Abstract
The photostabilization of poly(methyl methacrylate) (PMMA) films having 2-(6-methoxynaphthalen-2-yl)propanoate and Sn(II), Ni(II), Zn(II) and Cu(II) complexes was investigated. The production of PMMA films containing such complexes (0.5% by weight) was carried out by the casting method using chloroform. The photostabilization activities of the compounds were determined by monitoring the hydroxyl index with irradiation time. The quantum yield of the chain scission (Φcs) for the complexes in PMMA films and the changes in the viscosity average molecular weight of PMMA with irradiation time were evaluated. The rate of photostabilization for PMMA in the presence of the additives was found to follow the order NiL2 > CuL2 > ZnL2 > SnL2 (L, ligand). Depending on the structure of the additive, such as a peroxide decomposer, UV absorption or a radical scavenger for the photostabilizer, several mechanisms are suggested.
1. Introduction
Ultraviolet radiation (UV) can cause degradation of certain materials if exposed. Chemical materials, known as ultraviolet absorbers (UVAs) can be used to protect materials from the damaging effects of UV radiation. A UVA can be incorporated into a material to protect such a material from UV radiation, or a composition that contains UVA can be applied to a UV-sensitive substrate to protect the substrate. Ultraviolet light stabilizers are used in important materials, including films, plastics and cosmetics [1]. UV solar radiation has sufficient energy to initiate photochemical degradation, through chemical reactions, of materials in outdoor applications. The deterioration of plastics can be avoided by the addition of antioxidants, heat and light stabilizers [1].
The UV-stabilizer prevents the photodegradation of polymeric materials and photo-crosslinking caused by artificial light and ultraviolet sunlight. There are several types of ultraviolet light stabilizers that contain organic and inorganic moieties. Inorganic UV-stabilizers, such as the ones containing chromic, titanium and iron oxides and carbon black, are usually incompatible with the polymer matrix because of their uneven distribution within plastic substrates. The final effects of the UV-stabilizer depend on the concentration and particle sizes [2]. Such limitations hindered the commercial applications of inorganic UV-stabilizers. Organic UV-stabilizers contain benzotriazoles, hydroxybenzophenone, phenyl benzoate and fluorescent compounds, which usually have a low molecular weight. The addition of organic UV-stabilizers to plastic materials creates many problems, such as incompatibility, volatility, migration and solvent extraction. Such problems could lead to strong diminution of the material utilized. Several approaches to overcome such a limitation have been developed, including synthesis of reactive UV-stabilizers [3] that have compatible side chains or chemically anchoring of the additive to the polymeric material backbone [4]. For example, the synthesis of a high molecular weight UV-stabilizer is highlighted because, for most polymer materials, blending is the first choice to enhance their UV-resistance. Meanwhile, various high molecular weight UV-stabilizers can be synthesized via copolymerization of the monomers along with a reactive UV-stabilizer. It is very convenient to ameliorate the compatibility between the plastic matrix and a suitable high molecular weight UV-stabilizer [5]. Synthetic polymers have to be stabilized against adverse effects. Therefore, it is essential to find a mechanism to prevent or reduce the damaging effect that can be caused by environmental parameters, such as air, heat and light. The photostabilization of polymers has to be considered, which involves the retardation of photochemical reactions in polymeric materials that occur during the irradiation process. Various stabilizing systems have been developed that depend on the stabilizer action, such as excited state quenchers, light screeners, peroxide decomposers, radical scavengers, UV absorbers and luminescent shifters [6,7,8,9,10,11,12,13,14]. As part of our on-going research on the synthesis of polymeric materials [15,16,17,18] and polymer stabilization [19,20], we became interested in the photostabilization of PMMA using 2-(6-methoxynaphthalen-2-yl)propanoate with Sn(II), Ni(II), Zn(II) and Cu(II) complexes. To the best of our knowledge, this is the first attempt to investigate the photostabilization of PMMA films by complexes containing a naphthalene moiety.
2. Experimental Section
2.1. Materials
Several metal ions of 2-(6-methoxynaphthalen-2-yl)propanoate complexes were prepared from the reaction of potassium salt of 2-(6-methoxynaphthalen-2-yl)propanoic acid (2 mole equivalents) and metal acetate or chloride (1 mole equivalent) in ethanol under reflux conditions for 30 min [21]. The solid obtained upon cooling was collected by filtration, washed with ethanol and recrystallized from ethanol to give the colored complexes that have been dried under vacuum at 50 °C. The metal contents were determined by the use of flame atomic absorption techniques and were in agreement with the calculated ones. The FT infrared spectra for the complexes (Table 1 and Figure 1) show three characteristics bands that have appeared within the regions of 432–480, 1545−1606 and 1392−1460 cm−1, which have been attributed to ν for M–O, COO− asymmetrical and COO− symmetrical bonds, respectively. The magnetic moment was zero for ZnL2 (L, ligand), CdL2 and SnL2 and 2.91 and 1.53 Bohr Magnetons (B.M.), B.M. = 9.27 × 10−24 Amperes/Joules (J.T−1), for NiL2 and CuL2, respectively. The conductivity of complexes was measured, at room temperature in dimethylformamide, to be in the range of 10–15 ohm−1 cm2 mol−1, which is an indication that all complexes are non-electrolytes [21].

Table 1.
Structure of metal ligand complexes (ML2) used along with a ligand (L).
| Symbol | Name |
|---|---|
| L | Potassium 2-(6-methoxynaphthalen-2-yl)propanoate |
| NiL2 | bis[2-(6-Methoxynaphthalen-2-yl)propanoate] nickel(II) |
| CuL2 | bis[2-(6-Methoxynaphthalen-2-yl)propanoate] copper(II) |
| ZnL2 | bis[2-(6-Methoxynaphthalen-2-yl)propanoate] zinc(II) |
| SnL2 | bis[2-(6-Methoxynaphthalen-2-yl)propanoate] tin(II) |
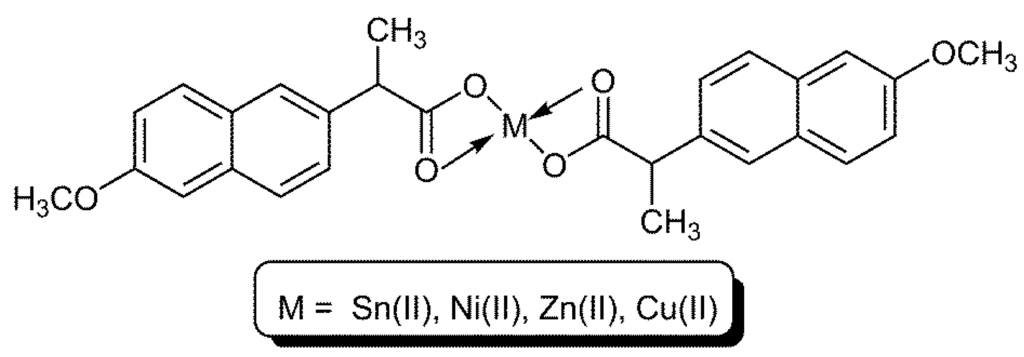
Figure 1.
Structure of ML2 used.
2.2. Film Preparation
The dropwise addition of alcohol to the commercial PMMA solution in chloroform leads to its re-precipitation. The solid was filtered and dried at room temperature for 24 h under vacuum. The polymer films with a 40-μm thickness were prepared, measured by a micrometer Type 2610 A, Germany, using a fixed concentration of PMMA solution in chloroform (5 g/100 mL). The films and prepared complexes (0.5% by weight) were mixed at different concentrations of complexes. Furthermore, a blank was used in which the concentration of complexes was 0. It was essential to control the solvent evaporation rate and the hygrometry during casting to have very limited turbidity and, more importantly, to maintain high optical quality. The film transmission has to be more than 80% in the near-UV range. The evaporation technique at room temperature for 24 h was used to prepare the films. The films were dried for 3 h at room temperature to remove any chloroform residues. Stands with an aluminum plate (0.6 mm in thickness) were used to fix the films and were supplied by the Q-Panel Company (Homestead, FL, USA) [22].
2.3. Irradiation Experiments: Accelerated Testing Technique
An accelerated weather-meter Q-panel Laboratory ultraviolet (QUV; Q-Panel Company, Homestead, Florida, USA) tester was used for the irradiation of polymeric films and was supplied by the Q-Panel Company. The accelerated weathering tester contains a stainless steel plate that has one hole in the back side and two others in the front. Each side contains a UV-B 313 fluorescent ultraviolet lamp (40 W) (Q-Panel Company) giving a spectrum within a 290–360 nm range, with 313 nm as the maximum wavelength. The samples of polymeric film were fixed parallel to the lamps and vertically to ensure that the UV incident radiation is perpendicular to the samples. To ensure that the intensity of incident light is the same on all sample, samples were rotated from time to time [23].
2.4. Photodegradation Measuring Methods
2.4.1. Measuring the Photodegradation Rate of Polymer Films Using Infrared Spectrophotometry
An FTIR 8300 Shimadzu Spectrophotometer (400−4000 cm−1) (Shimadzu, Tokyo, Japan) was used to monitor the degree of photodegradation of polymeric film samples in which hydroxyl group absorption took place at 3430 cm−1 [23]. The changes in the hydroxyl group’s absorption peaks indicated the progress of photodegradation at different irradiation times. The comparison between the FTIR hydroxyl group’s absorption peak (3430 cm−1) and the reference peak (1450 cm−1) allows the calculation of the hydroxyl index (IOH), as shown in Equation (1) [23].
2.4.2. Determination of Average Molecular Weight () Using the Viscometry Method
The polymer average molecular weight was determined in terms of the viscosity property by the use of the Mark–Houwink relation [24].
Equation (2) was used for convert the single-point measurements to the intrinsic viscosities.
where C = the polymer solution concentration (g/100 mL). The molecular weight of the undergirding and polymers can be calculated by the use of Equation (5). The PMMA molecular weights, with and without additives, were calculated from intrinsic viscosities that have been measured in benzene by the use of Equation (2). The main chain scission quantum yield (Φcs) [25] was calculated from the viscosity measurement by the use of Equation (6).
where A = Avogadro’s number, C = The concentration, = The initial viscosity average molecular weight, Io = The incident intensity, t = The irradiation time in seconds and [ηo] = The intrinsic viscosity of PMMA before irradiation [26].
3. Results and Discussion
3.1. Photodegradation Mechanism for PMMA
The PMMA photochemical degradation steps are summarized as shown below (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8):
(1) The formation of free radicals, A1 and A2, via homolytic cleavage of the C–CH3 and C–COOCH3 bonds, respectively (Figure 2).
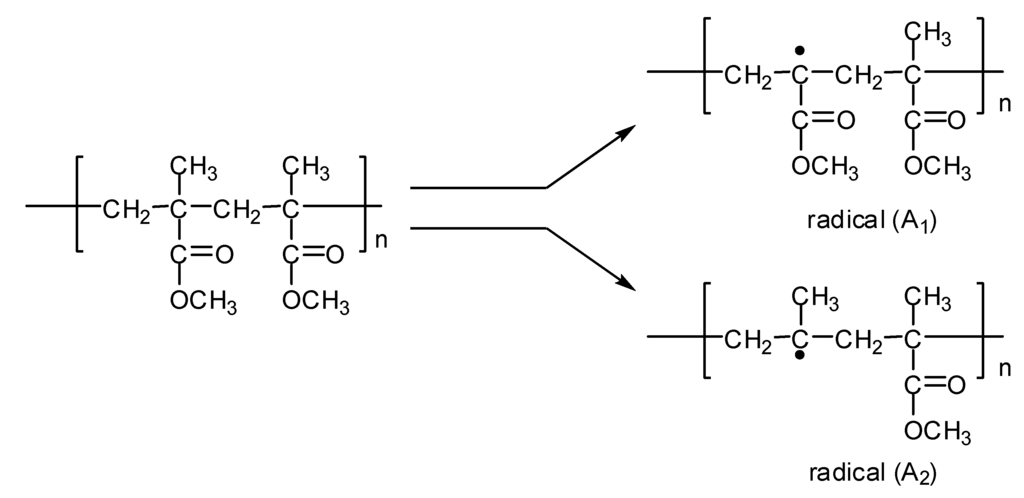
Figure 2.
Formation of radicals A1 and A2.
(2) The formation of peroxy radicals B1 and B2 via reaction of A1 and A2 free radicals with oxygen, respectively (Figure 3).
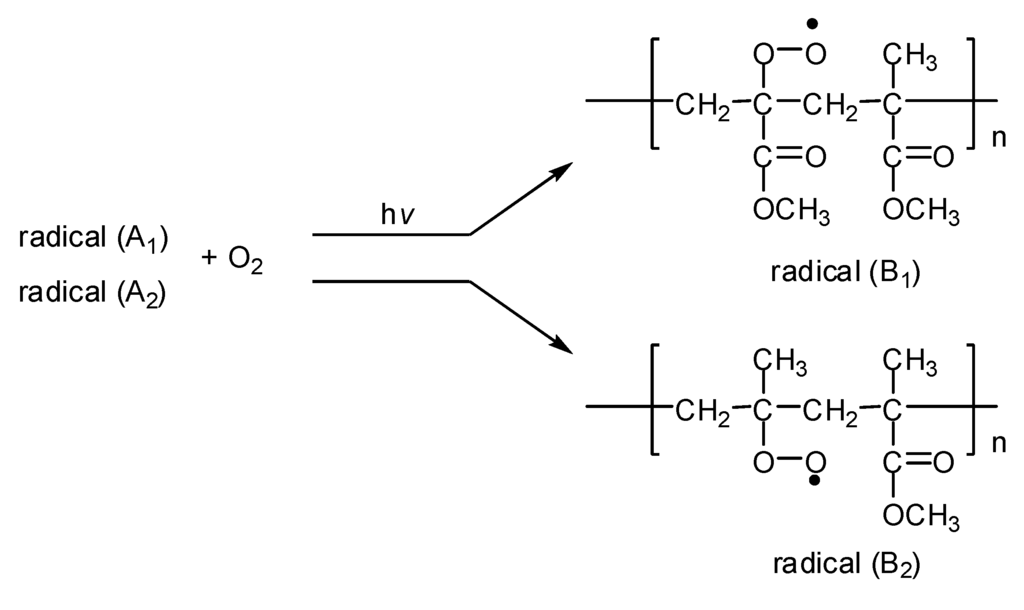
Figure 3.
Formation of radicals B1 and B2.
(3) The abstraction of the hydrogen atom from (P−H) polymer chain by the peroxy radicals B1 and B2 afforded the hydroperoxide polymers C1 and C2, respectively (Figure 4)).
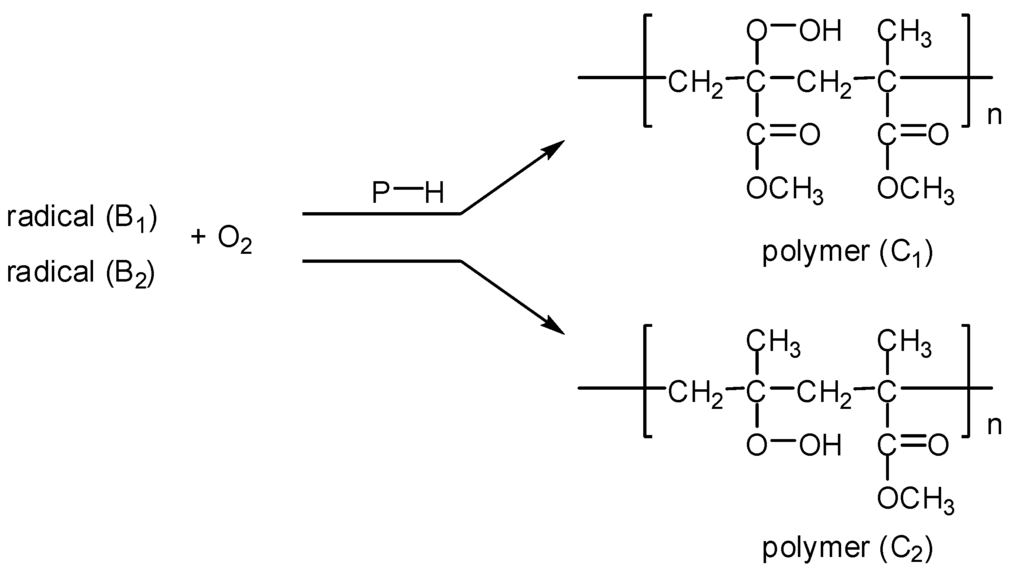
Figure 4.
Formation of hydroperoxide polymers C1 and C2.
(4) The homolytic cleavage of the O−OH bonds of the hydroperoxy polymers C1 and C2 to produce the polymer oxy radicals D1 and D2, respectively (Figure 5).
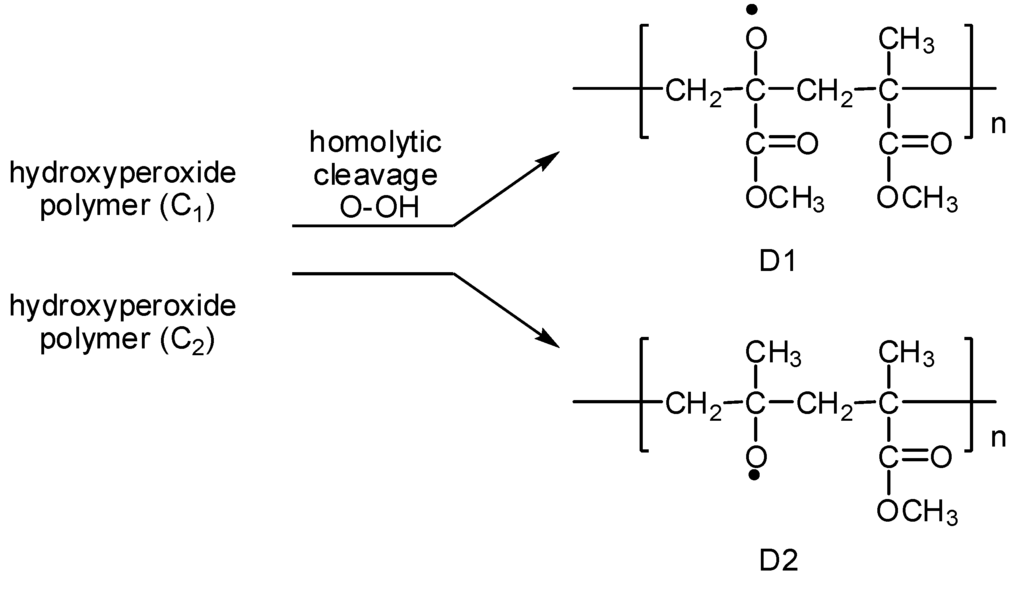
Figure 5.
Formation of polymer oxy radicals D1 and D2.
(5) The alkoxy radicals D1 and D2 further react via abstraction of the hydrogen atom to produce polymeric alcohols E1 and E2, respectively (Figure 6), or the β-scission reaction to produce ketonic polymers F1 and F2, respectively (Figure 7).
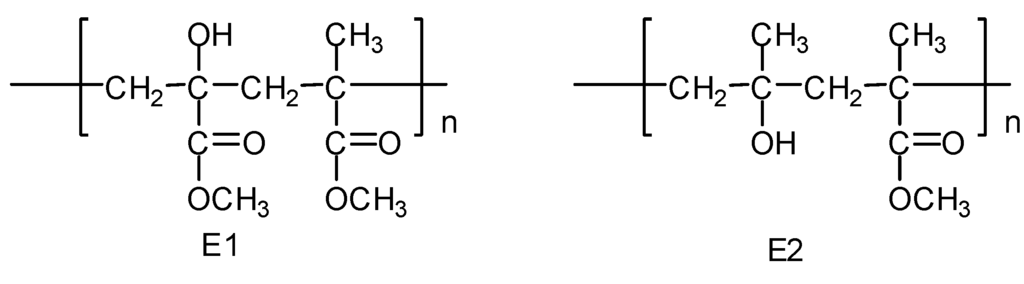
Figure 6.
Formation of polymeric alcohols E1 and E2.
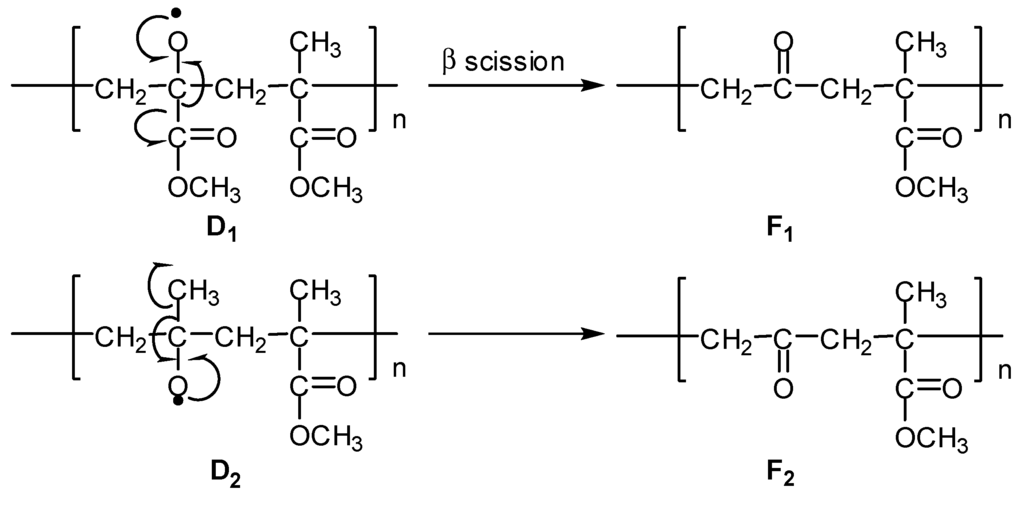
Figure 7.
Formation of ketonic polymers F1 and F2.
The carbonyl groups in the polymeric chain play an essential role in the formation of acyl radicals (Figure 8) via oxidative degradation of the main polymeric chain.
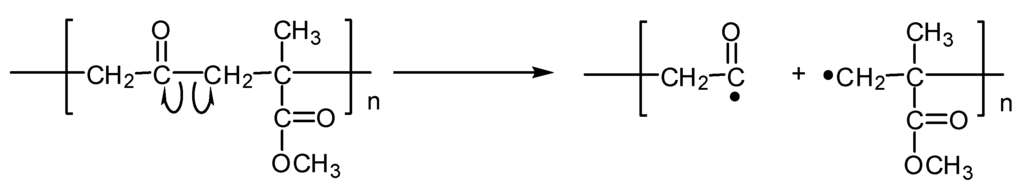
Figure 8.
Formation of acyl radicals via degradation of the main polymeric chain.
Photostabilization of poly(vinyl chloride) (PVC) films having different concentrations (0.1%–0.5% by weight) of various additive metal complexes has shown that a complex concentration of 0.5% by weight gave the best results [19]. Such a concentration has been applied successfully for photostabilization of PVC and polystyrene films with other additive metal complexes [27,28]. Therefore, additive metal complexes have been used in a concentration of 0.5% by weight.
2-(6-Methoxynaphthalen-2-yl)propanoate complexes were used as additives for the PMMA films’ photostabilization. The hydroxyl group index was monitored with irradiation time using IR spectrophotometry to allow the photochemical activity of these additives for the photostabilization of PMMA films to be studied. The PMMA films was irritated with a UV light (λ = 313 nm) and produced a clear change in the FTIR spectrum. The absorption bands appearing at 3430 cm−1 was attributed to the formation of the OH groups [29]. Such an absorption band was used to follow the progress of the degradation of the polymer with irradiation and has been calculated as the hydroxyl index. It is fair to link the polymer degradation degree with the growth of the hydroxyl index. However, in Figure 9, the IOH of SnL2, ZnL2, CuL2 and NiL2 indicated a lower OH growth rate with irradiation time for the PMMA control film in the absence of any additives. Since the growth of the hydroxyl index with irradiation time was lower than the PMMA blank (Figure 9), it was conclude that such additives could be considered as photostabilizers for the PMMA polymer. In principle, a longer induction period is a sign of an efficient photostabilizer. Therefore, the NiL2 is considered to be the most efficient photostabilizer, followed by CuL2, ZnL2 and SnL2.
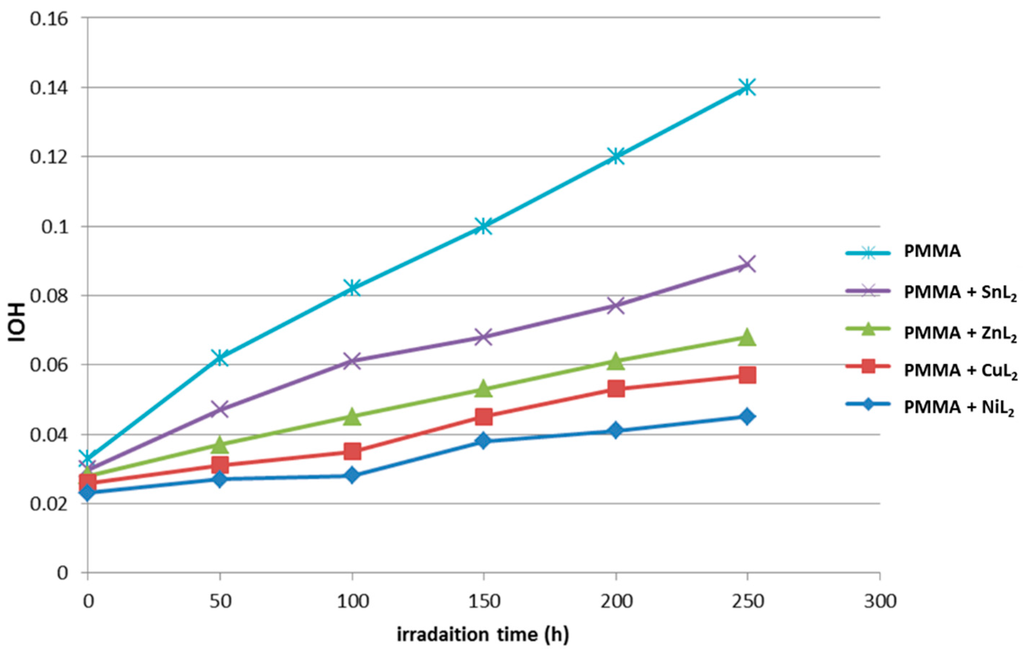
Figure 9.
The relationship between the hydroxyl index and irradiation time for PMMA films (40-μm thickness) with different additives (0.5% by weight).
3.2. Variation of PMMA Molecular Weight during Photolysis in the Presence of bis[2-(6-Methoxynaphthalen-2-yl)propanoate] Complexes
A versatile test for random chain scission was provided from the analysis of the relative changes in viscosity average molecular weight . Figure 10 shows the changes in versus irradiation time for PMMA film (0.5% by weight) in the absence of selected additives at a light absorption intensity of 1.121 × 10−8 ein dm−3 s−1. Equation 3 was used to measure in benzene at room temperature. It is worth mentioning that insoluble film traces with additives indicate that some degree of PMMA branching or cross-linking took place during the photolysis process [30]. To prove that, Equation (7) was used to calculate the number of average chain scissions (average number of cuts per single chain; S) [31].

Table 2.
Quantum yield (Φcs) for the chain scission for PMMA films (40-μm thickness) with and without additive (0.5% by weight) after irradiation (250 h).
| Additive (0.5 wt%) | Quantum Yield of Main Chain Scission (Φcs) |
|---|---|
| PMMA +NiL2 | 5.96 × 10−5 |
| PMMA + CuL2 | 5.35 × 10−5 |
| PMMA + ZnL2 | 7.11 × 10−5 |
| PMMA + SnL2 | 7.21 × 10−5 |
| PMMA (blank) | 4.55 × 10−6 |
The Φcs values for PMMA films with additives are less than those for the corresponding ones in the absence of additives (blank) and increase in the order: NiL2 > CuL2 > ZnL2 > SnL2.
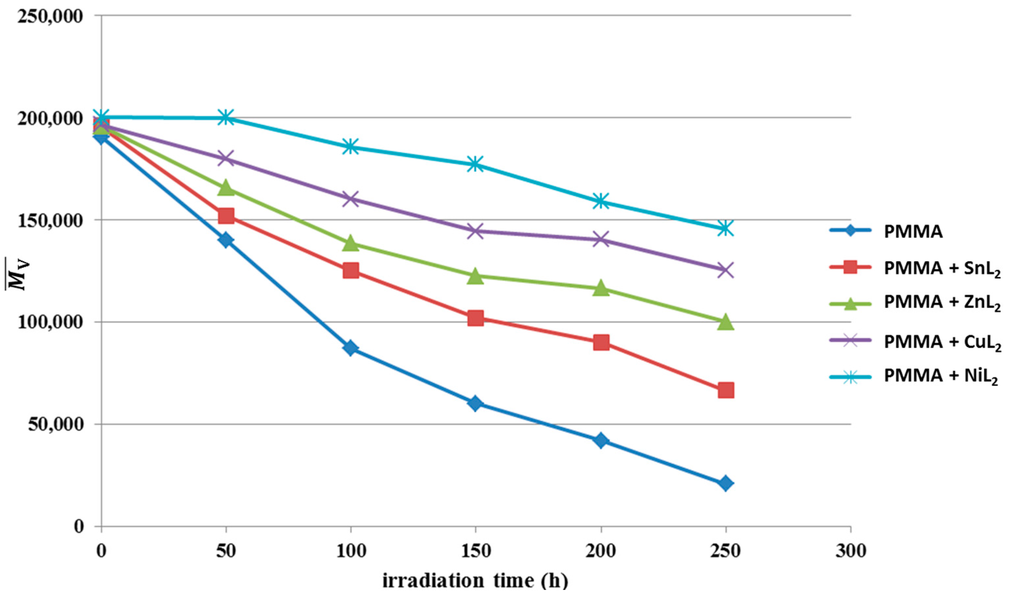
Figure 10.
Changes in the viscosity average molecular weight during irradiation of PMMA films (40 μm; control) and additives (0.5% by weight).
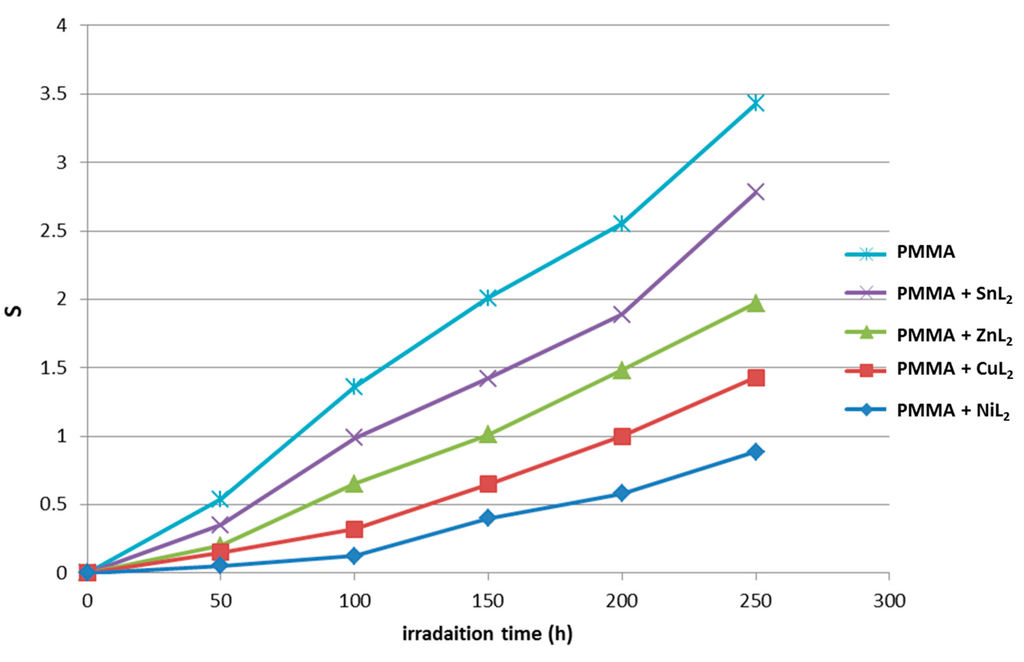
Figure 11.
Changes in the main chain scission (S) during irradiation of PMMA films (40 μm; control) and additives (0.5% by weight).
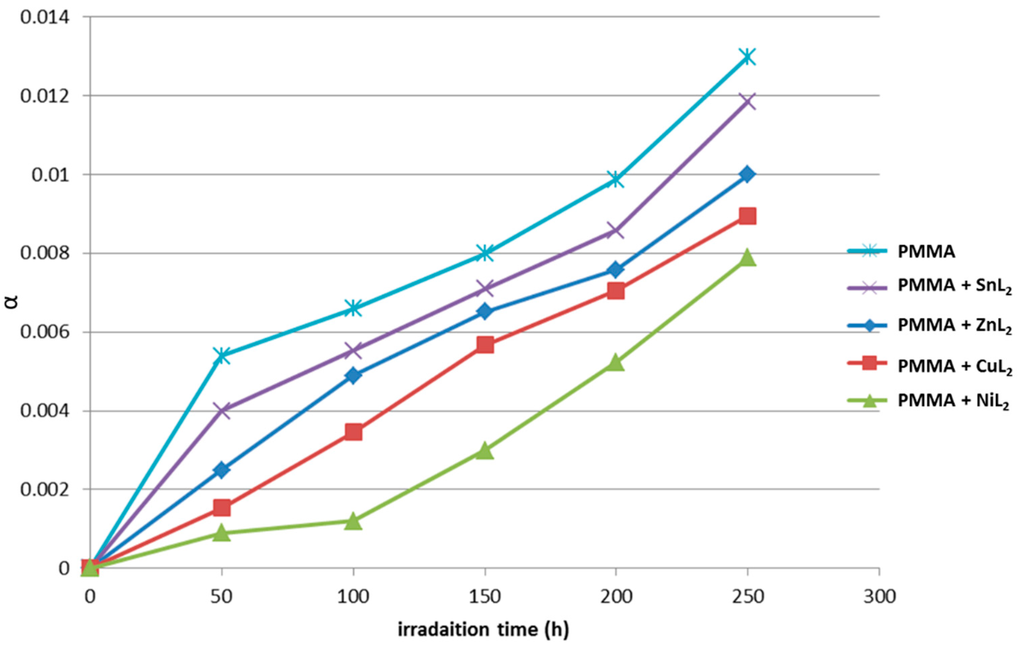
Figure 12.
Changes in the degree of deterioration (α) during irradiation of PMMA films (40 μm; control) and additives (0.5% by weight).
3.3. Suggested Mechanisms of Photostabilization of PMMA by the bis[2-(6-Methoxynaphthalen-2-yl)propanoate] Complexes
The results obtained showed that the efficiency of bis[2-(6-methoxynaphthalen-2-yl)propanoate] complexes as stabilizers for PMMA films can be ordered according to the change in the hydroxyl content as a reference for comparison (Figure 9), where NiL2 > CuL2 > ZnL2 > SnL2 > PMMA.
Each additive in the polymeric material plays a role in the photostability of such a material. Therefore, various mechanisms for the photostabilization could take place, which were mainly based on the additive chemical structure, the functional groups present, in particular the ones that resonate, such as aromatic and heteroaromatics moieties, and the compatibility of the additive with the polymer matrix. For example, the metal complexes might stabilize PMMA by dissipation of energy as heat via direct absorption of the UV radiation [33]. However, obviously, the nature of the metal will have a significant effect on the efficiency of the additive as a stabilizer. Clearly, both the metal and the ancillary ligands could play a role in absorbing the UV light [34,35,36].
In a similar way, the aromatic rings in complexes play an important role in the stabilization mechanism by acting as UV absorbers. The UV light absorption by such additives containing aromatic rings dissipates the UV energy to harmless heat energy (Scheme 1). Moreover, the aromatic rings play a role in the resonating structure conjugation of the radical in the peroxide decomposer, which supports this compound as being a photostabilizer [37].
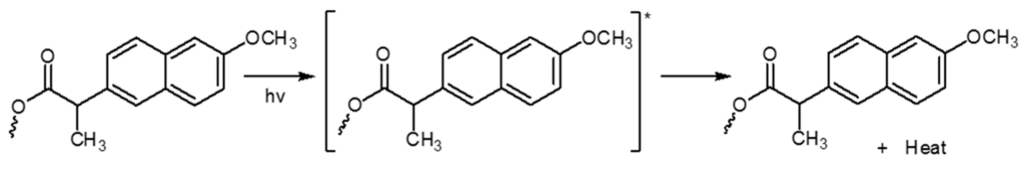
Scheme 1.
Possible photostabilization of aromatic moiety as a UV absorber.
Hydroperoxides play an important role in the degradation of polymeric materials by photooxidation. The use of typical UV stabilizers protecting polymer films, through peroxide decomposition, has been reported [38]. Metal chelate complexes are known as PMMA photostabilizers through both a peroxide decomposer and excited state quencher. Therefore, it was expected that such complexes could act as peroxide decomposers (Scheme 2) [19].
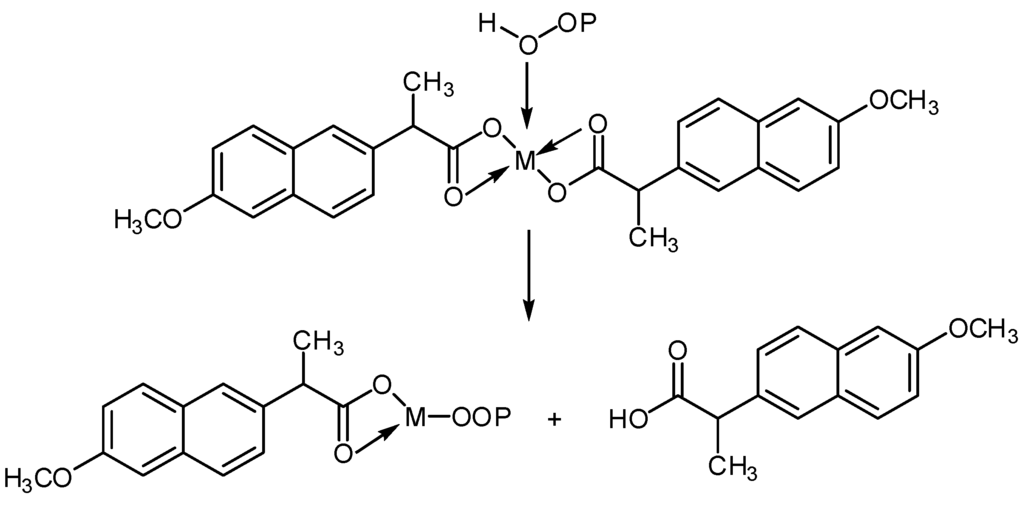
Scheme 2.
Possible photostabilization of ML2 complexes as peroxide decomposers.
These metal chelate complexes also act as radical scavengers through energy transfer, form un-reactive charge transfer complexes between the metal chelate and excited state of the chromophore (POO●) and stabilize through resonating structures, as shown in Scheme 3 [26].
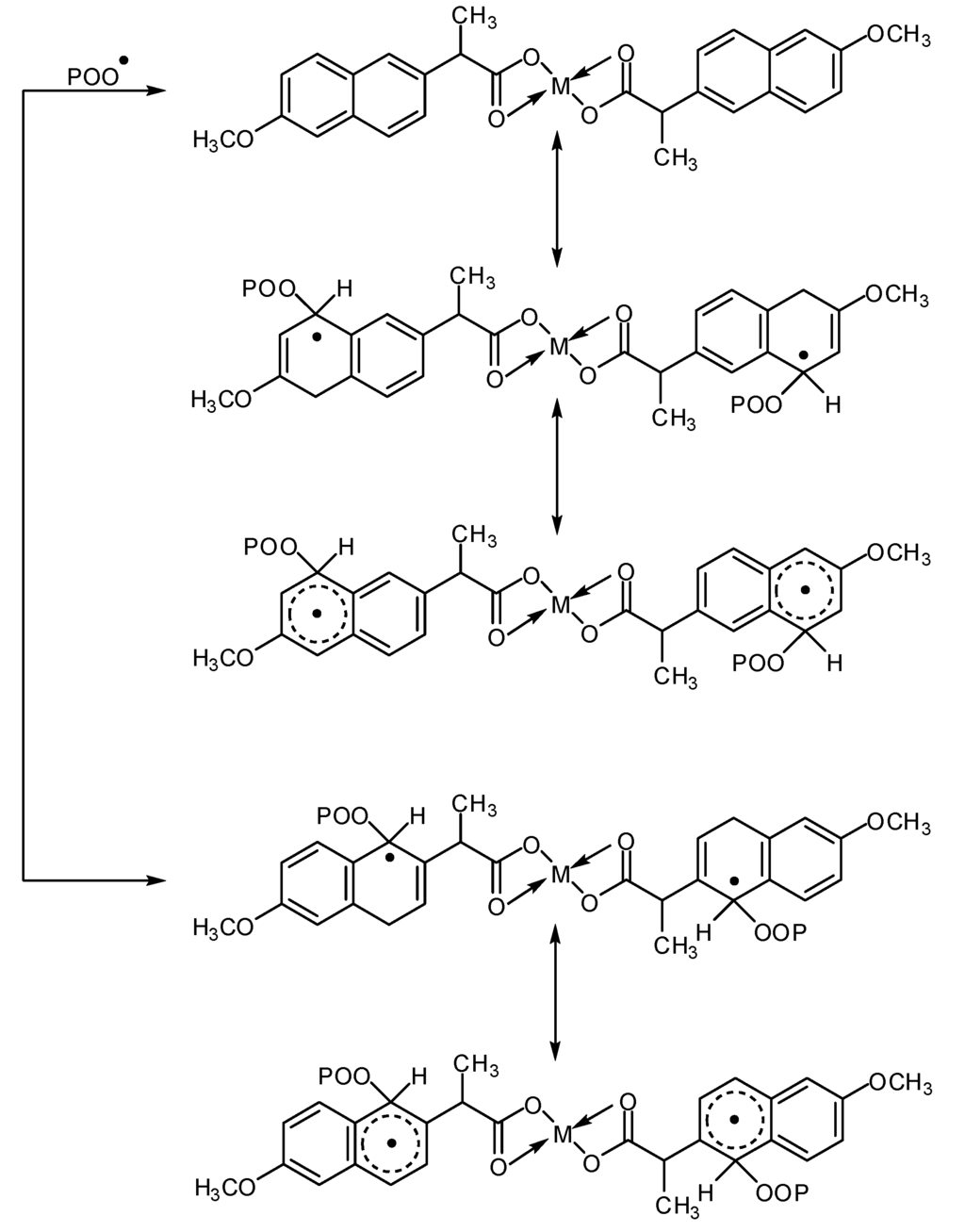
Scheme 3.
Possible photostabilization of carboxylates complexes as radical scavengers through energy transfer.
4. Conclusions
The photostabilization of PMMA films of bis[2-(6-methoxynaphthalen-2-yl)propanoate] complexes was studied in which additives act successfully as PMMA film photostabilizers. The order of photostabilization activity of the additives was NiL2 > CuL2 > ZnL2 > SnL2 as a result of the decrease in hydroxyl index for PMMA films.
The additives stabilize the PMMA films through different mechanisms, including a peroxide decomposer, a radical scavenger, UV absorption or screening. Such mechanisms support the aim to use bis[2-(6-methoxynaphthalen-2-yl)propanoate] complexes as commercial stabilizers for PMMA. Based on the photostability, the complex containing NiL2 was found to be the most efficient in the photostabilization process.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for its funding for this research through the research group Project RGP-VPP-239 and to the Department of Chemistry, College of Science, Al-Nahrain University, for continued support.
Author Contributions
Emad Yousif conceived of and designed the experiments. Raghad Haddad performed the experiments and analyzed the data. Gamal A El-Hiti provided the funds and revised the paper. Emad Yousif, Gamal A El-Hiti and Raghad Haddad wrote the paper. All of the authors discussed the results and improved the final text of the paper.
Conflicts of Interests
The authors declare no conflict of interest.
References
- Chmela, Š.; Lajoie, P.; Hrdlovič, P.; Lacoste, J. Combined oligomeric light and heat stabilizers. Polym. Degrad. Stabil. 2001, 71, 171–177. [Google Scholar]
- Zhao, Y.; Dan, Y. Preparation and characterization of a high molecular weight UV-stabilizer based on a derivative of 2,4-dihydroxybenzophenone and its application in polymer aterials. J. Appl. Polym. Sci. 2006, 102, 2203–2211. [Google Scholar] [CrossRef]
- Grassie, N.; Scott, G. Polymer Degradation and Stabilization; Cambridge University Press: London, UK, 1985. [Google Scholar]
- Andrady, A.L.; Hamid, S.H.; Hu, X.; Torikai, A. Effects of increased solar ultraviolet radiation on materials. In environmental effects of ozone depletion. J. Photochem. Photobiol. B 1998, 46, 96–103. [Google Scholar] [CrossRef]
- Harper, D.J.; McKellar, J.F.; Turner, P.H. Photostabilizing effect of Ni(II) chelates in polymers. II. Effect of diamagnetic chelates on polypropylene phosphorescence. J. Appl. Polym. Sci. 1974, 18, 2805–2807. [Google Scholar]
- Shneshil, M.K.; Redayan, M.A. Photostabilization of PVC films by using some novel tetra Schiff’s bases derived from1,2,4,5-tetra-[5-amino-1,3,4-thiadiazole-2-yl]benzene. Diyala J. Pure Sci. 2010, 7, 34–77. [Google Scholar]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398–460. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, C.T. Irradiation crosslinking of PVC–ENR blend effect of efficient radical scavenger. Plast. Rubber Compos. 2001, 30, 416–420. [Google Scholar] [CrossRef]
- Griffini, G.; Bella, F.; Nisic, F.; Dragonetti, C.; Roberto, D.; Levi, M.; Bongiovanni, R.; Turri, S. Multifunctional luminescent down shifting fluoropolymer coatings: A straightforward strategy to improve the UV-light harvesting ability and long term outdoor stability of organic dye sensitized solar cells. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Bella, F.; Griffini, G.; Gerosa, M.; Turri, S.; Bongiovanni, R. Performance and stability improvements for dye-sensitized solar cells in the presence of luminescent coatings. J. Power Sources 2015, 283, 195–203. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photochemical production of interpenetrating polymer networks; SimultaneousiInitiation of radical and cationic polymerization reactions. Polymers 2014, 6, 2588–2610. [Google Scholar] [CrossRef]
- Mosnáček, J.; Kundys, A.; Andicsová, A. Reversible-deactivation radical polymerization of methyl methacrylate induced by photochemical reduction of various copper catalysts. Polymers 2014, 6, 2862–2874. [Google Scholar] [CrossRef]
- Shih, H.-K.; Chen, Y.H.; Chu, Y.L.; Cheng, C.-C.; Chang, F.-C.; Zhu, C.-Y.; Kuo, S.-W. Photo-crosslinking of pendent uracil units provides supramolecular hole injection/transport conducting polymers for highly efficient light-emitting diodes. Polymers 2015, 7, 804–818. [Google Scholar] [CrossRef]
- Buruaga, L.; Pomposo, J.A. Metal-free polymethyl methacrylate (PMMA) nanoparticles by enamine “click” chemistry at room temperature. Polymers 2011, 3, 1673–1683. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Poly(propylene sulfide)-borane: Convenient and versatile reagent for organic synthesis. Tetrahedron 2012, 68, 7834–7839. [Google Scholar] [CrossRef]
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A.; Alshammari, M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015, 36, 74–85. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; Pardasani, R.T.; El-Hiti, G.A. New polymeric sulfide–borane complexes: Convenient hydroborating and reducing reagents. J. Sulfur Chem. 2011, 32, 287–295. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A.; Al-Zuhairi, A.J. The synthesis of polymeric sulfides by reaction of dihaloalkanes with sodium sulphide. J. Sulfur Chem. 2011, 32, 521–531. [Google Scholar] [CrossRef]
- Yousif, E.A.; Aliwi, S.M.; Ameer, A.A.; Ukal, J.R. Improved photostability of PVC films in the presence of 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes. Turk. J. Chem. 2009, 33, 399–410. [Google Scholar]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Ahmed, A.; Shneine, J.; Yousif, E.; Salman, H. Synthesis and characterization on some metal ions of 2-(6-methoxynaphthalen-2-yl)propanoate complexes. J. Al-Nahrain Univ. 2013, 16, 46–50. [Google Scholar]
- Yousif, E.; Salimon, J.; Salih, N. Improvement of the photostabilization of PVC films in the presence of thioacetic acid benzothiazole complexes. Malaysian J. Anal. Sci. 2011, 15, 81–92. [Google Scholar]
- Rabek, J.; Ranby, B. Photodegradation, Photooxidation and Photostabilization of Polymers; John Wiley: New York, NY, USA, 1975. [Google Scholar]
- Mark, J. Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
- Nakajima, N.; Sadeghi, M.R.; Kyu, T. Swelling of PVC particles during gelation and fusion of plastisols as observed with small-angle light scattering. J. Appl. Polym. Sci. 1990, 41, 889–363. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N. Mechanism of photostabilization of poly(methy methacrylate) films by 2-thioacetic acid benzothiazol complexes. Arabian J. Chem. 2014, 7, 306–311. [Google Scholar] [CrossRef]
- Yousif, E.; Salih, N.; Salimon, J. Improvement of the photostabilization of PVC films in the presence of 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole. J. Appl. Polym. Sci. 2011, 120, 2207–2214. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N. New stabilizers for polystyrene based on 2-thioacetic acid benzothiazol complexes. J. Appl. Polym. Sci. 2012, 125, 1922–1927. [Google Scholar] [CrossRef]
- Andrady, A.L.; Searle, N.D. Photodegradation of rigid PVC formulations. II. Spectral sensitivity to light-induced yellowing by polychromatic light. J. Appl. Polym. Sci. 1989, 37, 2789–2802. [Google Scholar]
- Mori, F.; Koyama, M.; Oki, Y. Studies on photodegradation of poly(vinyl chloride (part I). Die Angew. Makromol. Chem. 1997, 64, 89–99. [Google Scholar] [CrossRef]
- Shyichuk, A.V.; White, J.R. Analysis of chain-scission and crosslinking rates on the photo–oxidation of polystyrene. J. Appl. Polym. Sci. 2000, 77, 3015–3023. [Google Scholar] [CrossRef]
- Gugumus, F. Mechanism of Polymer Degradation and Stabilization; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Pospíšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Shahroosvand, H.; Rezaei, S.; Mohajerani, E.; Mahmoudi, M.; Kamyabi, M.A.; Nasiri, S.; Affiliations, S. Key role of ancillary ligands in imparting blue shift in electroluminescence wavelength in ruthenium polypyridyl light-emitting diodes. New J. Chem. 2014, 38, 5312–5323. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal-organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef] [PubMed]
- Anthonysamy, A.; Lee, Y.; Karunagaran, B.; Ganapathy, V.; Rhee, S.-W.; Karthikeyan, S.; Kim, K.S.; Ko, M.J.; Park, N.-G.; Ju, M.-J.; et al. Molecular design and synthesis of ruthenium(II) sensitizers for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 12389–12397. [Google Scholar]
- Yousif, E.; Hameed, A.; Rasheed, R.; Mansoor, H.; Farina, Y.; Graisa, A.; Salih, N.; Salimon, J. Synthesis and photostability study of some modified poly(vinyl chloride) containing pendant benzothiazole and benzimidozole ring. Int. J. Chem. 2010, 2, 65–80. [Google Scholar] [CrossRef]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; Volume 1, pp. 48–49. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).