Thermo-Responsive Injectable MPEG-Polyester Diblock Copolymers for Sustained Drug Release
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
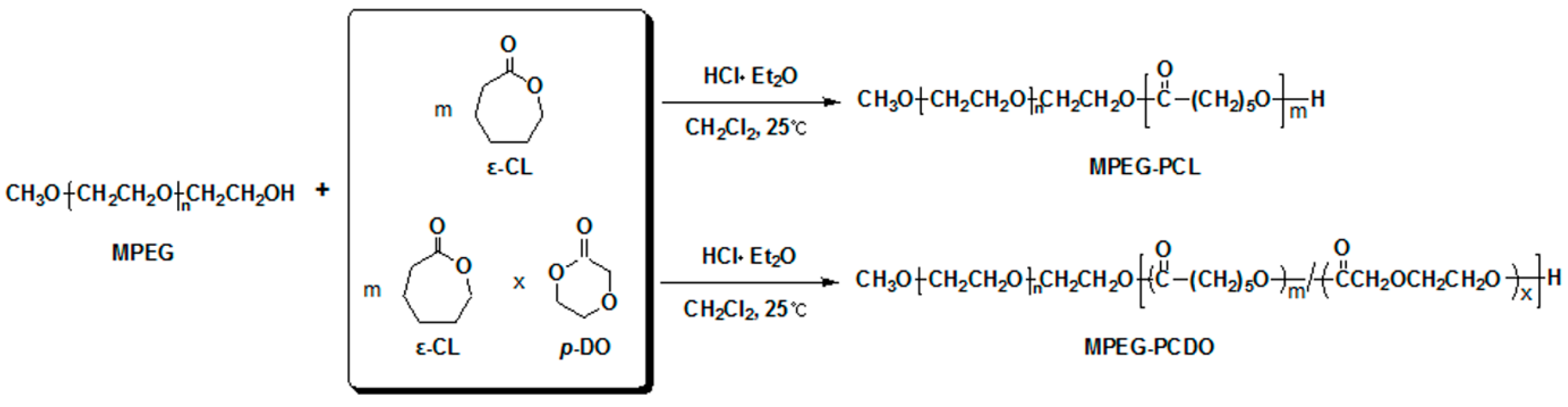
2.3. Synthesis of MPEG-b-poly(ε-caprolactone) (MPEG-b-PCL) and MPEG-b-poly(ε-caprolactone-ran-p-dioxanone) (MPEG-b-PCDO) Diblock Copolymers
2.4. In Vitro Gel Formation and Protein Release Study
2.5. In Vivo Gel Formation and Protein Release Study
3. Results and Discussion
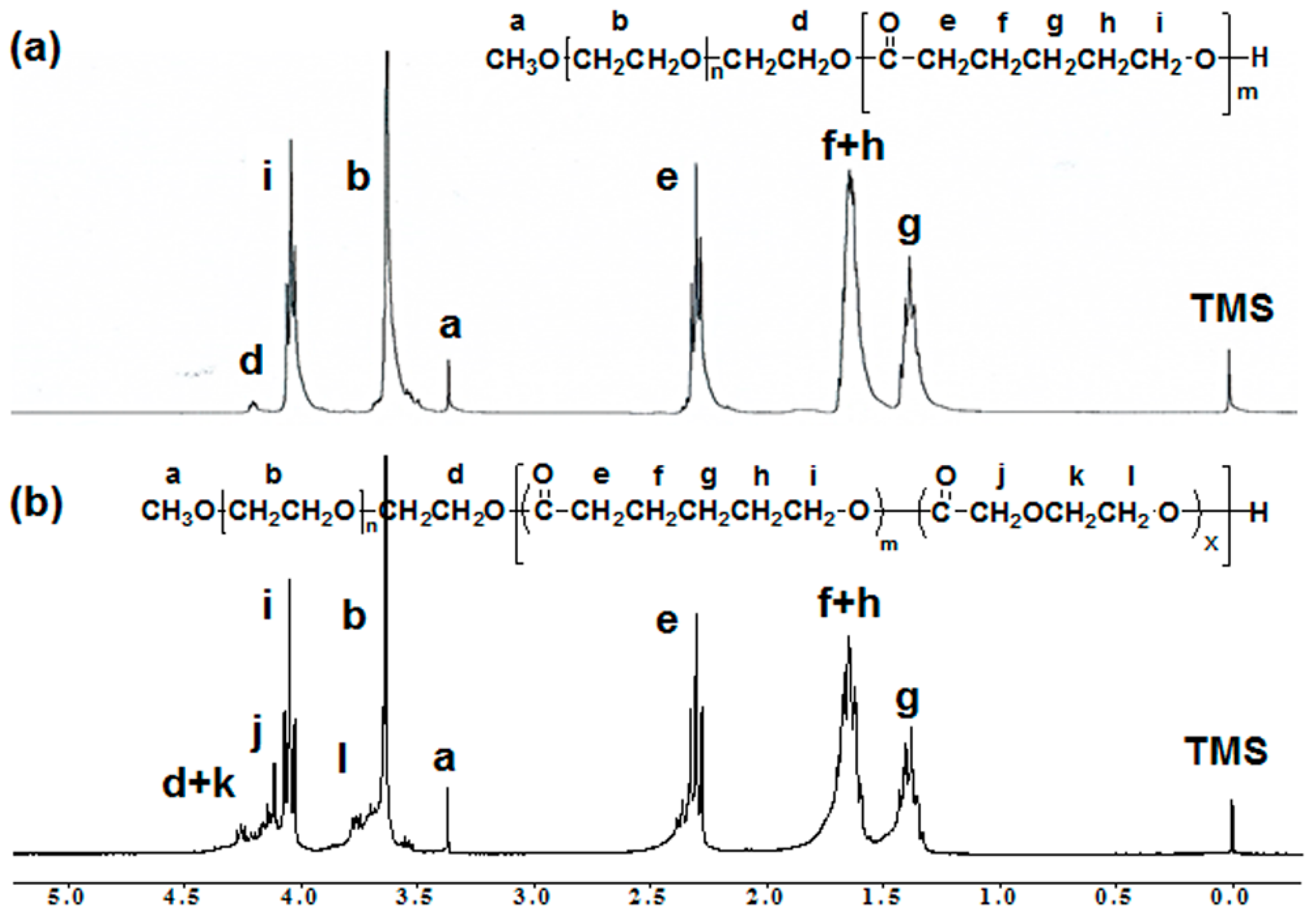
3.1. Synthesis of MPEG-b-PCL and MPEG-b-PCDO Diblock Copolymers
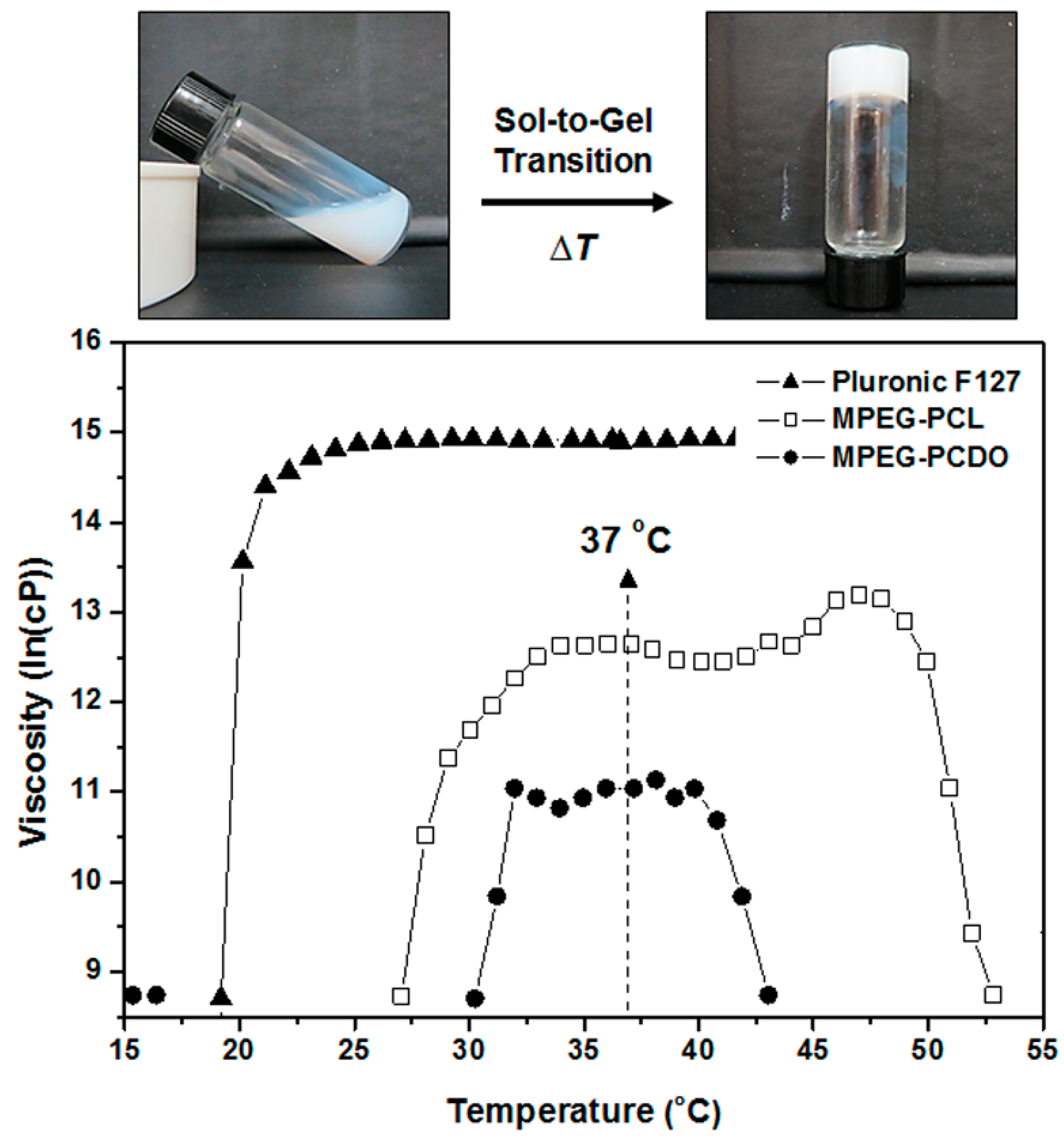
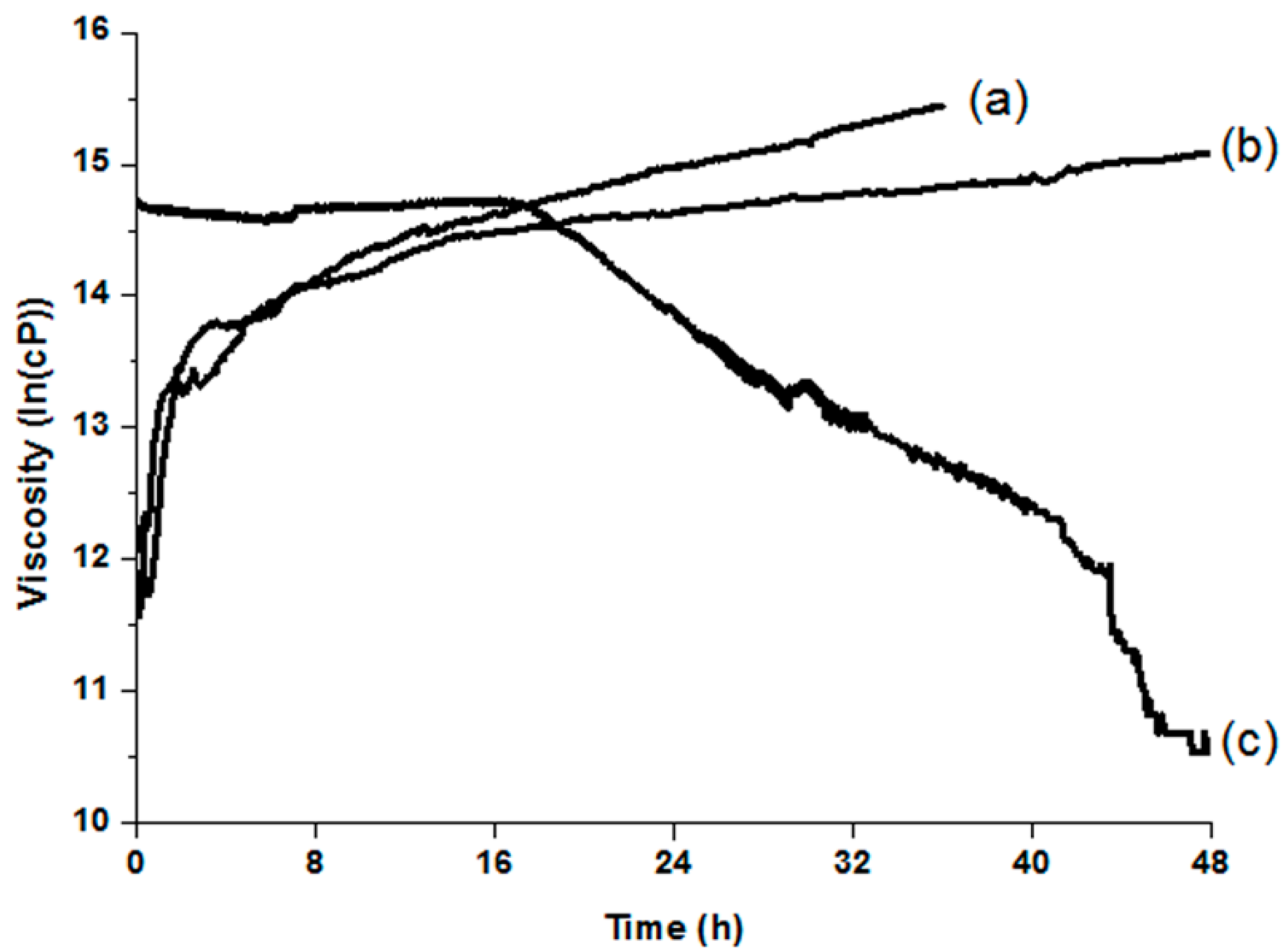
3.2. Temperature- and Time-Dependent Changes in the Viscosity of MPEG-b-PCL and MPEG-b-PCDO Diblock Copolymers
3.3. In Vitro and in Vivo Release of Proteins from MPEG-b-PCL and MPEG-b-PCDO Hydrogels

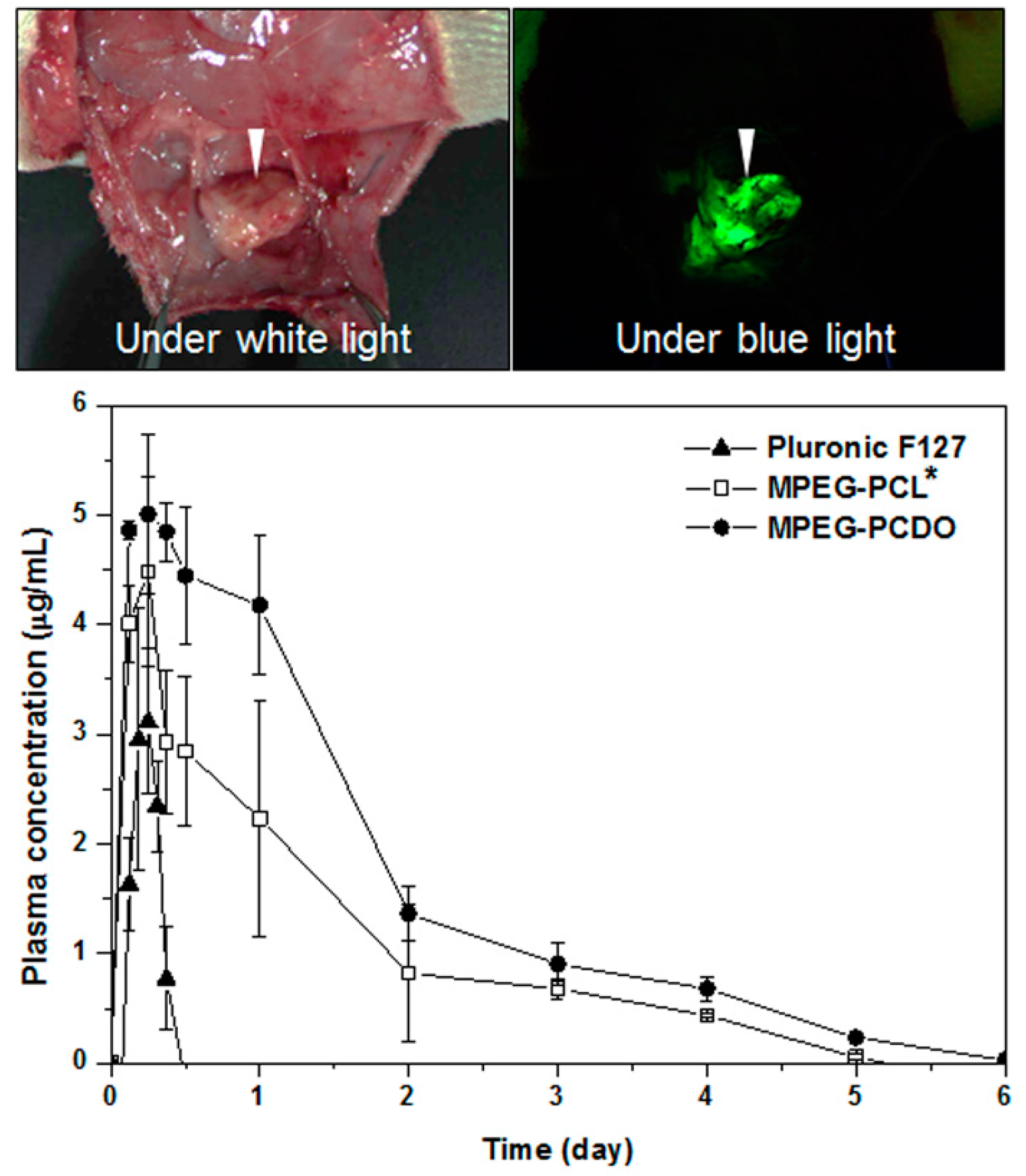
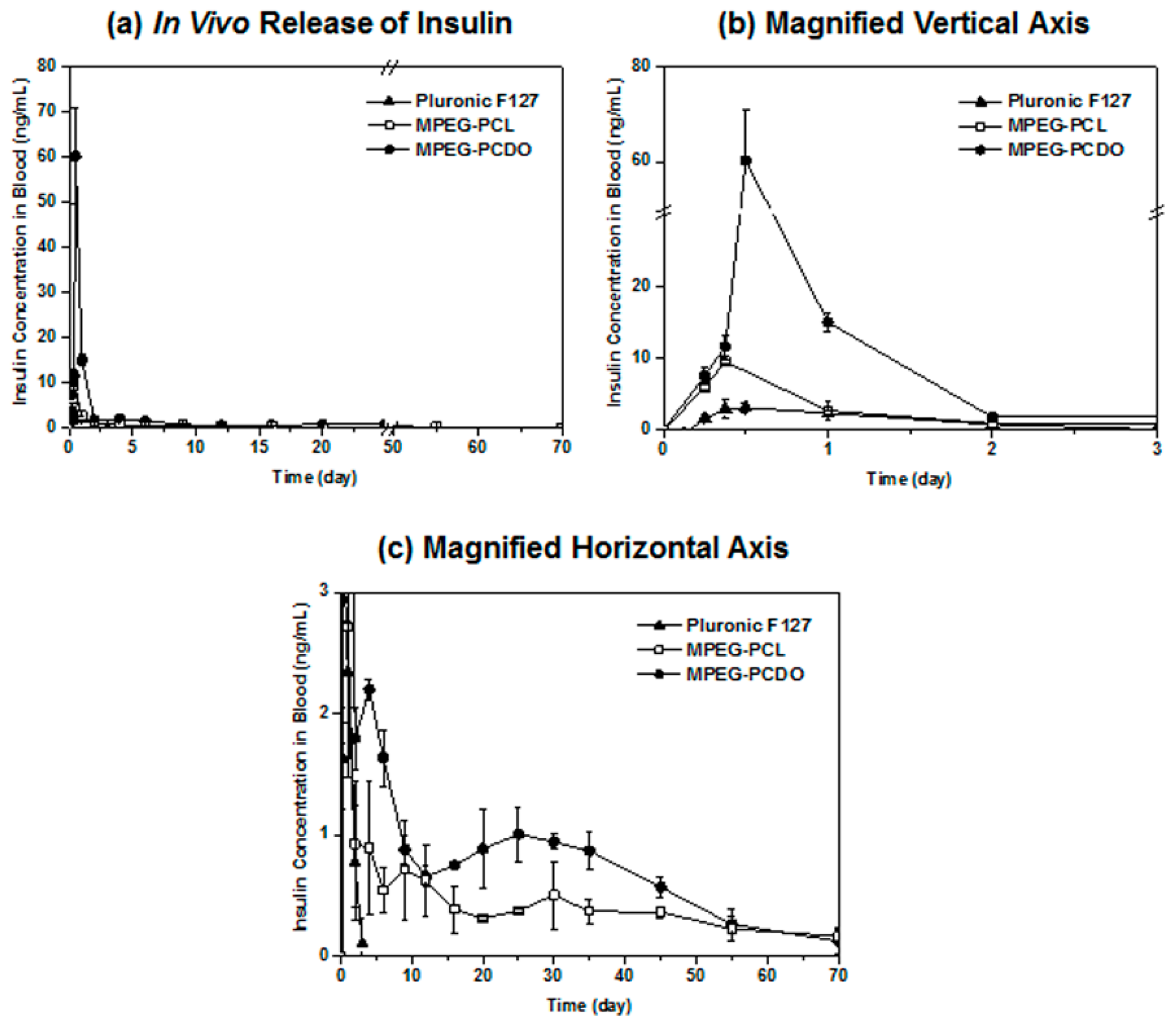
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, M.S.; Park, K. Injectable hydrogel. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherland, 2012; Volume 2, pp. 1091–1096. [Google Scholar]
- Aucoin, H.; Wilson, A.N.; Wilson, A.M.; Ishihara, K.; Guiseppi-Elie, A. Release of potassium ion and calcium ion from phosphorylcholine group bearing hydrogels. Polymers 2013, 5, 1241–1257. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, D.Y.; Kwon, D.Y.; Kang, H.J.; Kim, J.H.; Min, B.H.; Kim, M.S. An injectable biodegradable temperature-responsive gel with an adjustable persistence window. Biomaterials 2012, 33, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Jin, L.M.; Lee, B.; Kim, J.H.; Min, B.H.; Kim, M.S. Injectable intratumoral hydrogel as 5-fluorouracil drug depot. Biomaterials 2013, 34, 2748–2757. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, J.; Li, J.; Fei, Y.; Dong, J.; Pan, W. Optimization of thermosensitive chitosan hydrogels for the sustained delivery of venlafaxine hydrochloride. Int. J. Pharm. 2013, 441, 482–490. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 2008, 127, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.K.; Sanabria-DeLong, N.; Tew, G.N.; Bhatia, S.R. Structural characterization of PLA-PEO-PLA solutions and hydrogels: Crystalline vs. amorphous PLA domains. Macromolecules 2008, 41, 1774–1784. [Google Scholar] [CrossRef]
- Lee, J.; Bae, Y.H.; Sohn, Y.S.; Jeong, B. Thermogelling aqueous solutions of alternating multiblock copolymers of poly(l-lactic acid) and poly(ethylene glycol). Biomacromolecules 2006, 7, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev. 2011, 51, 23–52. [Google Scholar] [CrossRef]
- Tsai, Y.; Jheng, L.; Hsu, S.L. Thermogelling polymers composed of poly (cyclohexylenedimethylene adipate) and poly(ethylene glycol). Eur. Polym. J. 2012, 48, 541–548. [Google Scholar] [CrossRef]
- Wang, F.; Shen, Y.; Xu, X.; Lv, L.; Li, Y.; Liu, J.; Li, M.; Guo, A.; Guo, S.; Jin, F. Selective tissue distribution and long circulation endowed by paclitaxel loaded PEGylated poly(ε-caprolactone-co-l-lactide) micelles leading to improved anti-tumor effects and low systematic toxicity. Int. J. Pharm. 2013, 456, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.E.; Baek, W.Y.; Lim, J.O. Regional delivery of vancomycin using pluronic F-127 to inhibit methicillin resistant Staphylococcus aureus (MRSA) growth in chronic otitis media in vitro and in vivo. J. Control. Release 2004, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Kim, S.W. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. J. Biomed. Mater. Res. 2000, 50, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Hyun, H.; Khang, G.; Lee, H.B. Preparation of thermosensitive diblock copolymers consisting of MPEG and polyesters. Macromolecules 2006, 39, 3099–3102. [Google Scholar] [CrossRef]
- Jiang, Z.; Hao, J.; You, Y.; Gu, Q.; Cao, W.; Deng, Z. Biodegradable thermogelling hydrogel of P(CL-GL)-PEG-P(CL-GL) triblock copolymer: Degradation and drug release behavior. J. Pharm. Sci. 2009, 98, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.W.; Gong, C.Y.; Shi, S.; Fu, S.Z.; Men, K.; Zeng, S. Self-assembled honokiol-loaded micelles based on poly(caprolactone)-poly(ethylene glycol)-poly(caprolactone) copolymer. Int. J. Pharm. 2009, 369, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.N.; Kim, D.Y.; Yoon, S.M.; Lee, J.Y.; Lee, B.N.; Kwon, J.S.; Seo, H.W.; Lee, I.W.; Shin, H.C.; Kim, Y.M.; et al. Tissue engineered regeneration of completely transected spinal cord using human mesenchymal stem cells. Biomaterials 2012, 33, 4828–4835. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Kim, Y.H.; Song, I.B.; Lee, J.W.; Kim, M.S.; Khang, G.; Park, K.; Lee, H.B. In vitro and in vivo release of albumin using a biodegradable MPEG-PCL diblock copolymer as an in situ gel-forming carrier. Biomacromolecules 2007, 8, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Hyun, H.; Seo, K.S.; Cho, Y.H.; Lee, J.W.; Lee, C.R.; Khang, G.; Lee, H.B. Preparation and characterization of MPEG-PCL diblock copolymers with thermo-responsive sol-gel-sol phase transition. J. Polym. Sci. Part A 2006, 44, 5413–5423. [Google Scholar] [CrossRef]
- Cho, M.H.; Hyun, H.; Shin, Y.N.; Lee, J.W.; Lee, M.S.; Ahn, H.H.; Kim, M.S.; Lee, B.; Khang, G.; Lee, H.B. Release profile of BSA or BDNF from temperature sensitive hydrogels. Key Eng. Mat. 2007, 342–343, 473–476. [Google Scholar] [CrossRef]
- Kwon, J.S.; Yoon, S.M.; Shim, S.W.; Park, J.H.; Min, K.J.; Oh, H.J.; Kim, J.H.; Kim, Y.J.; Yoon, J.J.; Choi, B.H.; et al. Injectable extracellular matrix hydrogel developed using porcine articular cartilage. Int. J. Pharm. 2013, 454, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Kim, M.S.; Khang, G.; Lee, H.B. Ring-opening polymerization of trimethylene carbonate by poly(ethylene glycol) in the presence of HCl∙Et2O as a monomer activator. J. Polym. Sci. Part A 2006, 44, 4235–4241. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, S.K.; Kim, S.H.; Hyun, H.; Khang, G.; Lee, H.B. In vivo osteogenic differentiation of rat bone marrow stromal cells in thermosensitive MPEG-PCL diblock copolymer gels. Tissue Eng. 2006, 12, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.W.; Kwon, D.Y.; Park, J.H.; Kim, J.H.; Chun, H.J.; Koh, Y.J.; Kim, M.S. Preparation of zwitterionic sulfobetaine end-functionalized poly(ethylene glycol)-b-poly(caprolactone) diblock copolymers and examination of their thermogelling properties. J. Polym. Sci. A 2014, 52, 2185–2191. [Google Scholar] [CrossRef]
- Davies, A.G. Organotin Chemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Pitt, C.G.; Schindler, A. Long-Acting Contraceptive Delivery Systems; Zatuchni, G.L., Goldsmith, A., Shelton, J.D., Sciarra, J.J., Eds.; Harper and Row Publishers: Philadelphia, PA, USA, 1984; pp. 48–63. [Google Scholar]
- Kim, M.S.; Seo, K.S.; Khang, G.; Lee, H.B. Ring-opening polymerization of ε-caprolactone by poly(ethylene glycol) by an activated monomer mechanism. Macromol. Rapid Commun. 2005, 26, 643–648. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, H.; Park, S.H.; Kwon, D.Y.; Khang, G.; Lee, H.B.; Kim, M.S. Thermo-Responsive Injectable MPEG-Polyester Diblock Copolymers for Sustained Drug Release. Polymers 2014, 6, 2670-2683. https://doi.org/10.3390/polym6102670
Hyun H, Park SH, Kwon DY, Khang G, Lee HB, Kim MS. Thermo-Responsive Injectable MPEG-Polyester Diblock Copolymers for Sustained Drug Release. Polymers. 2014; 6(10):2670-2683. https://doi.org/10.3390/polym6102670
Chicago/Turabian StyleHyun, Hoon, Seung Hun Park, Doo Yeon Kwon, Gilson Khang, Hai Bang Lee, and Moon Suk Kim. 2014. "Thermo-Responsive Injectable MPEG-Polyester Diblock Copolymers for Sustained Drug Release" Polymers 6, no. 10: 2670-2683. https://doi.org/10.3390/polym6102670
APA StyleHyun, H., Park, S. H., Kwon, D. Y., Khang, G., Lee, H. B., & Kim, M. S. (2014). Thermo-Responsive Injectable MPEG-Polyester Diblock Copolymers for Sustained Drug Release. Polymers, 6(10), 2670-2683. https://doi.org/10.3390/polym6102670




