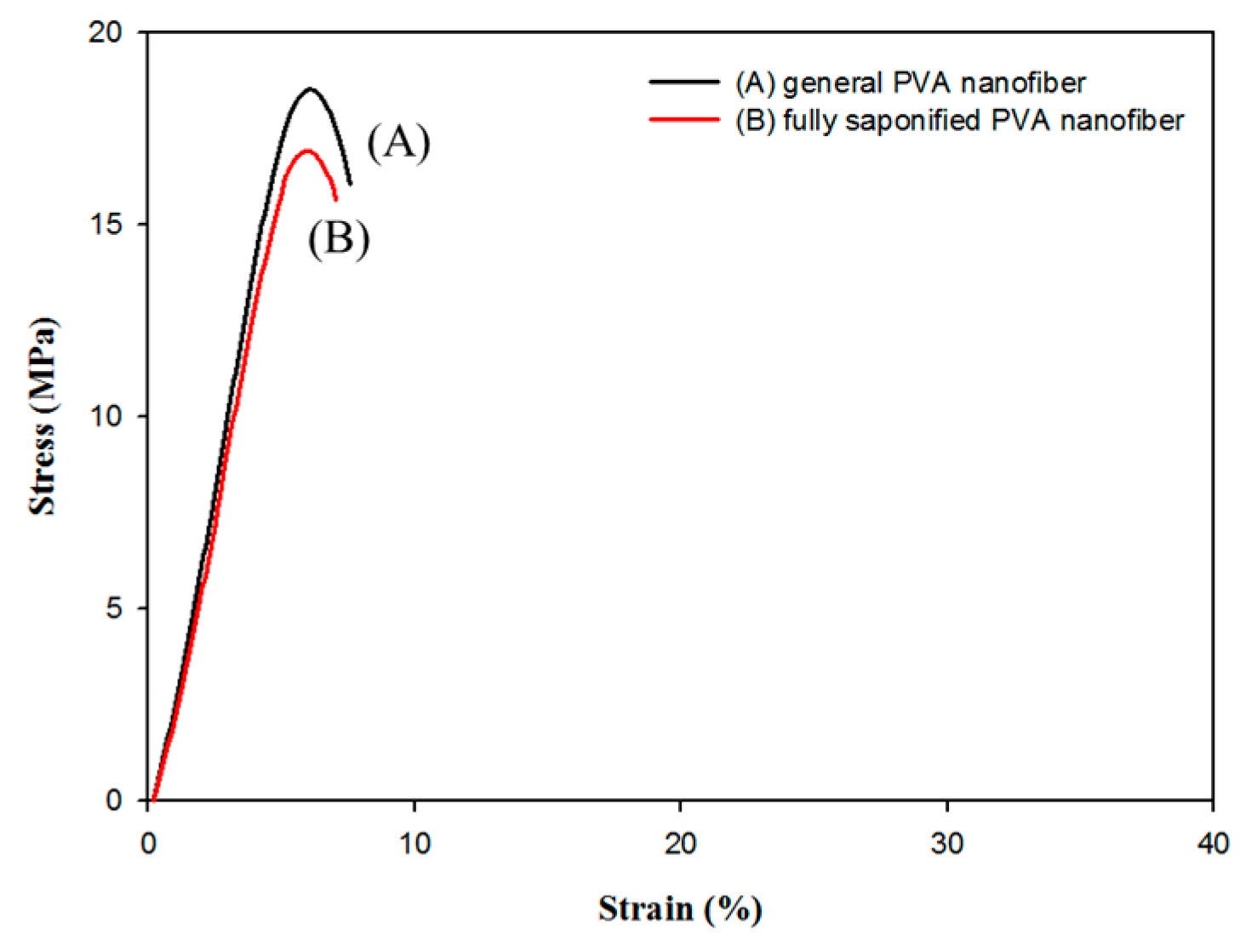
| Condition | Value |
|---|---|
| Type of initiator | ADMVN |
| Type of suspending agent | PVA |
| Initiator concentration | 0.0001 mol/mol of VAc |
| Suspending agent concentration | 1.5 g/dL of water |
| VAc/water | 0.5 L/L |
| rpm | 400 |
| Temperature | 60 °C |
| Figure | NaOH (g) | Na2SO4 (g) | MeOH (g) | H2O (g) | Temparature (°C) | Time (h) | DS (%) |
|---|---|---|---|---|---|---|---|
| Figure 2A | 10 | 10 | 10 | 100 | 25 | 12 | 70.8 |
| Figure 2B | 10 | 10 | 10 | 100 | 35 | 12 | 98.17 |
| Figure 2C | 10 | 10 | 10 | 100 | 45 | 12 | 99.72 |
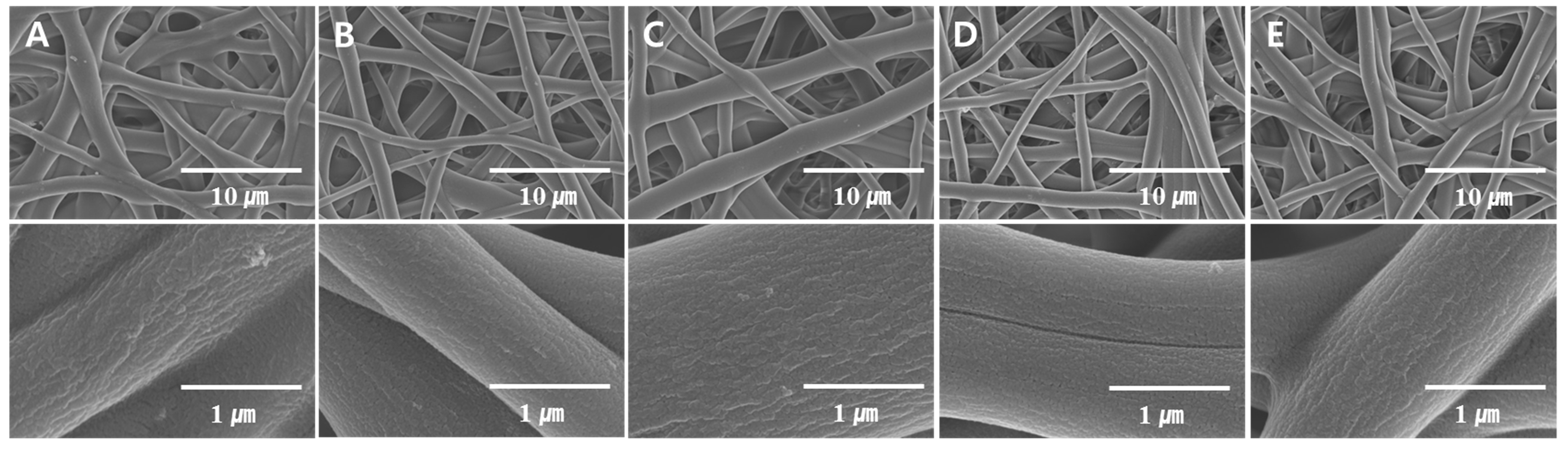
| Figure 3A | 10 | 10 | 10 | 100 | 25 | 6 | 0 |
| Figure 3B | 10 | 10 | 10 | 100 | 25 | 12 | 70.8 |
| Figure 3C | 10 | 10 | 10 | 100 | 25 | 18 | 79.14 |
| Figure 3D | 10 | 10 | 10 | 100 | 25 | 24 | 95.64 |
| Figure 3E | 10 | 10 | 10 | 100 | 25 | 30 | 99.93 |
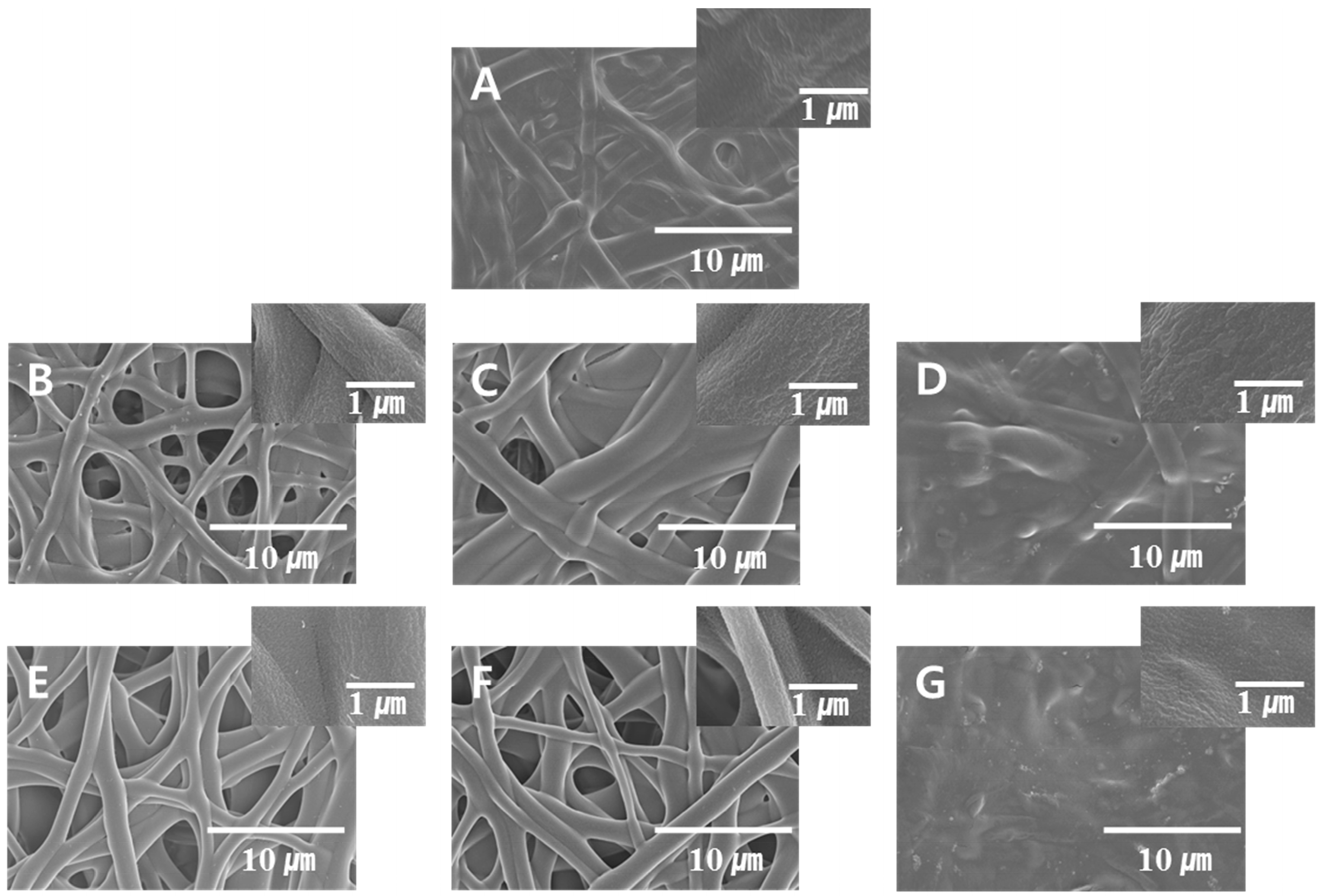
| Figure 4A | 5 | 5 | 5 | 100 | 25 | 24 | 45.8 |
| Figure 4B | 7.5 | 5 | 5 | 100 | 25 | 24 | 80.85 |
| Figure 4C | 5 | 7.5 | 5 | 100 | 25 | 24 | 88.86 |
| Figure 4D | 5 | 5 | 7.5 | 100 | 25 | 24 | 69.64 |
| Figure 4E | 10 | 5 | 5 | 100 | 25 | 24 | 61.08 |
| Figure 4F | 5 | 10 | 5 | 100 | 25 | 24 | 68.94 |
| Figure 4G | 5 | 5 | 10 | 100 | 25 | 24 | 59.26 |
| Sample | Mw (g/mol) | Mn (g/mol) | Polydispersity index (Mw/Mn) |
|---|---|---|---|
| General PVA nanofibers | 82,125 | 10,841 | 7.57 |
| Saponified PVA nanofibers | 84,216 | 9,868 | 8.53 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.B.; Kim, J.W.; Yeum, J.H. Effect of Saponification Condition on the Morphology and Diameter of the Electrospun Poly(vinyl acetate) Nanofibers for the Fabrication of Poly(vinyl alcohol) Nanofiber Mats. Polymers 2016, 8, 376. https://doi.org/10.3390/polym8100376
Yang SB, Kim JW, Yeum JH. Effect of Saponification Condition on the Morphology and Diameter of the Electrospun Poly(vinyl acetate) Nanofibers for the Fabrication of Poly(vinyl alcohol) Nanofiber Mats. Polymers. 2016; 8(10):376. https://doi.org/10.3390/polym8100376
Chicago/Turabian StyleYang, Seong Baek, Jong Won Kim, and Jeong Hyun Yeum. 2016. "Effect of Saponification Condition on the Morphology and Diameter of the Electrospun Poly(vinyl acetate) Nanofibers for the Fabrication of Poly(vinyl alcohol) Nanofiber Mats" Polymers 8, no. 10: 376. https://doi.org/10.3390/polym8100376
APA StyleYang, S. B., Kim, J. W., & Yeum, J. H. (2016). Effect of Saponification Condition on the Morphology and Diameter of the Electrospun Poly(vinyl acetate) Nanofibers for the Fabrication of Poly(vinyl alcohol) Nanofiber Mats. Polymers, 8(10), 376. https://doi.org/10.3390/polym8100376