Abstract
Feather keratin (FK) extracted from feathers represents a valuable source of biodegradable and biocompatible polymer. The aim of this study was the development and characterization of blended films based on FK and polyvinyl alcohol (PVA) cross-linked by dialdehyde starch (DAS) for a potential drug release application. The compatibility of FK/PVA was improved when cross-linked by DAS: the relative crystallinity of the PVA/FK film slightly decreased, and the enthalpy value for the melting peak decreased by about 50% for the cross-linked films. The total soluble mass of all blend films in water was below 35% at 37 °C, indicating a good stability of the films in water. The results of the Rhodamine B dye (as a model drug) release tests showed that the release rates decreased with increasing DAS content. DAS-induced cross-linking improves several important properties of the FK/PVA films, such as the compactness, the compatibility, and the stability in water. These improvements offer the potential to expand the application of FK films in the biomaterial field.
1. Introduction
Over the last 20 years, an increased number of research publications focused on biomaterials obtained from renewable resources such as proteins, polysaccharides, and lipids [1,2]. Most materials of biological origin degrade into water, carbon dioxide, and inorganic compounds, and are considered to be environmentally friendly [3]. Using biodegradable materials is considered the most effective solution for a number of environmental pollution problems caused by synthetic polymers. However, there are concerns that the potential source of such material could compete with food resources. Thus, there is an increasing interest in finding new sources of biomaterials that do not originate from potential food sources [4]. One of the best choices is feather keratin (FK), obtainable in large quantities from the poultry industry, which produces millions of tons of feathers annually throughout the world [5,6]. Although feathers are cheap, a renewable resource, biodegradable, and easily available, their utilization in industrial applications is limited [7]. Currently, they are used for the production of animal feed, as well as duvets and down coats. However, most of the feathers end up in landfills as solid waste, resulting in environmental and economic problems [8,9]. Furthermore, feathers in duvets and down coats could lead to secondary pollution when these down products are eventually abandoned. In order to lower the solid waste pollution, several research groups around the world are working on improving the processing of feathers and improving FK extraction methods.
Feathers contain more than 90% FK, with a cysteine residue content of about 7%, which is higher than the content of other proteins. The cysteine residues can be oxidized to generate inter- or intramolecular disulfide bonds, and form a cross-link network, which leads to the high thermal stability, high mechanical strength, and hydrophobicity of feathers, and their good stability in the solid state [10,11]. Films developed only from keratin exhibit relatively poor mechanical properties, are very fragile, and show a high moisture sensitivity, like other protein-based films. A variety of studies has been devoted to the improvement of the properties of keratin films by blending keratin with both natural [12,13,14,15,16] and synthetic polymers [6,17]. Nevertheless, using natural polymer/keratin blend films would just enhance the film-forming ability but not fundamentally improve the water resistance, due to the high hydrophilicity of natural polymers. On the other hand, using synthetic polymers in the keratin blends would not completely eliminate the problem of environmental contamination, since most of the synthetic polymers are not biodegradable.
In our work, we have used polyvinyl alcohol (PVA) as the coalescent agent for the preparation of FK-based films. PVA is a nontoxic, biodegradable, synthetic, water-soluble polymer with outstanding chemical stability and good film-forming capacity [18,19]. PVA-protein films are produced as materials for food packing, tissue engineering, and drug delivery systems [20,21,22,23]. PVA hydrogel materials containing keratin from wool and hair were recently synthesized by electron beam irradiation [24]. To improve the mechanical properties, moisture sensitivity, and thermal stability of these protein/PVA blend films, the polymers were cross-linked with glutaraldehyde. Glutaraldehyde interacts with both PVA and the protein and binds them together [20,25]. However, there are always concerns about the toxicity of glutaraldehyde residuals in materials, which limits the application of glutaraldehyde in the biomaterial and food packing industries [26]. Dialdehyde starch (DAS) has been suggested as an alternative to glutaraldehyde due to its lower toxicity. DAS is a polymeric aldehyde obtained by controlled periodate oxidative cleavage of the C2–C3 bond of the anhydroglucose units of native starch.
Although there are some reports on using DAS as cross-linking agent for various proteins, such as soy protein isolate [26,27], collagen [28], corn zein [29], flour starch–protein [30], and whey protein [31], to the best of our knowledge there are very few studies on keratin materials cross-linked with DAS. We previously reported that the mechanical properties, thermal stability, and water vapor barrier property of FK/PVA blended films could be improved by DAS-induced cross-linking [32].
This study extends our investigations to test the effect of DAS on the microstructure, crystallization behavior, compatibility, stability in water, and delayed drug release of FK/PVA films.
2. Materials and Methods
2.1. Materials
PVA with a degree of polymerization of roughly 1700 and a degree of alcoholysis of approximately. 99% was purchased from Aladdin Ltd. (Shanghai, China). The chicken feather keratin (FK) solution and the dialdehyde starch (DAS) were prepared as described in our previous work [32].
2.2. Preparation of the Blended Films and the Dye-Loaded Films
The pH value of the FK solution was adjusted to 9.5 using a 0.1 mol·L−1 NaOH solution under continuous stirring at 40 °C for 10 min. The DAS aqueous solution was prepared by adding 2 g of DAS powder to 48 g of deionized water. The pH value of the resulting solution was again adjusted to 9.5 by adding a 0.1 mol·L−1 NaOH solution under continuous stirring at 40 °C for 30 min. Mixtures of the FK solution and different weight ratios of the 15% PVA aqueous solution and the 4% DAS solution were produced as the film-forming solution. The pH value of the mixture was adjusted to 9.5 using a 0.1 mol·L−1 NaOH solution, followed by an annealing step at 60 °C under continuous stirring for 20 min. The mixture was placed in an ultrasonic bath to remove any air bubbles, and then poured into a polypropylene dish to prepare the FK films. The sample was dried in an oven at 60 °C for 6 h, and then cooled down to room temperature. The resulting films could be easily peeled from the dish.
A series of FK/PVA films labeled as P-10, P-20, P-30 and P-40 were fabricated with FK to PVA wt% ratios of 90:10, 80:20, 70:30, and 60:40, respectively. Another series of FK/PVA films with added DAS were labeled as P-PVA-n, where n denotes the weight percent of DAS relative to the total weight of FK and PVA in the film. For example, P-30-10 corresponds to a weight ratio of FK to PVA of 70:30 in the film, and the DAS content relative to the total weight of FK and PVA was 10 wt%. One weight percent of Rhodamine B (RB) (wt% based on the weight of the blended film) was added to the film-forming solution after the addition of DAS to prepare the dye-loaded film according to the procedure described above.
2.3. Characterization
The surface of the resulting films was investigated by scanning electron microscopy (SEM) at an acceleration voltage of 20 kV using a Quanta 400 SEM (Oxford, UK). Prior to the SEM observations, the samples were coated with a fine gold layer.
An X-ray diffraction (XRD) analysis was performed using an X-ray diffractometer (D/max 2200 UPC, Rigaku, Osaka, Japan) operated at 30 kV and 30 mA.
The temperature co-ordinates of the characteristic peaks were assessed using a differential scanning calorimeter (DSC, Instruments 910s, Mettler, Zurich, Switzerland) and open aluminum pans. The samples were first heated from 25 to 105 °C at a heating rate of 20 °C·min−1, then kept at this temperature for 5 min, and then cooled from 100 to −60 °C, again at a rate of 20 °C·min−1. The second heating step was from −60 to 250 °C at a heating rate of 10 °C·min−1.
The total soluble mass (TSM) of the films in water at 37 °C was obtained using the following method: first, the rectangular specimens (40 mm × 10 mm) were preconditioned by drying in an air oven at 70 °C for 24 h, then cooled in a desiccator for a few minutes and then immediately weighed (W1). Under oscillation, the preconditioned specimens were immersed in distilled water at 37 ± 2 °C for 24 h with shaking. The resulting wet specimens were dried again in an oven at 70 °C for 24 h, cooled in a desiccator, and immediately weighed again (W2). Afterwards, the TSM of the films was calculated using the following equation:
TSM (%) = (W1 − W2)/W1 × 100%
2.4. Dye Release
To investigate the release of Rhodamine B (RB) from the blended films, the dye-loaded films were processed in ultrapure water in a shaking water bath with the temperature regulated at 37 °C, with a film to solution ratio of 1:100 (g/mL). The amounts of RB released from the dye-loaded films were calculated by measuring the absorbance at 553 nm using a UV-VIS spectrophotometer (UV-2450, Shimadzu Corporation, Suzhou, China).
3. Results and Discussion
3.1. Morphology of the Films
After blending PVA with FK, macroscopically uniform, yellowish, optically semitransparent films were obtained. The surfaces of the FK/PVA blend films look very smooth, without visible cracks or holes. The appearance of these films did not change when different DAS and PVA ratios were used. A fragile film was formed for PVA ratios lower than 10%. All films could be easily peeled from the dish. However, the appearance of the two sides of the film was different both for films containing DAS and films not containing DAS. The film side facing the casting plate was shiny, while the other side was dull, probably due to the polymer arrangement during solvent evaporation. This phenomenon was also observed for whey protein isolate films by Óscar et al. [33].
The surface and fracture morphology of the cast PVA/FK blend films was examined by SEM (Figure 1). The SEM images reveal that the samples (keratin film, P-40 and P-40-10) have a compact and continuous structure, and voids were not found even at higher magnifications. The SEM images reveal a very similar surface morphology for the blended samples both for films containing DAS and not containing DAS, showing the formation of compact and continuous films with good structural integrity. However, compared with the pure keratin film, the surface of all samples (P-40 and P-40-10) is less smooth, with some teardrop-shaped bulges revealed at higher magnifications, which can probably be attributed to the different coacervation kinetics of FK compared to PVA. This phenomenon is further verified by the fracture morphology of the blended films. The keratin films exhibit a relatively smooth and homogeneous fracture surface. A fluctuating and rough fracture surface with a few small particles inside the matrix was observed for the blend films without cross-linking (P-40), suggesting a phase separation caused by PVA aggregates in the FK matrix. This phase separation phenomenon was also observed for a PVA/silk fibroin blended system by Tsukada et al. [34]. On the other hand, the blend film cross-linked by DAS (P-40-10) did not show any particles, and a more uniform and compact fracture surface was observed. However, some cross-linked agglomerates were found in the form of spherical particles in these FK/PVA blends. In conclusion, the SEM results show that a phase separation occurred in the PVA/FK blended system, which could be suppressed by adding DAS as cross-linking agent. This indicates that the PVA and FK polymer chains are mostly entangled due to hydrogen bonds prior to the chemical cross-linking, but the formation of covalent bonds has occurred due to the DAS-induced cross-linking [35].
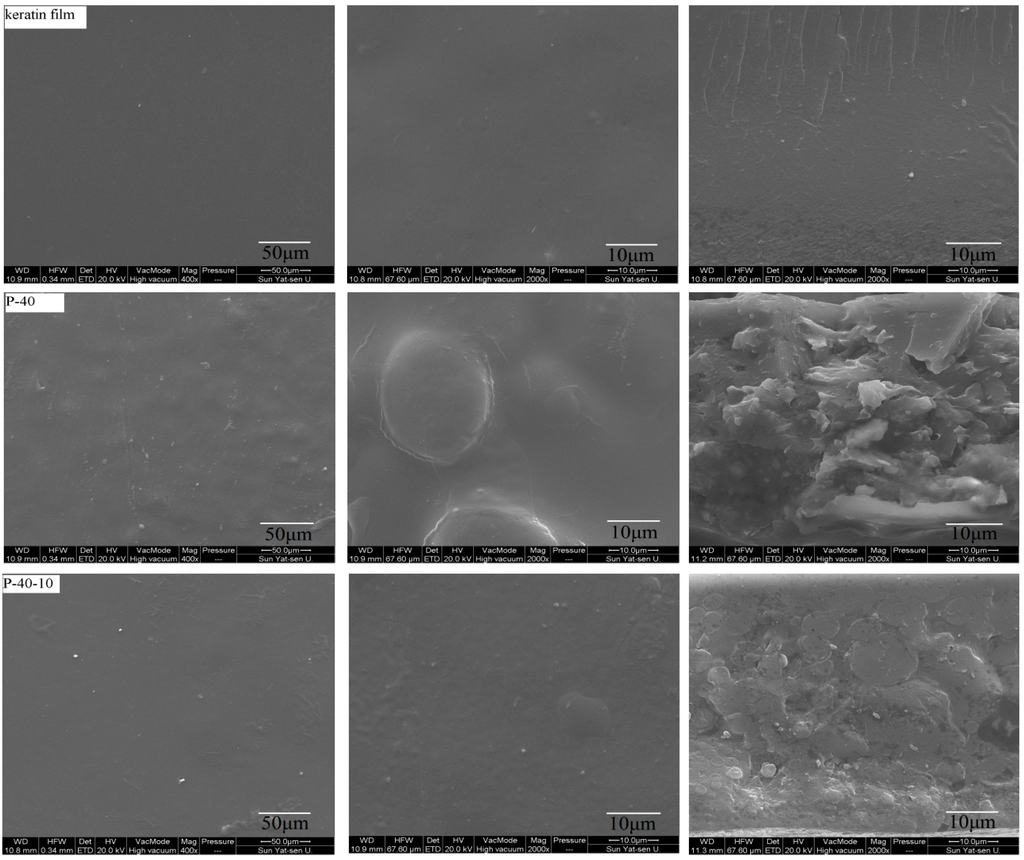
Figure 1.
Representative SEM images revealing the surface morphology (left, 400×; center, 2000×) and the fracture morphology (right, 2000×) of the FK/PVA blend films.
3.2. X-Ray Diffraction (XRD) Analysis
The XRD analysis revealed that the inclusion of DAS into the FK/PVA matrix slightly reduced the degree of crystallinity, probably due to the formation of covalent bonds. The XRD patterns of pure PVA, pure FK, and the film P-30 are shown in Figure 2a. The XRD pattern obtained for pure FK exhibits broad diffraction peaks at 2θ = 9° and 20°, which are typical fingerprints of partially crystalline materials, whereas sharp diffraction peaks at 2θ = 19° were observed for pure PVA, indicating a high crystallinity. The blend film (P-30) also exhibits similar diffractograms for the films based on pure keratin (2θ = 9°) or pure PVA (2θ = 19°), respectively, indicating partially crystalline structures. However, the intensity of these peaks is lower than the intensity of the peaks observed for PVA, suggesting that the overall crystallinity of the blend films is slightly lower than the crystallinity of the PVA film due to the addition of FK, which contains a small crystalline and a large amorphous region. Figure 2b shows that the intensity of the characteristic peak slightly decreased after DAS was added to the FK/PVA blend (P-30-5 and P-30-15), indicating that the cross-linking induced by the addition of DAS reduces the relative crystallinity of the FK/PVA blend to some extent. However, some microcrystals remain in the polymer chain, probably due to the high crystallinity of PVA. Therefore, the cross-links partly reduce the degree of freedom in the polymer chains and limit the formation of crystalline regions.
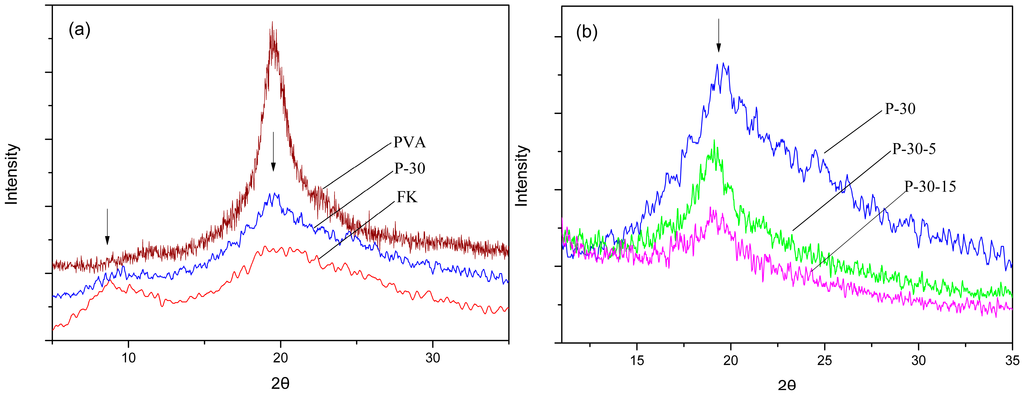
Figure 2.
XRD patterns obtained for (a) pure PVA and pure FK, and (b) the blend films P-30, P-30-5, and P-30-15.
3.3. DSC Analysis
Figure 3 reveals the DSC traces of the films dried at 70 °C for 24 h in an oven. Overall, the DSC plots of the pure keratin and the blended films correspond to partially crystalline structures. In the DSC curves, the endothermic peak at approximately 140 °C can be attributed to the evaporation of bound water (Te), representing the energy required to vaporize bound water present in the films, followed by another endothermic peak observed at about 220 °C related to the crystalline melting (Tm) of the films, and the peak area representing the crystallinity of the films [9,36]. At a PVA content of 30% in the blended film, the ∆He value for the vaporization of bound water increased from 232.0 to 303.7 J·K−1 (cp. Table 1), suggesting a higher water binding ability of the blended film, which may be due to the higher hydrophilicity of PVA. The addition of DAS caused a reduction of the ∆He value to about 149.7 J·K−1 due to the chemical cross-linking effect. This suggests that the FK/PVA blend films with DAS-induced cross-linking are less hydrophilic. The melting peak of the pure keratin film at 214.8 °C changed into two peaks: one at about 213.6 °C, in shoulder form, and the second at 217.2 °C. The value of ∆Hm obviously increases from 267.5 to 314.2 J·K−1 after adding 30 wt% PVA. Therefore, we suggest that FK and PVA are partly compatible and that the crystallinity of the blended film is higher than the crystallinity of the keratin film. It is worth noting that, after adding DAS to the mixture, the melting peak changed back to a single peak at 216 °C, and the ∆Hm value sharply decreased to 150.5 J·K−1, which could be caused by the formation of covalent bonds between the components and the cross-link network in the blended film. This indicates that adding DAS could improve the compatibility of FK with PVA and decrease the overall crystallinity of the blended films, which is consistent with the XRD results described above.
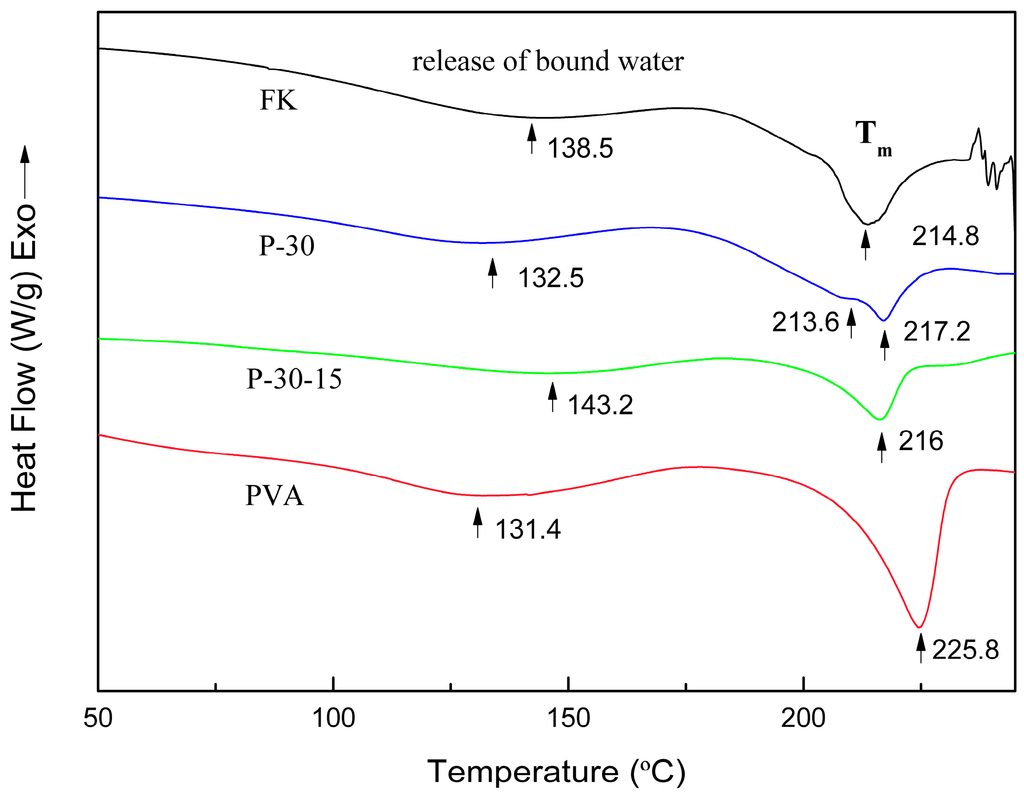
Figure 3.
Comparison of the DSC curves obtained for pure FK, P-30, P-30-15, and pure PVA, respectively.

Table 1.
Temperatures (°C) and enthalpy values (J·K−1) corresponding to the endothermic peaks observed for the different FK films.
| Sample | Te (°C) | ∆He (J·K−1) | Tm (°C) | ∆Hm (J·K−1) |
|---|---|---|---|---|
| FK | 138.5 | 232.0 | 214.8 | 267.5 |
| P-30 | 132.5 | 303.7 | 217.2 | 314.2 |
| P-30-15 | 143.2 | 149.7 | 216.0 | 150.5 |
| PVA | 131.4 | 296.1 | 225.8 | 461.2 |
An erratic peak above 240 °C was observed for the pure FK film, which is attributed to the decomposition of FK. However, this decomposition peak was not observed for the blended films (P-30 and P-30-15), suggesting that PVA interferes with the keratin self-assembling, causing the protein chains to organize in a thermally more stable secondary structure. The cross-link network formed in the blended film after DAS was added to the mixture improved the thermal stability of the blended film.
3.4. TSM of the Blend Films
The stability in water is an important property of protein-based films. For example, in drug-release applications, a high film integrity and stability in a humid environment are required over a relatively long period of time. Generally, cross-linking is the most common and useful method to reduce the TSM of protein-based films in water [37,38,39]. The TSM values obtained for the FK/PVA blended films cross-linked by DAS in water at 37 °C are listed in Figure 4. If DAS was not added, an increase of the PVA concentration in the blended films resulted in a slight decrease of the film’s solubility in water. This is in agreement with the results published by Elizondo et al., who reported that the TSM of films consisting of PVA and amaranth flour decreased to 44% as the PVA content was increased to 50% [40]. The interaction between FK and PVA is probably dominated by the FK/PVA ratio, and the interaction could prevent the penetration of water molecules into the films. Adding 5% of DAS sharply reduces the TSM of the film. However, adding more than 5% DAS has little effect on the TSM. The reduction of the TSM indicates an increase in the degree of cross-linking. Rhim et al. reported that the TSM of cast soy protein isolate films was significantly reduced by up to 50% by adding DAS [26]. Martucci et al. also reported that gelatin films with DAS-induced cross-linking exhibited a substantially lower solubility in water than films without a DAS treatment [39]. The soluble substance in the FK/PVA blend films could be the PVA, excessive DAS, and/or low molecular weight peptide chains that could not be cross-linked by DAS. According to the analyses above, it is clear that DAS, FK, and PVA certainly formed a cross-linked network structure.
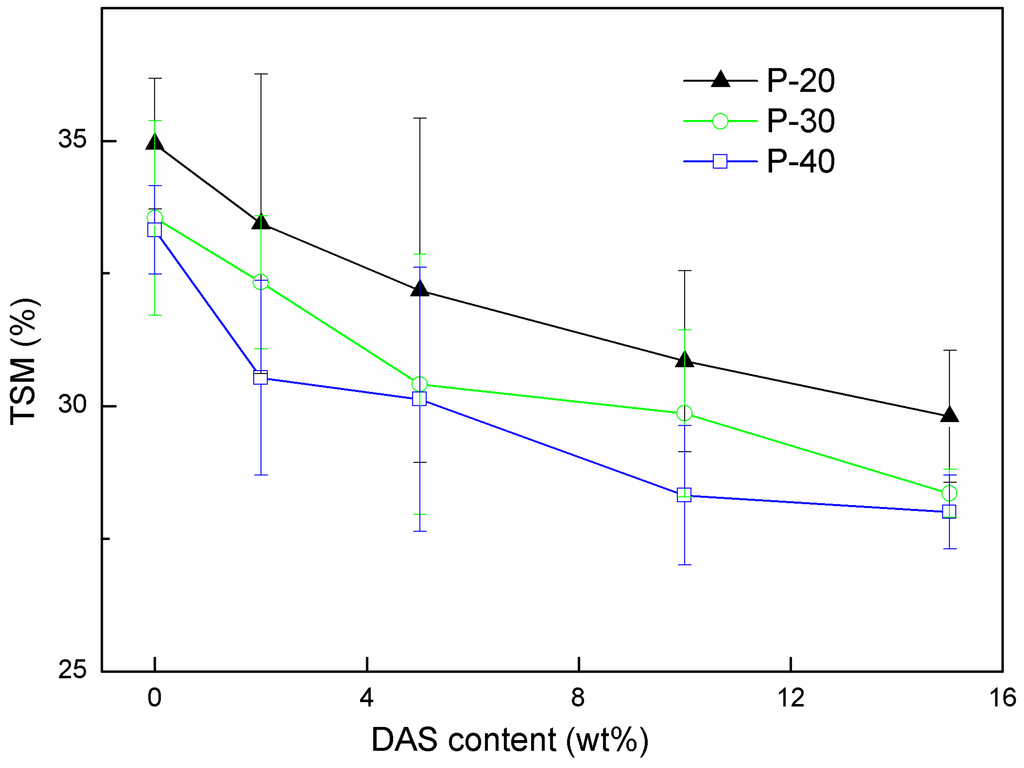
Figure 4.
The total soluble mass observed for the FK/PVA blended films in deionized water at 37 °C for 24 h, plotted as a function of the DAS content.
3.5. Rhodamine B Release from the PVA/FK Film
So far, little is known about the effect of materials produced from FK on the sustained release of medicine. Rhodamine B (RB) was used in this study as a model drug to investigate the effect of the DAS modification on the dye release rate from FK/PVA blended films in ultrapure water. According to the results of the solubility experiments, the blended films show a relatively low TSM and are more stable in water. As a consequence, we chose the blended films containing PVA at a ratio of 40% and cross-linked by DAS (5%, 10%, and 15%, respectively) as the potential drug delivery material. The effect of the concentration of the cross-linking agent on the cumulative release rate of RB is shown in Figure 5. Obviously, the amount of the drug released from the cross-linked blended film P-40-15 was lower than the amount released from the film P-40-5. Almost 42% of the RB was released from the film P-40-15 within 6 h, which was about 30% less than the amount released from the film P-40-5. The release rates were significantly reduced with increasing DAS concentration in the blended film. According to the results of the solubility experiments (cp. Figure 4), compared to the P-40-15 film, a larger fraction of non-cross-linked components of the P-40-5 film was dissolved in water. The DAS cross-linking reaction decreased the solubility of the films and formed interpenetrating networks, trapping the RB molecules and thereby reducing the drug release rate. This indicates that the RB release rate is closely related to the degree of cross-linking.
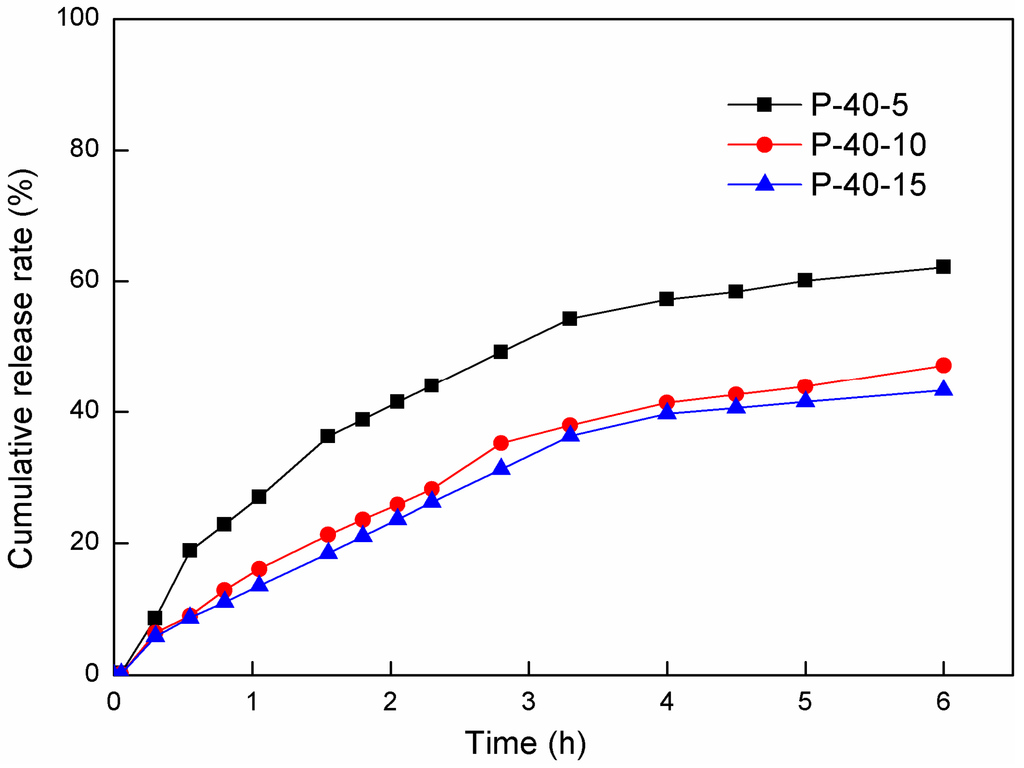
Figure 5.
Comparison of the RB release rate time curves measured for the release of RB from P-40 series films with different DAS content at 37 °C with shaking.
4. Conclusions
Extending our previous studies [32], FK/PVA films cross-linked by DAS were produced and their properties were investigated. In general, the properties of the films can be controlled by altering the ratio of PVA, FK, and DAS in the mixed system. The analysis of the film’s morphology, degree of crystallization, and thermal properties showed that the DAS-induced cross-linking improved the compactness and compatibility of the FK/PVA films due to the formation of covalent bonds. The total soluble mass of the blended films slightly decreased as the amount of DAS was increased. An investigation of the Rhodamine B release rate from the film revealed a reduction of the release rate as the DAS content was increased. In summary, the FK/PVA films cross-linked by DAS were produced from inexpensive, biodegradable, and eco-friendly materials and have the potential for a broad range of applications in the packing industry and the biomaterial field.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21176269) and the EU FP7-PEOPLE-2009-IRSES project ABREM (247599). Furthermore, the support from the Higher School Science and Technology Innovation Project of Guangdong Province (2013KJCX0102) and the Science and Technology Plan Project of Guangdong Province (2013B010403029) is also acknowledged.
Author Contributions
Guoqiang Yin and Yingde Cui conceived and directed the research; Yao Dou and Buning Zhang performed the experiments and data analysis and wrote the manuscript; Ming He contributed materials and analysis tools; and Irina Savina gave the important advice on the design of the experiment and the analysis of experimental data and reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rocha Plácido Moore, G.; Maria Martelli, S.; Gandolfo, C.; José do Amaral Sobral, P.; Borges Laurindo, J. Influence of the glycerol concentration on some physical properties of feather keratin films. Food Hydrocoll. 2006, 20, 975–982. [Google Scholar]
- Kundu, B.; Kurland, N.E.; Yadavalli, V.K.; Kundu, S.C. Isolation and processing of silk proteins for biomedical applications. Int. J. Biol. Macromol. 2014, 70, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-B.; Jo, W.-S.; Song, H.-Y.; Chung, K.-S.; Won, M.; Song, K.B. Effects of plasticizers and nano-clay content on the physical properties of chicken feather protein composite films. Food Hydrocoll. 2013, 31, 340–345. [Google Scholar] [CrossRef]
- Verbeek, C.J.R.; van den Berg, L.E. Recent developments in thermo-mechanical processing of proteinous bioplastics. Recent Pat. Mater. Sci. 2009, 2, 171–189. [Google Scholar] [CrossRef]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.; Sharif, A.; Kalaee, M.; Nouri, A.; Manafi, M. Morphology, nanomechanical and thermodynamic surface characteristics of nylon 6/feather keratin blend films: An atomic force microscopy investigation. Polym. Int. 2012, 61, 646–656. [Google Scholar] [CrossRef]
- Flores-Hernández, C.; Colín-Cruz, A.; Velasco-Santos, C.; Castaño, V.; Rivera-Armenta, J.; Almendarez-Camarillo, A.; García-Casillas, P.; Martínez-Hernández, A. All green composites from fully renewable biopolymers: Chitosan-starch reinforced with keratin from feathers. Polymers 2014, 6, 686–705. [Google Scholar] [CrossRef]
- Jin, E.; Reddy, N.; Zhu, Z.; Yang, Y. Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011, 59, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Vineis, C.; Ceria, A.; Tonin, C. Composite biomaterials from fibre wastes: Characterization of wool-cellulose acetate blends. Compos. A 2008, 39, 126–132. [Google Scholar] [CrossRef]
- Martelli, S.M.; Moore, G.R.P.; Laurindo, J.B. Mechanical properties, water vapor permeability and water affinity of feather keratin films plasticized with sorbitol. J. Polym. Environ. 2006, 14, 215–222. [Google Scholar] [CrossRef]
- Barone, J.R.; Schmidt, W.F. Nonfood applications of proteinaceous renewable materials. J. Chem. Educ. 2006, 83, 1003–1009. [Google Scholar] [CrossRef]
- Prasong, S.; Wasan, T. Preparation and characterization of hair keratin/gelatin blend films. Pak. J. Biol. Sci. 2011, 14, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Okitsu, N.; Tachibana, A.; Yamauchi, K. Preparation and characterization of keratin-chitosan composite film. Biomaterials 2002, 23, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Tian-Yu, K.; Dong-Xia, L.; Xu-Hong, Y. Preparation and properties of keratin/cmc blend membranes. Adv. Mater. Res. 2013, 647, 190–194. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, A.; Boopalan, K.; Radhakrishnan, G.; Sankaran, S.; Das, B.N.; Sastry, T.P. Preparation of feather keratin hydrolyzate-gelatin composites and their graft copolymers. J. Macromol. Sci. A. 2005, 42, 1703–1713. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Patrucco, A.; Zoccola, M. Wool keratin-polypropylene composites: Properties and thermal degradation. Polym. Degrad. Stab. 2013, 98, 980–987. [Google Scholar] [CrossRef]
- Subramanian, U.M.; Kumar, S.V.; Nagiah, N.; Sivagnanam, U.T. Fabrication of polyvinyl alcohol-polyvinylpyrrolidone blend scaffolds via electrospinning for tissue engineering applications. Int. J. Polym. Mater. 2014, 63, 462–470. [Google Scholar] [CrossRef]
- Mao, Y.; Mohanty, P.; Ghosh, G. Biomimetic apatite-coated porous pva scaffolds promote the growth of breast cancer cells. Mater. Sci. Eng. C 2014, 44, 310–316. [Google Scholar] [CrossRef]
- Alves, P.M.A.; Carvalho, R.A.; Moraes, I.C.F.; Luciano, C.G.; Bittante, A.M.Q.B.; Sobral, P.J.A. Development of films based on blends of gelatin and poly(vinyl alcohol) cross linked with glutaraldehyde. Food Hydrocoll. 2011, 25, 1751–1757. [Google Scholar] [CrossRef]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Properties of biodegradable blend films based on fish myofibrillar protein and polyvinyl alcohol as influenced by blend composition and ph level. J. Food Eng. 2010, 100, 85–92. [Google Scholar] [CrossRef]
- Su, J.-F.; Yuan, X.-Y.; Huang, Z.; Xia, W.-L. Properties stability and biodegradation behaviors of soy protein isolate/poly (vinyl alcohol) blend films. Polym. Degrad. Stab. 2010, 95, 1226–1237. [Google Scholar] [CrossRef]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/PVA blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, B.-S.; Shin, H.K.; Park, S.-J.; Kim, H.-Y. Preparation and characterization of keratin-based biocomposite hydrogels prepared by electron beam irradiation. Mater. Sci. Eng. C 2013, 33, 5051–5057. [Google Scholar] [CrossRef]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly(vinyl alcohol) films crosslinked by glutaraldehyde under mild conditions. J. Appl. Polym. Sci. 2009, 111, 3074–3080. [Google Scholar] [CrossRef]
- Rhim, J.W.; Gennadios, A.; Weller, C.L.; Cezeirat, C.; Hanna, M.A. Soy protein isolate dialdehyde starch films. Ind. Crop. Prod. 1998, 8, 195–203. [Google Scholar] [CrossRef]
- Rhim, J.W.; Gennadios, A.; Handa, A.; Weller, C.L.; Hanna, M.A. Solubility, tensile, and color properties of modified soy protein isolate films. J. Agric. Food Chem. 2000, 48, 4937–4941. [Google Scholar] [CrossRef] [PubMed]
- Langmaler, F.; Mokrejs, P.; Kolomamik, K.; Mladek, M. Biodegradable packing materials from hydrolysates of collagen waste proteins. Waste Manag. 2008, 28, 549–556. [Google Scholar] [CrossRef]
- Parris, N.; Coffin, D.R. Composition factors affecting the water vapor permeability and tensile properties of hydrophilic zein films. J. Agric. Food Chem. 1997, 45, 1596–1599. [Google Scholar]
- Mokrejs, P.; Langmaier, F.; Janacova, D.; Mladek, M.; Kolomaznik, K.; Vasek, V. Thermal study and solubility tests of films based on amaranth flour starch-protein hydrolysate. J. Therm. Anal. Calorim. 2009, 98, 299–307. [Google Scholar]
- Ustunol, Z.; Mert, B. Water solubility, mechanical, barrier, and thermal properties of cross-linked whey protein isolate-based films. J. Food Sci. 2004, 69, 129–133. [Google Scholar]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and physicochemical properties of dialdehyde starch crosslinked feather keratin/pva composite films. J. Macromol. Sci. A 2014, 51, 1009–1015. [Google Scholar] [CrossRef]
- Ramos, O.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Fatima Pocas, M.; Pintado, M.E.; Xavier Malcata, F. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Crighton, J.S. Structure and compatibility of poly(vinyl alcohol)-silk fibroin (PVA/SA) blend films. J. Polym. Sci. B Polym. Phys. 1994, 32, 243–248. [Google Scholar] [CrossRef]
- Costa-Júnior, E.S.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
- Nakano, Y.; Bin, Y.; Bando, M.; Nakashima, T.; Okuno, T.; Kurosu, H.; Matsuo, M. Structure and mechanical properties of chitosan/poly(vinyl alcohol) blend films. Macromol. Symp. 2007, 258, 63–81. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Effect of crosslinking on the properties of sodium caseinate films. J. Appl. Polym. Sci. 2010, 116, 18–26. [Google Scholar] [CrossRef]
- Hernandez-Munoz, P.; Villalobos, R.; Chiralt, A. Effect of cross-linking using aldehydes on properties of glutenin-rich films. Food Hydrocoll. 2004, 18, 403–411. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Tensile properties, barrier properties, and biodegradation in soil of compression-molded gelatin-dialdehyde starch films. J. Appl. Polym. Sci. 2009, 112, 2166–2178. [Google Scholar] [CrossRef]
- Elizondo, N.J.; Sobral, P.J.A.; Menegalli, F.C. Development of films based on blends of amaranthus cruentus flour and poly(vinyl alcohol). Carbohydr. Polym. 2009, 75, 592–598. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).