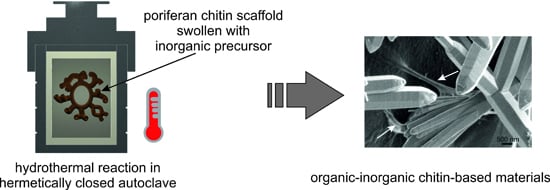
Poriferan Chitin as a Versatile Template for Extreme Biomimetics
Abstract
:1. Introduction
1.1. Historical Landmarks in the Discovery of Chitin
1.2. Discovery of Chitin in Sponges
2. Structural Properties of Chitin
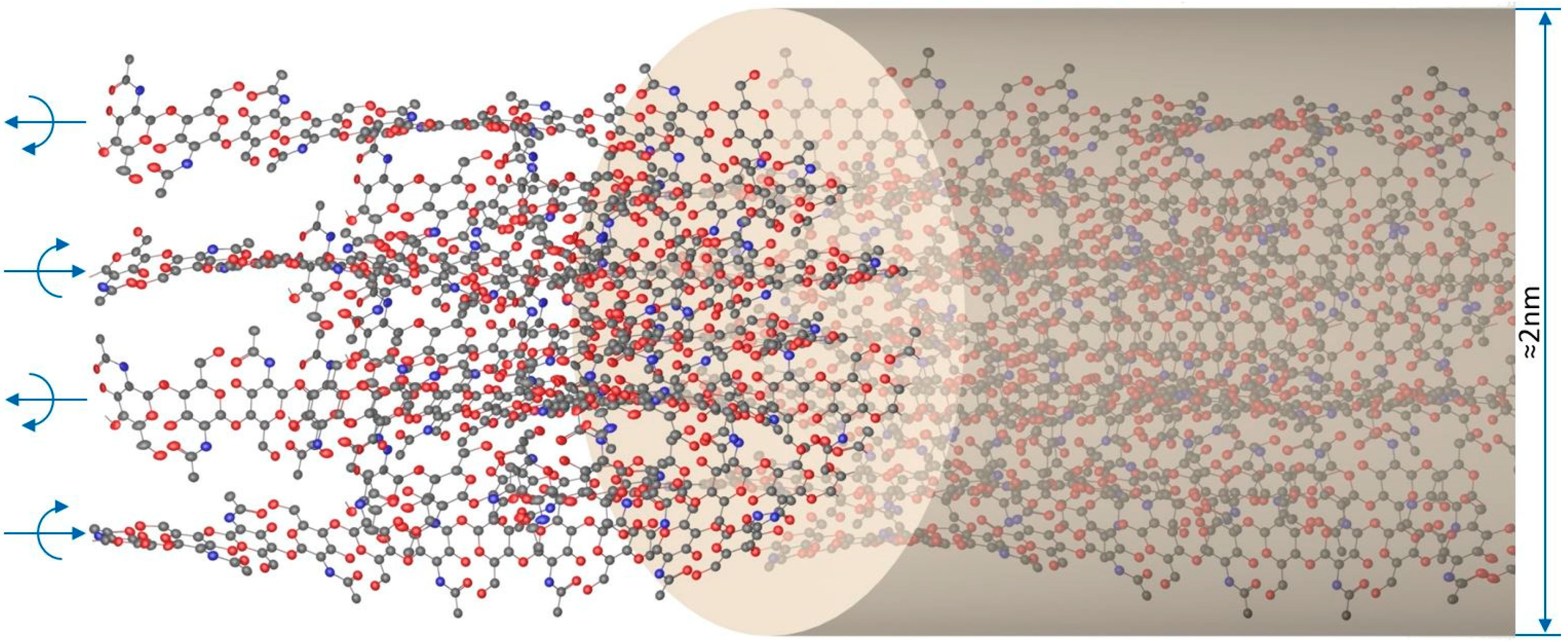
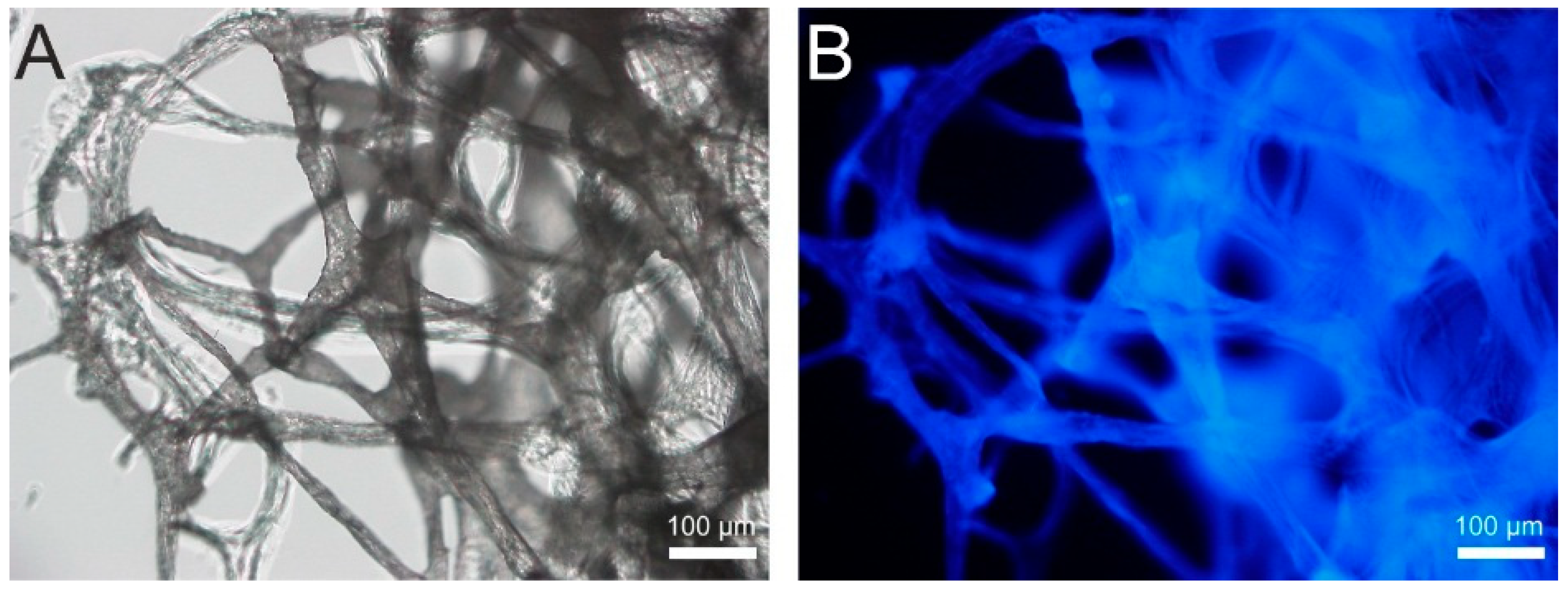
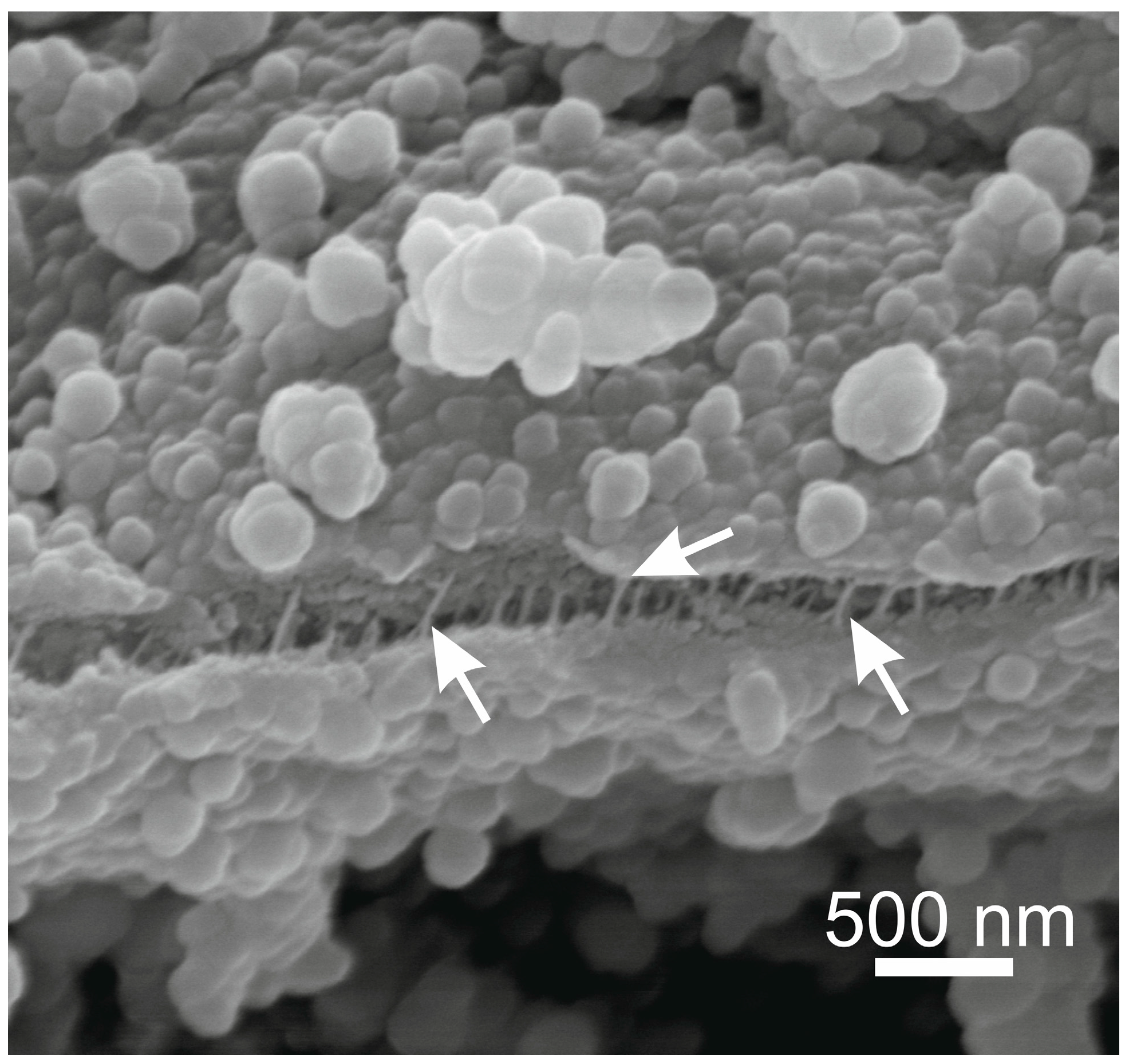
3. Mechanical Stability of Chitin-Based Materials
4. Thermal Stability of Chitin
| Chitin Source | Thermal Degradation (°C) | Atmosphere | Ref. |
|---|---|---|---|
| unknown | 287–427 | Inert | [131] |
| krill (α-chitin) | 250–410 | Inert | [133] |
| crab shells (α-chitin) | 220–400 | Inert | [130] |
| regenerated chitin—from crab shells (α-chitin) | 260–435 | Inert | [136] |
| Euphausia superba krill (α-chitin) | 320–460 | Inert | [49] |
| shrimp shells (α-chitin) | 290–440 | Inert | [49] |
| crab shells (α-chitin) | 285–450 | Inert | [49] |
| squid (β-chitin) | 210–400 | Inert | [49] |
| Ianthella basta marine sponge (α-chitin) | 325–530 | Inert | [114] |
| Aplysina cauliformis marine sponge (α-chitin) | 300–420 | Inert | [137] |
| crab shells (α-chitin) | 290–400 | Inert | [84] |
| crab shells (α-chitin) | 300–410 | Inert | [84] |
| Loligo pealeii squid (β-chitin) | 260–370 | Inert | [84] |
| Squid (γ-chitin) | 200–370 | Inert | [84] |
| crab shells (α-chitin) | 235–570 | oxidizing | [135] |
| Oedipoda miniata orthoptera (α-chitin) | 280–405 | Inert | [138] |
| Geolycosa vultuosa Hogna radiata spiders (α-chitin) | 275–400 | inert | [35] |
| Chelonibia patula (α-chitin) | 180–600 | Inert | [139] |
5. Chitin and Extreme Biomimetics
5.1. Hydrothermal Vents and Hot Springs as a Source of Inspiration
5.2. Hydrothermal Technology for Extreme Biomimetics
5.3. Extreme Biomimetics: Development of Chitin-Based Inorganic-Organic Materials under Hydrothermal Conditions
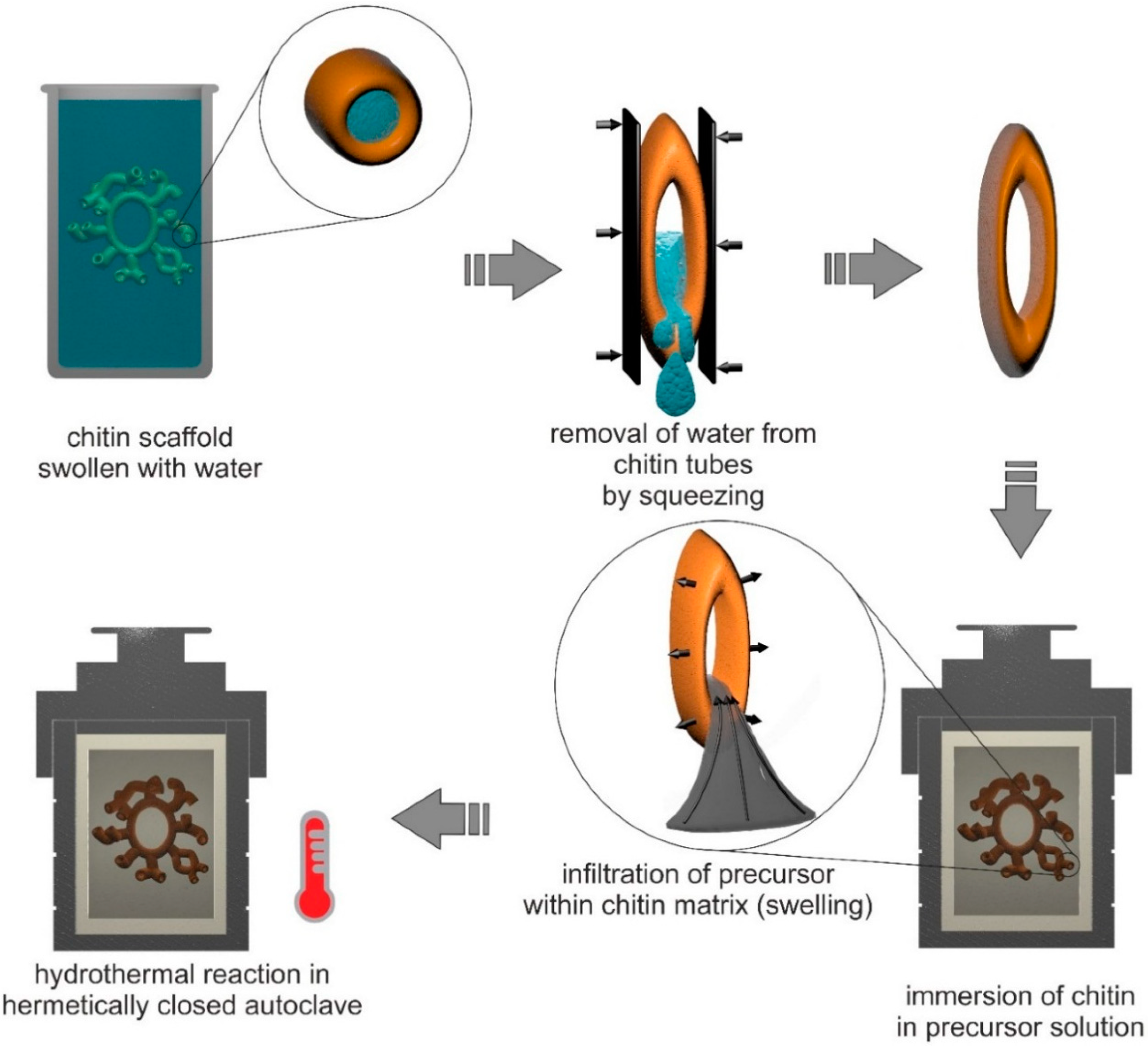
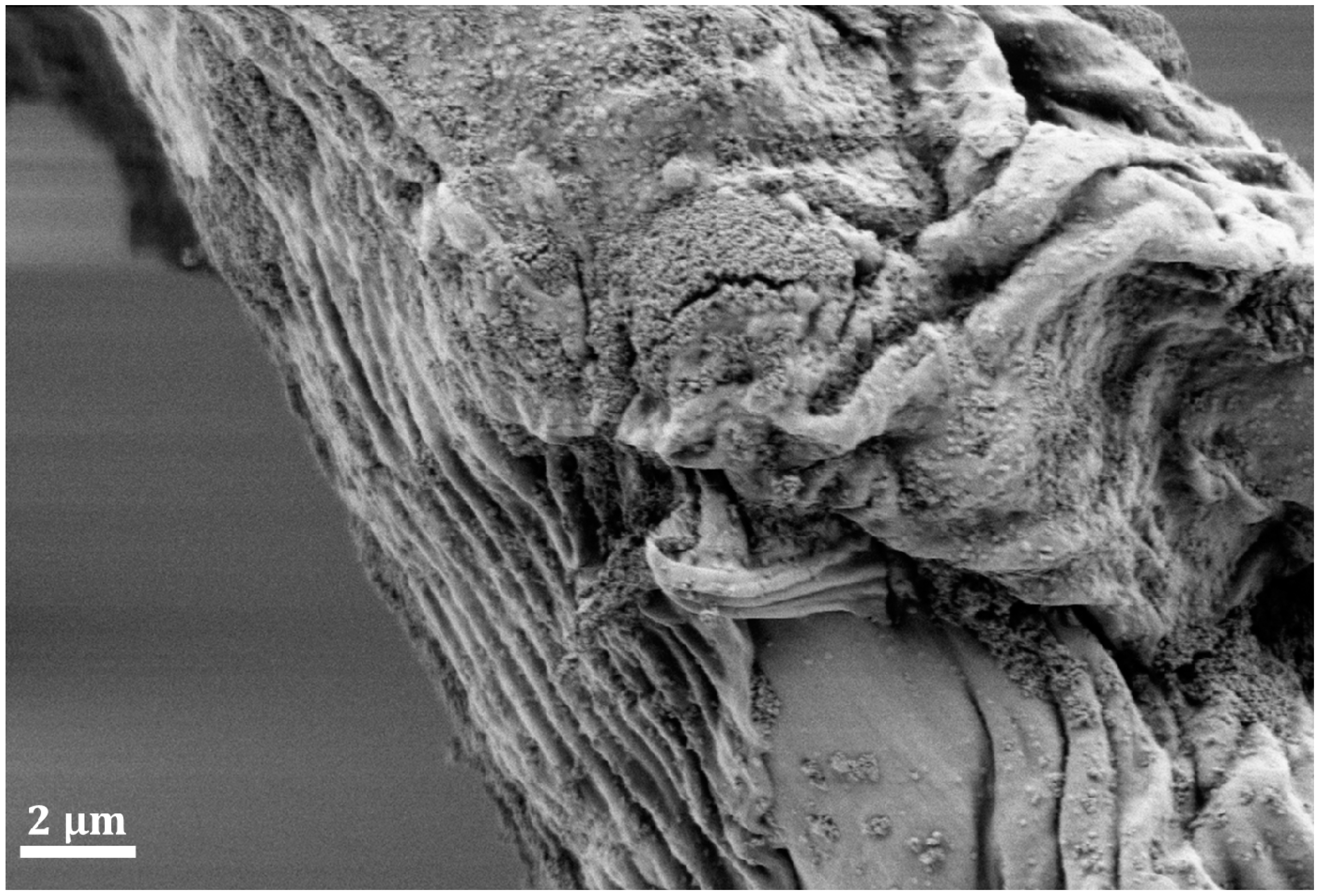
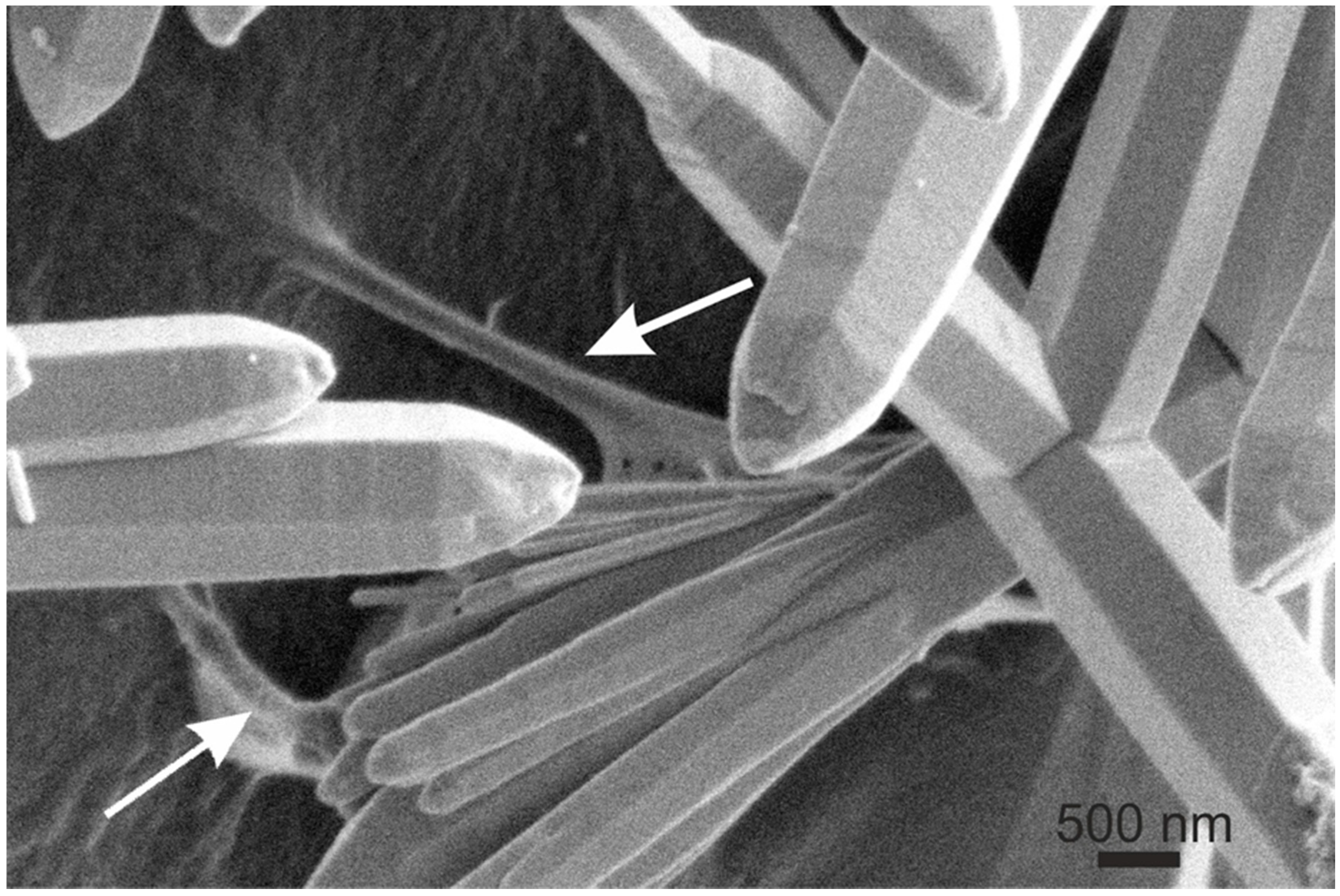
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khor, E. Chitin: Fulfiling a Biomaterials Promise, 1st ed.; Elsevier Science Ltd.: Oxford, UK, 2001. [Google Scholar]
- Roberts, G.A.F. Chitin Chemistry, 1st ed.; MacMillian: London, UK, 1992. [Google Scholar]
- Ledderhose, G. Ueber salzsaures glycosamin. Ber. Dtsch. Chem. Ges. 1876, 9, 1200–1201. (In German) [Google Scholar] [CrossRef]
- Ledderhose, G. Ueber glykosamin. Z. Physiol. Chem. 1880, 4, 139–159. (In German) [Google Scholar]
- Tiemann, F. Einiges über den abbau von salzsaurem glucosamin. Ber. Dtsch. Chem. Ges. 1884, 17, 241–251. (In German) [Google Scholar] [CrossRef]
- Tiemann, F. Ueber glucosamin. Ber. Dtsch. Chem. Ges. 1886, 19, 49–53. (In German) [Google Scholar] [CrossRef]
- Tiemann, F. Specifisches drehungsvermogen und krystallform des bromwasserstoffsauren Glucosamins. Ber. Dtsch. Chem. Ges. 1886, 19, 155–157. (In German) [Google Scholar] [CrossRef]
- Fränkel, S.; Kelly, A. Beiträge zur constitution des chitins. Monatsh. Chem. Verw. Teile Wiss. 1902, 23, 123–132. (In German) [Google Scholar] [CrossRef]
- Karrer, P.; Hofmann, A. Polysaccharide XXXIX. Über den enzymatischen abbau von chitin und chitosan I. Helv. Chim. Acta 1929, 12, 616–637. (In German) [Google Scholar] [CrossRef]
- Bergmann, M.; Zervas, L.; Silberkweit, E. Über chitin und chitobiose. Ber. Dtsch. Chem. Ges. 1931, 64, 2436–2440. (In German) [Google Scholar] [CrossRef]
- Zechmeister, L.; Tóth, G. Zur Kenntnis der hydrolyse von chitin mit salzsäure (I. Mitteil.). Ber. Dtsch. Chem. Ges. 1931, 64, 2028–2032. (In German) [Google Scholar] [CrossRef]
- Meyer, K.H.; Mark, H. Über den bau des krystallisierten anteils der cellulose. Ber. Dtsch. Chem. Ges. 1928, 61, 593–614. (In German) [Google Scholar]
- Meyer, K.H.; Mark, H. Über den aufbau des chitins. Ber. Dtsch. Chem. Ges. 1928, 61, 1936–1939. (In German) [Google Scholar] [CrossRef]
- Garcia Mendoza, C.; Novaes Ledieu, M. Chitin in the new wall of regenerating protoplasts of Candida utilis. Nature 1968, 220, 1035. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, H.J.; Roseman, S. Quantitative estimation of chitin in fungi. J. Bacteriol. 1957, 74, 222–224. [Google Scholar] [PubMed]
- Elorza, M.V.; Rico, H.; Sentandreu, R. Calcofluor white alters the assembly of chitin fibrils in Saccharomyces cerevisiae and Candida albicans cells. J. Gen. Microbiol. 1983, 129, 1577–1582. [Google Scholar] [PubMed]
- Peter, M.G. Chitin and chitosan in fungi. In Biopolymers Online; Steinbüchel, A., Ed.; John Wiley & Sons: Weinheim, UK, 2005; pp. 123–132. [Google Scholar]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Durkin, C.A.; Mock, T.; Armbrust, E.V. Chitin in diatoms and its association with the cell wall. Eukaryot. Cell 2009, 8, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.-H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira. Pseudonana. Angew. Chem. Int. Ed. 2009, 48, 9724–9727. [Google Scholar] [CrossRef]
- Rahman, M.A.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum. Compact. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Cauchie, H.-M. Chitin production by arthropods in the hydrosphere. Hydrobiologia 2002, 470, 63–96. [Google Scholar] [CrossRef]
- Das, S.; Gillin, F.D. Chitin synthase in encysting Entamoeba Invadens. Biochem. J. 1991, 280, 641–647. [Google Scholar] [PubMed]
- Campos-Góngora, E.; Ebert, F.; Willhoeft, U.; Said-Fernández, S.; Tannich, E. Characterization of chitin synthases from Entamoeba. Protist 2004, 155, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Bavestrello, G.; Kurek, D.; Paasch, S.; Brunner, E.; Born, R.; Galli, R.; Stelling, A.L.; Sivkov, V.N.; Petrova, O.V.; et al. Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa:Cnidaria). Int. J. Bol. Macromol. 2012, 51, 129–137. [Google Scholar] [CrossRef]
- Tóth, G.; Zechmeister, L. Chitin content of the mandible of the snail (Helix pomatia). Nature 1939, 144, 1049. [Google Scholar] [CrossRef]
- Goffinet, G.; Jeuniaux, C. Distribution et importance quantitative de u chitine dans les coquilles de mollusques. Cah. Biol. Mar. 1979, 20, 341–349. (In French) [Google Scholar]
- Peters, W. Occurrence of chitin in mollusca. Comp. Biochem. Physiol. B 1972, 41, 541–544. [Google Scholar]
- Weiss, I.M.; Schönitzer, V. The distribution of chitin in larval shells of the bivalve mollusk Mytilus galloprovincialis. J. Struct. Biol. 2006, 153, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Winkler, L.R. Localization and proof of chitin in the opisthobranch mollusks Aplysia californica Cooper and Bulla gmtldiana (Pilsbry), with an enzymochromatographic method for chitin demonstration. Pac. Sci. 1960, 14, 304–307. [Google Scholar]
- Weiss, I.M.; Schönitzer, V.; Eichner, N.; Sumper, M. The chitin synthase involved in marine bivalve mollusk shell formation contains a myosin domain. FEBS Lett. 2006, 580, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Brunet, P.C.J.; Carlisle, D.B. Chitin in Pogonophora. Nature 1958, 182, 1689. [Google Scholar] [CrossRef] [PubMed]
- Gaill, F.; Shillito, B.; Lechaire, J.P.; Chanzy, H.; Goffinet, G. The chitin secreting system from deep sea hydrothermal vent worms. Biol. Cell 1992, 76, 201–204. [Google Scholar] [CrossRef]
- Krishnan, G.; Ramachandran, G.N.; Santanam, M.S. Occurrence of chitin in the epicuticle of an arachnid Palmneus swammerdami. Nature 1955, 178, 557–558. [Google Scholar] [CrossRef]
- Kaya, M.; Seyyar, O.; Baran, T.; Erdoğan, S.; Kar, M. A physicochemical characterization of fully acetylated chitin structure isolated from two spider species: With new surface morphology. Int. J. Bol. Macromol. 2014, 65, 553–558. [Google Scholar] [CrossRef]
- Neville, A.C.; Parry, D.A.; Woodhead-Galloway, J. The chitin crystallite in arthropod cuticle. J. Cell Sci. 1976, 21, 73–82. [Google Scholar] [PubMed]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. Insect chitin synthases: A review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2006, 176, 1–15. [Google Scholar] [CrossRef]
- Glenn Richards, A. Studies on arthropod cutilce. III. The chitin of Limulus. Science 1949, 109, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Giraud, M.-M. Fine structure of the chitin-protein system in the crab cuticle. Tissue Cell 1984, 16, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Giraud, M.-M.; Chanzy, H.; Vuong, R. Chitin crystals in arthropod cuticles revealed by diffraction contrast transmission electron microscopy. J. Struct. Biol. 1990, 103, 232–240. [Google Scholar] [CrossRef]
- Ando, Y.; Fukada, E.; Glimcher, M.J. Piezoelectricity of chitin in lobster shell and apodeme. Biorheology 1977, 14, 175–179. [Google Scholar] [PubMed]
- Nieto Akosta, O.M.; Enriques Rodriguez, R.D.; Vitivskaya, G.A.; Elinov, N.P. Analysis of chitin from the skeleton of the spiny lobster Panulirus Argus. Appl. Biochem. Microbiol. 1978, 13, 604–607. [Google Scholar]
- Fabritius, H.-O.; Sachs, C.; Triguero, P.R.; Raabe, D. Influence of structural principles on the mechanics of a biological fiber-based composite material with hierarchical organization: The exoskeleton of the lobster Homarus americanus. Adv. Mater. 2009, 21, 391–400. [Google Scholar] [CrossRef]
- Horst, M.N. The Biosynthesis of crustacean chitin by a microsomal enzyme from larval brine shrimp. J. Biol. Chem. 1981, 256, 1412–1419. [Google Scholar] [PubMed]
- Rodde, R.; Einbu, A.; Varum, K.M. A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr. Polym. 2008, 71, 388–393. [Google Scholar] [CrossRef]
- Nicol, S.; Hosie, G.W. Chitin production by krill. Biochem. Syst. Ecol. 1993, 21, 181–184. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Y.; Yu, L.; Zhang, C.; Xu, X.; Xue, Y.; Li, Z.; Xue, C. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr. Polym. 2013, 92, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Stawski, D.; Rabiej, S.; Herczyńska, L.; Draczyński, Z. Thermogravimetric analysis of chitins of different origin. J. Therm. Anal. Calorim. 2008, 93, 489–494. [Google Scholar] [CrossRef]
- Wagner, G.P.; Lo, J.; Laine, R.; Almeder, M. Chitin in the epidermal cuticle of vertebrate (Paralipophorys trigloides, Blenniidae, Teleostei). Experientia 1993, 49, 317–319. [Google Scholar] [CrossRef]
- Ehrlich, H.; Maldonado, M.; Spindler, K.; Eckert, C.; Hanke, T.; Born, R.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J. Exp. Zool. B 2007, 308, 347–356. [Google Scholar] [CrossRef]
- Ehrlich, H.; Krautter, M.; Hanke, T.; Simon, P.; Knieb, C.; Heinemann, S.; Worch, H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexctinellida: Porifera). J. Exp. Zool. B 2007, 308, 473–483. [Google Scholar] [CrossRef]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Rohde, S.; Schupp, P.J. Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera). Hydrobiologia 2012, 687, 219–226. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Bol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef]
- Cruz-Barraza, J.A.; Carballo, J.L.; Rocha-Olivares, A.; Ehrlich, H.; Hog, M. Integrative taxonomy and molecular phylogeny of genus Aplysina (Demospongiae: Verongida) from Mexican Pacific. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina. fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Kaluzhnaya, O.V.; Tsurkan, M.V.; Ereskovsky, A.; Tabachnick, K.R.; Ilan, M.; Stelling, A.; Galli, R.; Petrova, O.V.; Nekipelov, S.V.; et al. First report on chitinous holdfast in sponges (Porifera). Proc. R. Soc. B 2013, 280. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Kaluzhnaya, O.V.; Brunner, E.; Tsurkan, M.V.; Ereskovsky, A.; Ilan, M.; Tabachnick, K.R.; Bazhenov, V.V.; Paasch, S.; Kammer, M.; et al. Identification and first insights into the structure and biosynthesis of chitin from the freshwater sponge Spongilla lacustris. J. Struct. Biol. 2013, 183, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.; Werner, C.; Schwille, P.; Petrasek, Z.; Pisera, A.; Simon, P.; Sivkov, V.; et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, B.A. Preservation of chitin in 25-million-year-old fossils. Science 1997, 276, 1541–1543. [Google Scholar] [CrossRef]
- Wysokowski, M.; Zatoń, M.; Bazhenov, V.V.; Behm, T.; Ehrlich, A.; Stelling, A.L.; Hog, M.; Ehrlich, H. Identification of chitin in 200-million-year-old gastropod egg capsules. Paleobiology 2014, 40, 529–540. [Google Scholar] [CrossRef]
- Ehrlich, H. Biomimetic potential of chitin-based composite biomaterials of poriferan origin. In Biomimetic Biomaterials: Structure and Aplications; Ruys, A.J., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 47–67. [Google Scholar]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, O.Y.; Mutsenko, V.V.; Revenko, E.B.; Petrenko, Y.A.; Ehrlich, H.; Petrenko, A.Y. Culture and differentiation of human adipose tissue mesenchymal stromal cells within carriers based on sea sponge chitin skeletons. Stem Cell Day 2013, 23, 267–270. [Google Scholar]
- Steck, E.; Burkhardt, M.; Ehrlich, H.; Richter, W. Discrimination between cells of murine and human origin in xenotransplants by species specific genomic in situ hybridization. Xenotransplantaiton 2010, 17, 153–159. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Ehrlich, H.; Simon, P.; Carrillo-Cabrera, W.; Bazhenov, V.V.; Botting, J.P.; Ilan, M.; Ereskovsky, A.V.; Muricy, G.; Worch, H.; Mensch, A.; et al. Insights into chemistry of biological materials: Newly discovered silica-aragonite-chitin biocomposites in demosponges. Chem. Mater. 2010, 22, 1462–1471. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Tai, B.C.U. Chitin—A promising biomaterial for tissue engineering and stem cell technologies. Biotechnol. Adv. 2013, 31, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Westholm, L.J.; Repo, E.; Sillanpää, M. Filter materials for metal removal from mine drainage—A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 9109–9128. [Google Scholar] [CrossRef] [PubMed]
- Kousalya, G.N.; Gandhi, M.R.; Viswanathan, N.; Meenakshi, S. Preparation and metal uptake studies of modified forms of chitin. Int. J. Bol. Macromol. 2010, 47, 583–589. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three marine sources. Int. J. Bol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef]
- Focher, B.; Naggi, A.; Torri, G. Structural differences between chitin polymorphs and their precipitates from solutions—Evidence from CP-MAS 13 C-NMR, FT-IR and FT-Raman spectroscopy. Carbohydr. Polym. 1992, 17, 97–102. [Google Scholar] [CrossRef]
- Sikorski, P.; Hori, R.; Wada, M. Revisit of α-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009, 10, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Darmon, S.; Rudall, K. Infra-red and X-ray studies of chitin. Discuss. Faraday Soc. 1950, 9, 253–260. [Google Scholar]
- Rudall, K.; Kenchington, W. The chitin system. Biol. Rev. 1973, 49, 597–636. [Google Scholar] [CrossRef]
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Calström, D. The crystal structure of α-chitin (poly-N-acetyl-d-glucosamine). J. Biophys. Biochem. Cytol. 1957, 3, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kimura, S.; Wada, M.; Kuga, S. Crystal analysis and high-resolution imaging of microfibrillar α-chitin from Phaecystis. J. Struct. Biol. 2010, 171, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.; Lymperakis, L.; Friák, M.; Neugebauer, J. Ab initio based conformational study of the crystalline α-chitin. Biopolymers 2012, 99, 22–34. [Google Scholar] [CrossRef]
- Dweltz, N.E. The structure of β-chitin. Biochim. Biophys. Acta 1961, 51, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J. Structure of β-chitin or parallel chain system of poly-β-(1–4)-N-acetyl-d-glucosamine. Biopolymers 1969, 7, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Salmon, S.; Hudson, S.M. Crystal morphology, biosynthesis, and physical assembly of cellulose, chitin and chitosan. J. Macromol. Sci. C 1997, 37, 199–276. [Google Scholar]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Mattioli-Belmonte, M. Emerging biomedical application of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Okano, T.; Gaill, F.; Chanzy, H.; Putaux, J.L. Structural data on the intra-crystalline swelling of beta-chitin. Int. J. Biol. Macromol. 2000, 28, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kimura, S.; Togawa, E.; Wada, M. Crystal transition between hydrate and anhydrous β-chitin monitored by synchrotron X-ray fiber diffraction. Carbohydr. Polym. 2010, 79, 882–889. [Google Scholar] [CrossRef]
- Sawada, D.; Ogawa, Y.; Kimura, S.; Nishiyama, Y.; Langan, P.; Masahisa, W. Solid-solvent molecular interactions observed in crystal structures of β-chitin complexes. Cellulose 2014, 21, 1007–1014. [Google Scholar] [CrossRef]
- Sawada, D.; Nishiyama, Y.; Langan, P.; Forsyth, V.T.; Kimura, S. Water in crystalline fibers of dehydrate β-chitin results in unexpected absence of intramolecular hydrogen bonding. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Grunenfelder, L.K.; Suksangpanya, N.; Salinas, C.; Milliron, G.; Yaraghi, N.; Herrera, S.; Evans-Lutterodt, K.; Nutt, S.R.; Zavattieri, P.; Kisailus, D. Bio-inspired impact-resistant composites. Acta Biomater. 2014, 10, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Al-Sawalmih, A.; Yi, S.B.; Fabritius, H. Preferred crystallographic texture of α-chitin as a microscopic and macroscopic design principle of the exoskeleton of the lobster Homarus americanus. Acta Biomater. 2007, 3, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-C.; Peters, R.D.; Dies, H.; Rheinstӓdter, M.C. Hierarchical, self-similar structure in native squid pen. Soft Matter 2014, 10, 5541–5549. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Takahashi, M.; Tokura, S.; Tamura, H.; Nagano, A. Cartilage-scaffold composites produced by bioresorbable β-chitin sponge with cultured rabbit chondrocytes. Tissue Eng. 2004, 10, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Minami, S.; Matsuhashi, A.; Sashiwa, H.; Saimoto, H.; Shigemasa, Y.; Tanigawa, T.; Tanaka, Y.; Tokura, S. Application of polymeric N-acetyl-d-glucosamine (chitin) to veterinary practice. J. Vet. Med. Sci. 1993, 55, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Shigemasa, Y.; Minami, S. Application of chitin and chitosan for biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 383–420. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Gao, H.; He, M.; Zhang, L. Hydrophobic modification of surface of chitin sponges for highly effective separation of oil. ACS Appl. Mater. Interfaces 2014, 6, 19933–19942. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, M.M.; Kozlecki, T.; Gorak, A. Review of the application of ionic liquids as solvents for chitin. J. Polym. Eng. 2012, 32, 67–69. [Google Scholar] [CrossRef]
- Barber, P.S.; Griggs, C.S.; Bonner, J.R.; Rogers, R.D. Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells. Green Chem. 2013, 15, 601–607. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, X.; Sun, N.; Rogers, R.D. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968–971. [Google Scholar] [CrossRef]
- Sharma, M.; Chandrakant, M.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshoka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Simple preparation method of chitin nanofibers with a uniform width of 10–20 nm from prawn shell under neutral conditions. Carbohydr. Polym. 2011, 84, 762–764. [Google Scholar] [CrossRef]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, Q.; She, X.; Xia, Y.; Liu, Y.; Li, J.; Yang, D. Fabrication and characterization of α-chitin nanofibers and highly transparent hitin films by pulsed ultrasonication. Carbohydr. Polym. 2013, 98, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Bentov, S.; Zaslansky, P.; Al-Sawalmih, A.; Masic, A.; Fratzl, P.; Sagi, A.; Berman, A.; Aichmayer, B. Enamel-like apatite crown covering amorphous mineral in a crayfish mandible. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef]
- Grunenfelder, L.; Herrera, S.; Kisailus, D. Crustacean-derived biomimetic components and nanostructured composites. Small 2014, 10, 3207–3232. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Behm, T.; Born, R.; Bazhenov, V.V.; Meiβner, H.; Richter, G.; Szwarc-Rzepka, K.; Makarova, A.; Vyalikh, D.; Schupp, P.; et al. Preparation of chitin-silica composites by in vitro silicification of two-dimensional Ianthella basta demosponge chitinous scaffolds under modified Stöber conditions. Mater. Sci. Eng. C 2013, 33, 3935–3941. [Google Scholar] [CrossRef]
- Michels, J.; Vogt, J.; Gorb, S.N. Tools for crushing diatoms—Opal teeth in copepod feature a rubber-like bearing composted of resilin. Sci. Rep. 2012, 2, 465. [Google Scholar] [CrossRef] [PubMed]
- Politi, Y.; Priewasser, M.; Pippel, E.; Zaslansky, P.; Hartman, J.; Siegel, S.; Li, C.; Barth, F.G.; Fratzl, P. A spider’s fang: How to design an injection needle using chitin-based composite material. Adv. Funct. Mater. 2012, 22, 2519–2528. [Google Scholar] [CrossRef]
- Fernandez, J.G.; Ingber, D.E. Bioinspired chitinous material solutions for environmental sustainability and medicine. Adv. Funct. Mater. 2013, 23, 4454–4466. [Google Scholar] [CrossRef]
- Yousof, N.L.B.M.; Lim, L.Y.; Khor, E. Flexible chitin films: Structural studies. Carbohydr. Res. 2004, 339, 2701–2711. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Chang, C.; Ding, B.; Cai, J.; Xu, M.; Feng, S.; Ren, J.; Shi, X.; Du, Y.; Zhang, L. High strength films with gas-barrier fabricated from chitin solution dissolved at low temperature. J. Mater. Chem. A 2013, 1, 1867–1874. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Sun, W.; de Silva, J.P.; Jin, J.; Makhnejia, K.; Cross, G.L.W.; Rolandi, M. Mechanical properties of self-assembled chitin nanofiber networks. J. Mater. Chem. B 2014, 2, 2461–2466. [Google Scholar] [CrossRef]
- Vincent, J.F.W.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arth. Struct. Dev. 2004, 187–199. [Google Scholar] [CrossRef]
- Ifuku, S.; Morooka, S.; Nakagato, A.N.; Morimoto, M.; Saimoto, H. Preparation and characterization of optically transparent chitin nanofiber/(meth)acrylic resin composites. Green Chem. 2011, 13, 1708–1711. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chang, S.H.; Jo, N.J. Synthesis and properties of biodegradable chitin-graf-poly(l-lactide) copolymers. Polym. Int. 2002, 51, 1123–1128. [Google Scholar] [CrossRef]
- Chen, B.; Sun, K.; Ren, T. Mechanical and viscoelastic properties of chitin fiber reinforced poly(ε-caprolactone). Eur. Polym. J. 2005, 41, 453–457. [Google Scholar] [CrossRef]
- Jayakumar, R.; Tamura, H. Synthesis, characterization and thermal properties of chitin-g-poly(γ-caprolactone) copolymers by using chitin gel. Int. J. Biol. Macromol. 2008, 43, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.C.; He, X. Thermal stability of proteins. Ann. N. Y. Acad. Sci. 2005, 1066, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Bershtein, V.A.; Egorov, V.M.; Egorova, L.M.; Ryzhov, V.A. The role of thermal analysis in revealing the common molecular nature of transitions in polymers. Thermochim. Acta 1994, 238, 41–73. [Google Scholar] [CrossRef]
- Kittur, F.S.; Harish Prashanth, K.V.; Udaya Sankar, K.; Tharanathan, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Köll, P.; Metzger, J. Nachweis von acetamid beim thermischen abbau von chitin. Z. Lebensm. Unters. Forsch. 1979, 113, 111–113. (In German) [Google Scholar] [CrossRef]
- Köll, P.; Borchers, G.; Metzger, J.O. Thermal degradation of chitin and cellulose. J. Anal. Appl. Pyrolysis 1991, 19, 119–129. [Google Scholar] [CrossRef]
- Wanjun, T.; Cunxin, W.; Donghua, C. Kinetic studies on the pyrolysis of chitin and chitosan. Polym. Degrad. Stable 2005, 87, 389–394. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. C 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Stolarek, P.; Ledakowicz, S. Pyrolysis kinetics of chitin by non-isothermal thermogravimetry. Thermochim. Acta 2005, 433, 200–208. [Google Scholar] [CrossRef]
- Nam, Y.S.; Park, W.H.; Ihm, D.; Hudson, S.M. Effect of the degree of deacetylation on the thermal decomposition of chitin and chitosan nanofibers. Carbohydr. Polym. 2010, 80, 291–295. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Non-isothermal kinetics of thermal degradation of chitin. J. Therm. Anal. Calorim. 2013, 111, 763–771. [Google Scholar] [CrossRef]
- Cardenas, G.; Etamal, J.; Tagle, L. Thermogravimetric studies of chitin derivatives I. Int. J. Polym. Mater. Polym. Biomater. 1993, 21, 137–146. [Google Scholar] [CrossRef]
- Ehrlich, H.; Simon, P.; Motylenko, M.; Wysokowski, M.; Bazhenov, V.V.; Galli, R.; Stelling, A.L.; Stawski, D.; Ilan, M.; Stöcker, H.; et al. Extreme biomimetics: Formation of zirconium dioxide nanophase using chitinous scaffolds under hydrothermal conditions. J. Mater. Chem. B 2013, 1, 5092–5099. [Google Scholar] [CrossRef]
- Kaya, M.; Erdogan, S.; Mol, A.; Baran, T. Comparison of chitin structures isolated from seven Orthoptera species. Int. J. Bol. Macromol. 2014, 72, 797–805. [Google Scholar] [CrossRef]
- Kaya, M.; Karaarslan, M.; Baran, T.; Can, E.; Ekemen, G.; Bitim, B.; Duman, F. The quick extraction of chitin from an epizoic crustacean species (Chelonibia patula). Nat. Prod. Res. 2014, 28, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E.; Darder, M.; Aranda, P.; Ariga, K. Advances in biomimetic and nanostructured biohybrid materials. Adv. Mater. 2010, 22, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chiu, C.-Y.; Huang, Y. Biomimetic synthesis of inorganic materials and their applications. Pure Appl. Chem. 2010, 83, 111–125. [Google Scholar] [CrossRef]
- Sanchez, C.; Arribart, H.; Guille, M.M.G. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.-W.; Ma, Y.; Cölfen, H. Biomimetic mineralization. J. Mater. Chem. 2007, 17, 415–449. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, D.; Jiao, Y.; Tian, Y.; Wang, Y.; Jiang, Z. Biomimetic synthesis of TiO2–SiO2–Ag nanocomposites with enhanced visible-light photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 3824–3832. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.; Tahir, M.N.; Natalio, F.; Tremel, W. Bioinspired synthesis of multifunctional inorganic and bio-organic hybrid materials. FEBS J. 2012, 279, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Blacklock, J.; Li, J.; Möhwald, H. One-pot synthesis of polypeptide—Gold nanoconjugates for in vitro gene transfection. ACS Nano 2012, 6, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, J.; Möhwald, H. Templating assembly of multifunctional hybrid colloidal spheres. Adv. Mater. 2012, 24, 2663–2667. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Niu, L.; Qi, Y.; Yiu, C.K.Y.; Ryou, H.; Arola, D.D.; Chen, J.; Pashley, D.H.; Tay, F.R. Subtleties of biomineralisation revealed by manipulation of the eggshell membrane. Biomaterials 2011, 32, 8743–8752. [Google Scholar] [CrossRef] [PubMed]
- Canabady-Rochelle, L.L.S.; Belton, D.J.; Deschaume, O.; Currie, H.A.; Kaplan, D.L.; Perry, C.C. Bioinspired silicification of silica-binding peptide-silk protein chimeras: Comparison of chemically and genetically produced proteins. Biomacromolecules 2012, 13, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.; Glawe, D.D.; Naik, R.R.; Hallinan, K.P.; Stone, M.O. Study of the chemical and physical influences upon in vitro peptide-mediated silica formation. Biomacromolecules 2004, 5, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V.; Maheshwari, R.; Mukherjee, N.; Kiick, K.L.; Clarson, S.J. Conformation and assembly of polypeptide scaffolds in templating the synthesis of silica: An example of a polylysine macromolecular “switch”. Biomacromolecules 2006, 7, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chu, C.C. Biomimetic mineralization of acid polysaccharide-based hydrogels: Towards porous 3-dimensional bone-like biocomposites. J. Mater. Chem. 2012, 22, 6080–6087. [Google Scholar] [CrossRef]
- Munro, N.H.; McGrath, K.M. Biomimetic mineralisation of polymeric scaffolds using a combined soaking approach: Adaptation with various mineral salts. Dalt. Trans. 2011, 40, 9269–9275. [Google Scholar] [CrossRef]
- Munro, N.H.; Green, D.W.; Dangerfield, A.; McGrath, K.M. Biomimetic mineralisation of polymeric scaffolds using a combined soaking and Kitano approach. Dalt. Trans. 2011, 40, 9259–9268. [Google Scholar] [CrossRef]
- Munro, N.H.; Green, D.W.; McGrath, K.M. In situ continuous growth formation of synthetic biominerals. Chem. Commun. 2013, 49, 3407–3409. [Google Scholar] [CrossRef]
- Spinde, K.; Kammer, M.; Freyer, K.; Ehrlich, H.; Vournakis, J.N.; Brunner, E. Biomimetic silicification of fibrous chitin from diatoms. Chem. Mater. 2011, 23, 2973–2978. [Google Scholar] [CrossRef]
- Tseng, Y.; Lin, H.; Liu, M. Biomimetic synthesis of nacrelike faceted mesocrystals of ZnO-gelatin composite. J. Phys. Chem. C 2009, 113, 18053–18061. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Stöcker, H.; Bazhenov, V.V.; Langer, E.; Dobrowolska, A.; Czaczyk, K.; Galli, R.; Stelling, A.L.; Behm, T.; et al. An extreme biomimetic approach: Hydrothermal synthesis of β-chitin/ZnO nanostructured composites. J. Mater. Chem. B 2013, 1, 6469–6476. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.L.; Jesionowski, T.; et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar] [CrossRef]
- Wysokowski, M.; Piasecki, A.; Bazhenov, V.V.; Paukszta, D.; Born, R.; Petrenko, I.; Jesionowski, T. Poriferan chitin as the scaffold for nanosilica deposition under hydrothermal synthesis conditions. J. Chitin Chitosan Sci. 2013, 1, 26–33. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Zabriskie, T.M.; Mcphail, K.L. Deep-sea hydrothermal vents: Potential hot spots for natural products discovery? J. Nat. Prod. 2010, 73, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, H.; Mottl, M. Geomicrobiology of deep-sea hydrothermal vents. Science 1985, 229, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, H.; Peng, X.; Wu, Z.; Chen, S.; Fang, J. Microbial diversity and biomineralization in low-temperature hydrothermal iron-silica-rich precipitates of the Lau Basin hydrothermal field. FEMS Microbiol. Ecol. 2012, 81, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Rentz, J.A.; Lilburn, T.G.; Davis, R.E.; Aldrich, H.; Chan, C.; Moyer, C.L. A novel lineage of proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS One 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Reysenbach, A.-L.; Liu, Y.; Banta, A.B.; Beveridge, T.J.; Kirshtein, J.D.; Schouten, S.; Tivey, M.K.; von Damm, K.L.; Voytek, M.A. A ubiquitous thermoacidophilic archaeon from deep-sea hydrothermal vents. Nature 2006, 442, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Islas, S.; Velasco, A.M.; Becerra, A.; Delaye, L.; Lazcano, A. Hyperthermophily and the origin and earliest evolution of life. Int. Microbiol. 2003, 6, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Biological Materials of Marine Origin; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Zbinden, M.; Le Bris, N.; Compère, P.; Martinez, I.; Guyot, F.; Gaill, F. Mineralogical gradients associated with alvinellids at deep-sea hydrothermal vents. Deep Sea Res. I Oceanogr. Res. Papers 2003, 50, 269–280. [Google Scholar] [CrossRef]
- Nidhin, M.; Sreeram, K.J.; Nair, B.U. Polysaccharide films as templates in the synthesis of hematite nanostructures with special properties. Appl. Surf. Sci. 2012, 258, 5179–5184. [Google Scholar] [CrossRef]
- Ercole, C.; Cacchio, P.; Botta, A.L.; Centi, V.; Lepidi, A. Bacterially induced mineralization of calcium carbonate: The role of exopolysaccharides and capsular polysaccharides. Microsc. Microanal. 2007, 13, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Fakra, S.C.; Edwards, D.C.; Emerson, D.; Banfield, J.F. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim. Cosmochim. Acta 2009, 73, 3807–3818. [Google Scholar] [CrossRef]
- Hedrich, R.; Machill, S.; Brunner, E. Biomineralization in diatoms-phosphorylated saccharides are part of Stephanopyxis turris biosilica. Carbohydr. Res. 2013, 365, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.U.P.A.; Abrecht, M.; Frazer, B.H. The Organic-mineral interface in biominerals. Rev. Mineral. Geochem. 2005, 59, 157–185. [Google Scholar] [CrossRef]
- Preat, A.; Mamet, B.; de Ridder, C.; Boulvain, F.; Gillan, D. Iron bacterial and fungal mats, Bajocian stratotype (Mid-Jurassic, northern Normandy, France). Sediment. Geol. 2000, 137, 107–126. [Google Scholar] [CrossRef]
- Djurišić, A.; Xi, Y.Y.; Hsu, Y.F.; Chan, W.K. Hydrothermal synthesis of nanostructures. Recent Pat. Nanotechnol. 2007, 1, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Riman, R.; Suchanek, W.; Lencka, M. Hydrothermal crystallization of ceramics. Ann. Chim. Sci. Matér. 2002, 27, 15–36. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Rabenau, B.A. The role of hydrothermal synthesis in preparative chemistry. Angew. Chem. Int. Ed. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2007, 43, 2085–2103. [Google Scholar] [CrossRef]
- Feng, S.; Xu, R. New materials in hydrothermal synthesis. Acc. Chem. Res. 2001, 34, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.; Chen, X.Y.; Leung, Y.H. Recent progress in hydrothermal synthesis of zinc oxide nanomaterials. Recent Pat. Nanotechnol. 2012, 6, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Namratha, K.; Byrappa, K. Novel solution routes of synthesis of metal oxide and hybrid metal oxide nanocrystals. Prog. Cryst. Growth Charact. Mater. 2012, 58, 14–42. [Google Scholar] [CrossRef]
- Aida, T.M.; Oshima, K.; Abe, C.; Maruta, R.; Iguchi, M.; Watanabe, M.; Smith, R.L. Dissolution of mechanically milled chitin in high temperature water. Carbohydr. Polym. 2014, 106, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, W.; Shenton, W.; Davis, S.A.; Mann, S. Template mineralization of ordered macroporous chitin-silica composites using a cuttlebone-derived organic matrix. Chem. Mater. 2000, 12, 2835–2837. [Google Scholar] [CrossRef]
- Peter, M.; Sudheesh Kumar, P.T.; Binulal, N.S.; Nair, S.V.; Tamura, H.; Jayakumar, R. Development of novel α-chitin/nanobioactive glass ceramic composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2009, 78, 926–931. [Google Scholar] [CrossRef]
- Copello, G.J.; Mebert, A.M.; Raineri, M.; Pesenti, M.P.; Diaz, L.E. Removal of dyes from water using chitosan hydrogel/SiO2 and chitin hydrogel/SiO2 hybrid materials obtained by the sol-gel method. J. Hazard. Mater. 2011, 186, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.A.-S.; Al-Akayleh, F.; Shubair, M.; Rashid, I.; Al Remawi, M.; Badwan, A. Evaluation of three chitin metal silicate co-precipitates as a potential multifunctional single excipient in tablet formulations. Mar. Drugs 2010, 8, 1699–1715. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Breedveld, V.; Deng, Y. Rheological study of self-crosslinking and co-crosslinking of ammonium zirconium carbonate and starch in aqueous solutions. J. Appl. Polym. Sci. 2011, 122, 1019–1029. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Schmidt, J.; Vesterinen, A.-H.; Tenkanen, M. Crosslinking with ammonium zirconium carbonate improves the formation and properties of spruce galactoglucomannan films. J. Mater. Sci. 2013, 48, 4205–4213. [Google Scholar] [CrossRef]
- Zong, S.; Cao, Y.; Zhou, Y.; Ju, H. Hydrogen peroxide biosensor based on hemoglobin modified zirconia nanoparticles-grafted collagen matrix. Anal. Chim. Acta 2007, 582, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Hristovski, K.D.; Westerhoff, P.K.; Crittenden, J.C.; Olson, L.W. Arsenate removal by nanostructured ZrO2 spheres. Environ. Sci. Technol. 2008, 42, 3786–3790. [Google Scholar] [CrossRef] [PubMed]
- Da Sacco, L.; Masotti, A. Chitin and chitosan as multipurpose natural polymers for groundwater arsenic removal and As2O3 delivery in tumor therapy. Mar. Drugs 2010, 8, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Huang, X.; Chen, X.; Zheng, N. Hollow mesoporous zirconia nanocapsules for drug delivery. Adv. Funct. Mater. 2010, 20, 2442–2447. [Google Scholar] [CrossRef]
- Ehrlich, H.; Brunner, E.; Richter, W.; Ilan, M.; Schupp, P. Two or Three-Dimensional Cleaned Chitin Skeleton of Dictyoceratid Sponges, Method for the Production and Use Thereof. WO2011023531 A2, 3 March 2011. [Google Scholar]
- Nata, I.F.; Wang, S.S.-S.; Wu, T.-M.; Lee, C.-K. β-Chitin nanofibrils for self-sustaining hydrogels preparation via hydrothermal treatment. Carbohydr. Polym. 2012, 90, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235-265. https://doi.org/10.3390/polym7020235
Wysokowski M, Petrenko I, Stelling AL, Stawski D, Jesionowski T, Ehrlich H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers. 2015; 7(2):235-265. https://doi.org/10.3390/polym7020235
Chicago/Turabian StyleWysokowski, Marcin, Iaroslav Petrenko, Allison L. Stelling, Dawid Stawski, Teofil Jesionowski, and Hermann Ehrlich. 2015. "Poriferan Chitin as a Versatile Template for Extreme Biomimetics" Polymers 7, no. 2: 235-265. https://doi.org/10.3390/polym7020235
APA StyleWysokowski, M., Petrenko, I., Stelling, A. L., Stawski, D., Jesionowski, T., & Ehrlich, H. (2015). Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers, 7(2), 235-265. https://doi.org/10.3390/polym7020235