Laccase Immobilization by Chelated Metal Ion Coordination Chemistry
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Metal Chelated Amidoxime PAN Nanofibrous Membranes
2.3. Immobilization of Laccase on Metal-Chelated AOPAN Nanofibrous Membranes
2.4. Structural Characterization
2.5. Activity Assays of Free and Immobilized Laccase
3. Results and Discussion
3.1. Characterization of the Amidoximized PAN

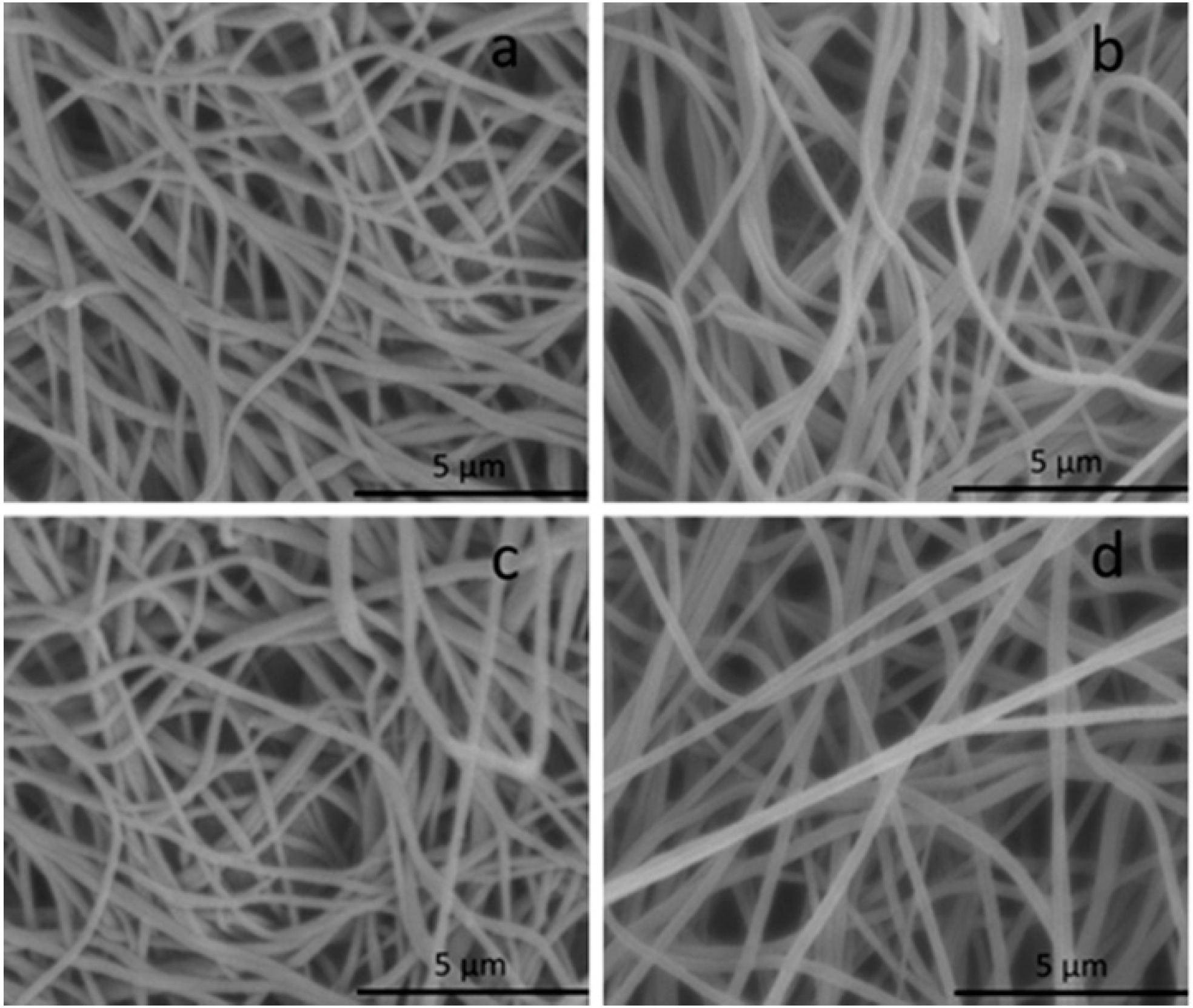
3.2. Characterization of the Metal Ion Chelated PAN Nanofibrous Membranes
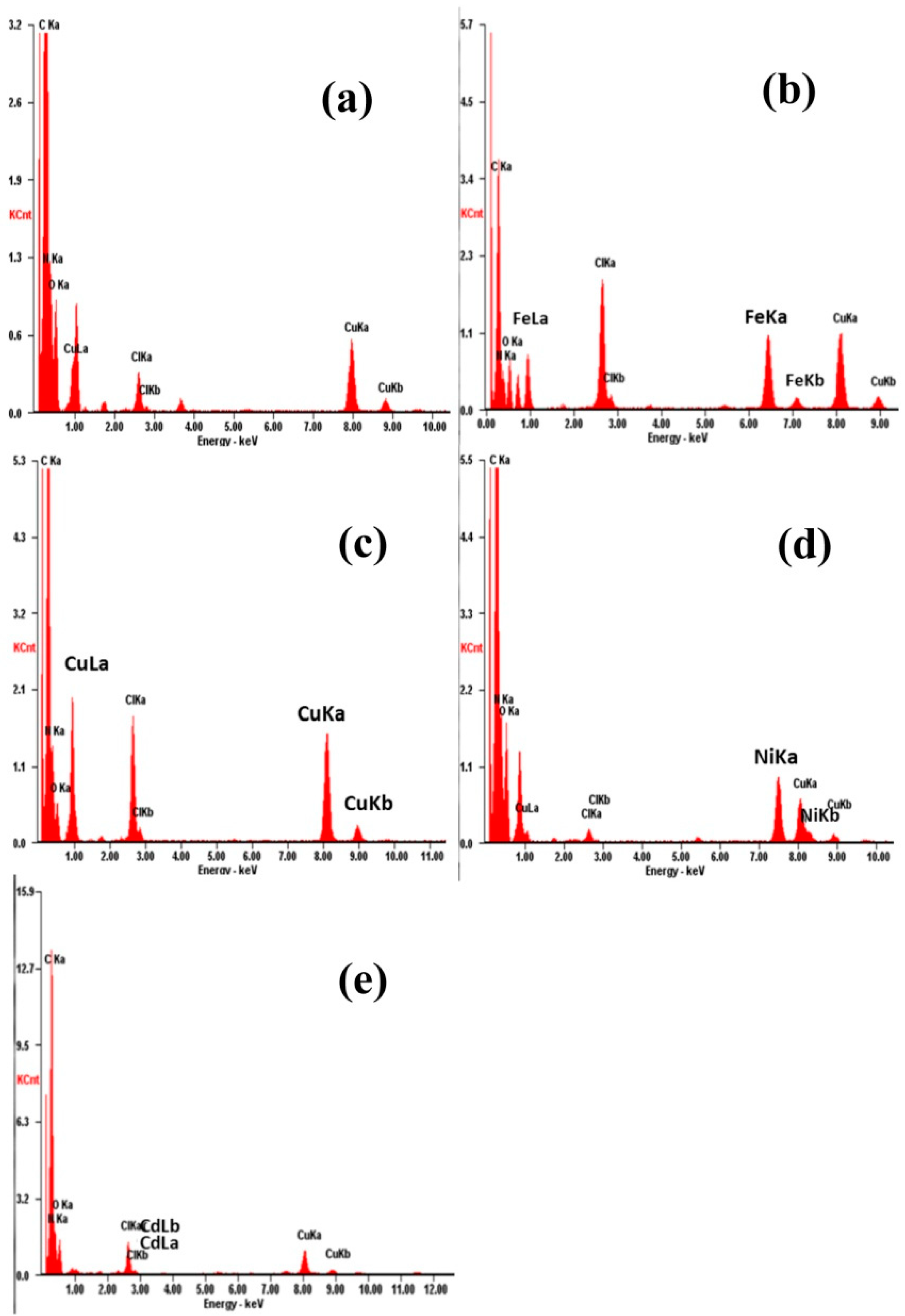
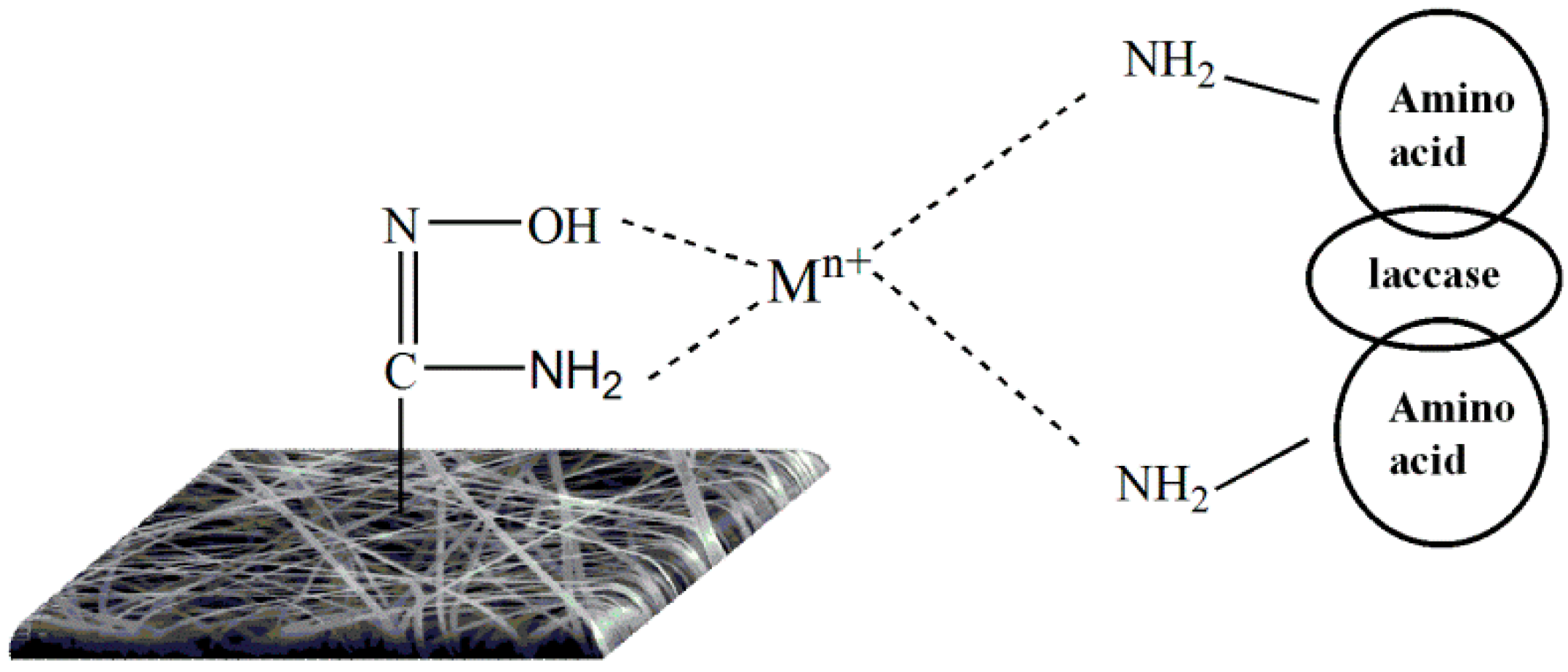
3.3. The Coordination of Metal Ions and Their Properties in Enzyme Immobilization
3.4. Properties of the Immobilized Enzyme
3.4.1. Kinetic Properties
3.4.2. Optimum pH
3.4.3. Optimum Temperature
3.4.4. Storage Stability
3.4.5. Operational Stability
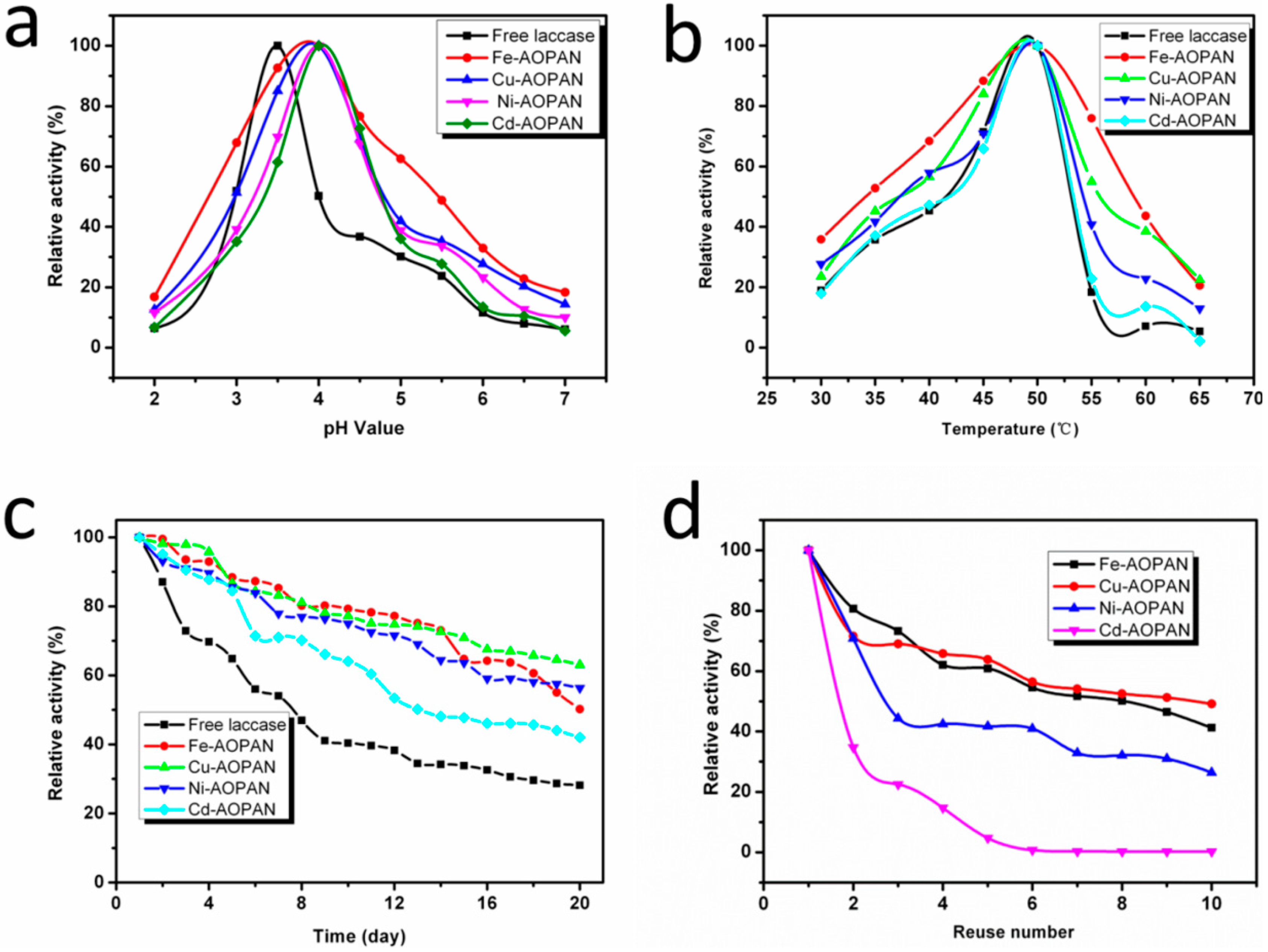
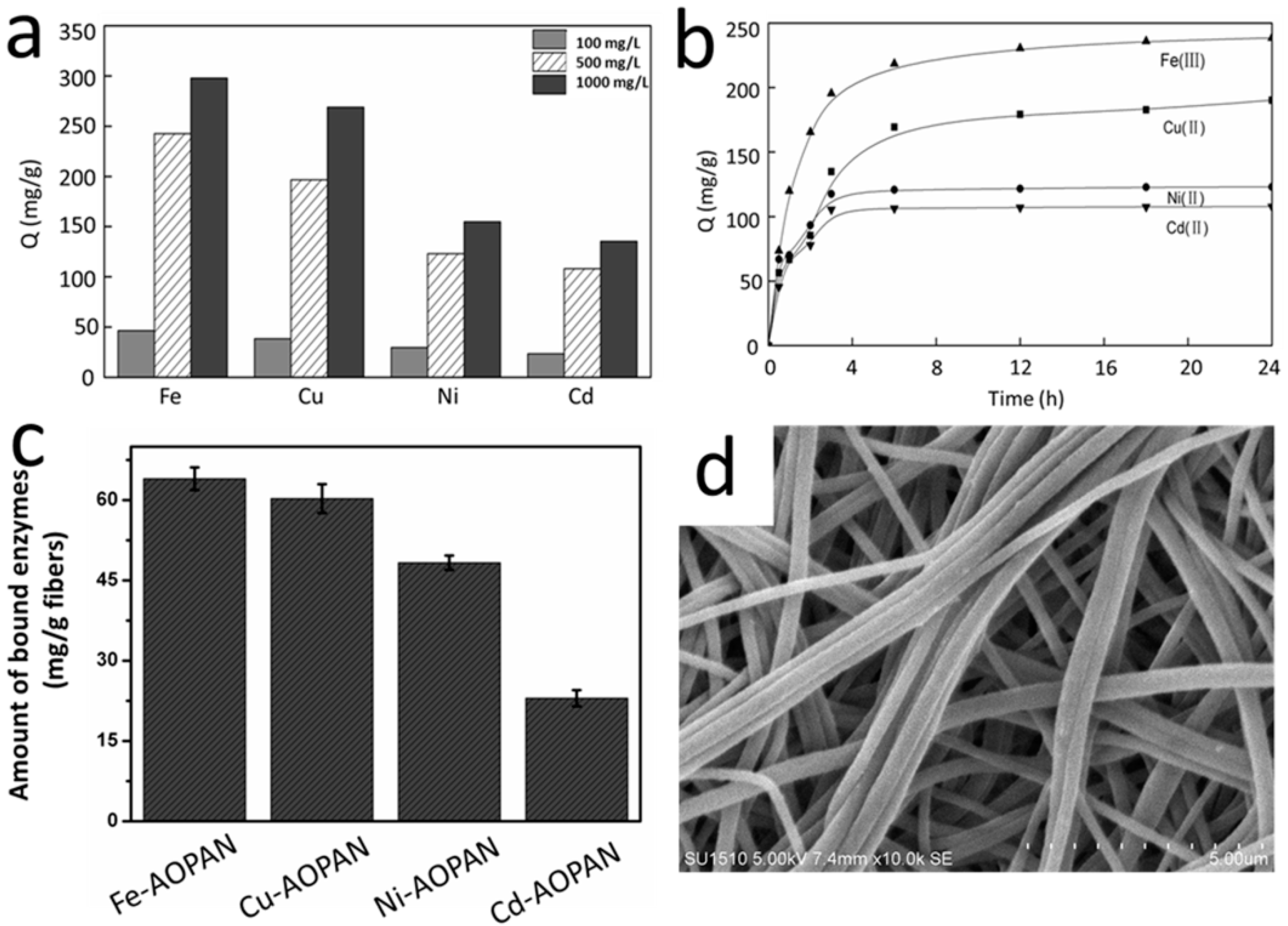
3.5. Enzyme Immobilization on the AOPAN Chelated with Four Metal Ions
3.5.1. Coordination with Mixed Metal Ions by AOPAN Nanofibers
| Metal Ions | Before Coordination Reaction (mg·L−1) | After Coordination Reaction (mg·L−1) |
|---|---|---|
| Fe3+ | 2.640 | 0.920 |
| Cu2+ | 2.899 | 0.613 |
| Ni2+ | 7.290 | 7.080 |
| Cd2+ | 5.560 | 5.360 |
3.5.2. Properties of the Immobilized Enzyme on the Mixed Metal Ion Chelated AOPAN Nanofibrous Membrane

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galhaup, C.; Goller, S.; Peterbauer, C.K.; Strauss, J.; Haltrich, D. Characterization of the major laccase isoenzyme from trametes pubescens and regulation of its synthesis by metal ions. Microbiology 2002, 148, 2159–2169. [Google Scholar] [PubMed]
- Madhavi, V.; Lele, S.S. Laccase: Properties and applications. Bioresources 2009, 4, 1694–1717. [Google Scholar]
- Flory, A.R.; Requesens, D.V.; Devaiah, S.P.; Teoh, K.T.; Mansfield, S.D.; Hood, E.E. Development of a green binder system for paper products. BMC Biotechnol. 2013, 13. [Google Scholar] [CrossRef]
- Fackler, K.; Kuncinger, T.; Ters, T.; Srebotnik, E. Laccase-catalyzed functionalization with 4-hydroxy-3-methoxybenzylurea significantly improves internal bond of particle boards. Holzforschung 2008, 62, 223–229. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Rezaei, S.; Forootanfar, H.; Mahvi, A.H.; Faramarzi, M.A. The enzymatic decolorization and detoxification of synthetic dyes by the laccase from a soil-isolated ascomycete, paraconiothyrium variabile. Int. Biodeterior. Biodegrad. 2013, 85, 173–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Z.T.; Zeng, G.M.; Liu, X.M.; Liu, Z.F.; Chen, M.; Liu, L.F.; Li, J.B.; Xie, G.X. Effect of triton x-100 on the removal of aqueous phenol by laccase analyzed with a combined approach of experiments and molecular docking. Colloid Surf. B 2012, 97, 7–12. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Flocculation and haze removal from crude beer using in-house produced laccase from trametes versicolor cultured on brewer’s spent grain. J. Agric. Food Chem. 2012, 60, 7895–7904. [Google Scholar] [CrossRef] [PubMed]
- Basto, C.; Tzanov, T.; Cavaco-Paulo, A. Combined ultrasound-laccase assisted bleaching of cotton. Ultrason. Sonochem. 2007, 14, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, M.; Sanroman, M.A.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar]
- Qiu, H.J.; Xu, C.X.; Huang, X.R.; Ding, Y.; Qu, Y.B.; Gao, P.J. Immobilization of laccase on nanoporous gold: Comparative studies on the immobilization strategies and the particle size effects. J. Phys. Chem. C 2009, 113, 2521–2525. [Google Scholar] [CrossRef]
- Feng, Q.A.; Xia, X.; Wei, A.F.; Wang, X.Q.; Wei, Q.F.; Huo, D.Y.; Wei, A.J. Preparation of Cu(II)-chelated poly(vinyl alcohol) nanofibrous membranes for catalase immobilization. J. Appl. Polym. Sci. 2011, 120, 3291–3296. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Yilmaz, M.; Arica, M.Y. Reversible immobilization of laccase to poly(4-vinylpyridine) grafted and Cu(II) chelated magnetic beads: Biodegradation of reactive dyes. Bioresour. Technol. 2010, 101, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Krysinski, P.; Michota-Kaminska, A.; Bukowska, J.; Rogalski, J.; Blanchard, G.J. Immobilization of laccase on gold, silver and indium tin oxide by zirconium-phosphonate-carboxylate (ZPC) coordination chemistry. Bioelectrochemistry 2007, 71, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Teerapatsakul, C.; Bucke, C.; Parra, R.; Keshavarz, T.; Chitradon, L. Dye decolorisation by laccase entrapped in copper alginate. World J. Microbiol. Biotechnol. 2008, 24, 1367–1374. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Wang, Y. Immobilization of laccase by alginate-chitosan microcapsules and its use in dye decolorization. World J. Microbiol. Biotechnol. 2007, 23, 159–166. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wan, L.S.; Liu, Z.M.; Huang, X.J.; Xu, Z.K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Xu, R.; Chi, C.L.; Li, F.T.; Zhang, B.R. Laccase-polyacrylonitrile nanofibrous membrane: Highly immobilized, stable, reusable, and efficacious for 2,4,6-trichlorophenol removal. ACS Appl. Mater. Int. 2013, 5, 12554–12560. [Google Scholar] [CrossRef]
- Saeed, K.; Haider, S.; Oh, T.J.; Park, S.Y. Preparation of amidoxime-modified polyacrylonitrile (pan-oxime) nanofibers and their applications to metal ions adsorption. J. Membr. Sci. 2008, 322, 400–405. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, Q.Q.; Tang, B.; Wei, A.F.; Wang, X.Q.; Wei, Q.F.; Huang, F.L.; Cai, Y.B.; Hou, D.Y.; Bi, S.M. Immobilization of catalases on amidoxime polyacrylonitrile nanofibrous membranes. Polym. Int. 2013, 62, 251–256. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Peng, L.; Chen, Y.Z.; Zhang, L.; Gu, Z.H.; Shi, G.Y.; Zhang, K.C. Production and characterization of thermostable laccase from the mushroom, ganoderma lucidum, using submerged fermentation. Afr. J. Microbiol. Res. 2012, 6, 1147–1157. [Google Scholar]
- Huang, F.L.; Xu, Y.F.; Liao, S.Q.; Yang, D.W.; Hsieh, Y.L.; Wei, Q.F. Preparation of amidoxime polyacrylonitrile chelating nanofibers and their application for adsorption of metal ions. Materials 2013, 6, 969–980. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Q.Q.; Zhang, J.N.; Li, G.H.; Wei, Q.F. Preparation of amidoxime-modified polyacrylonitrile nanofibers immobilized with laccase for dye degradation. Fiber. Polym. 2014, 15, 30–34. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Peng, L.; Du, Y.Z.; Xu, J.; Cai, Y.B.; Feng, Q.; Huang, F.L.; Wei, Q.F. Fabrication of hydrophilic nanoporous PMMA/o-MMT composite microfibrous membrane and its use in enzyme immobilization. J. Porous Mater. 2013, 20, 457–464. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Peng, L.; Li, G.H.; Zhang, P.; Li, D.W.; Huang, F.L.; Wei, Q.F. Activity of laccase immobilized on TiO2-montmorillonite complexes. Int. J. Mol. Sci. 2013, 14, 12520–12532. [Google Scholar] [CrossRef] [PubMed]
- Bilba, N.; Bilba, D.; Moroi, G. Synthesis of a polyacrylamidoxime chelating fiber and its efficiency in the retention of palladium ions. J. Appl. Polym. Sci. 2004, 92, 3730–3735. [Google Scholar] [CrossRef]
- Arica, M.Y. Immobilization of polyphenol oxidase on carboxymethylcellulose hydrogel beads: Preparation and characterization. Polym. Int. 2000, 49, 775–781. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zeng, Z.T.; Zeng, G.M.; Tang, L.; Pang, Y.; Li, Z.; Liu, C.; Lei, X.X.; Wu, M.S.; Ren, P.Y.; et al. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour. Technol. 2012, 115, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Yilmaz, M.; Senel, A.U.; Arica, M.Y. Preparation of nanofibrous polymer grafted magnetic poly(GMA-MMA)-g-MAA beads for immobilization of trypsin via adsorption. Biochem. Eng. J. 2008, 40, 262–274. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zhang, X.S.; Li, P.P.; Zhang, W.Q. Effective Cd2+ chelating fiber based on polyacrylonitrile. React. Funct. Polym. 2009, 69, 48–54. [Google Scholar] [CrossRef]
- Horzum, N.; Boyaci, E.; Eroglu, A.E.; Shahwan, T.; Demir, M.M. Sorption efficiency of chitosan nanofibers toward metal ions at low concentrations. Biomacromolecules 2010, 11, 3301–3308. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Q.; Cui, J.; Li, G.; Zhang, J.; Huang, F.; Wei, Q. Laccase Immobilization by Chelated Metal Ion Coordination Chemistry. Polymers 2014, 6, 2357-2370. https://doi.org/10.3390/polym6092357
Wang Q, Cui J, Li G, Zhang J, Huang F, Wei Q. Laccase Immobilization by Chelated Metal Ion Coordination Chemistry. Polymers. 2014; 6(9):2357-2370. https://doi.org/10.3390/polym6092357
Chicago/Turabian StyleWang, Qingqing, Jing Cui, Guohui Li, Jinning Zhang, Fenglin Huang, and Qufu Wei. 2014. "Laccase Immobilization by Chelated Metal Ion Coordination Chemistry" Polymers 6, no. 9: 2357-2370. https://doi.org/10.3390/polym6092357
APA StyleWang, Q., Cui, J., Li, G., Zhang, J., Huang, F., & Wei, Q. (2014). Laccase Immobilization by Chelated Metal Ion Coordination Chemistry. Polymers, 6(9), 2357-2370. https://doi.org/10.3390/polym6092357





