2-(Dimethylamino)ethyl Methacrylate/(2-Hydroxyethyl) Methacrylate/α-Tricalcium Phosphate Cryogels for Bone Repair, Preparation and Evaluation of the Biological Response of Human Trabecular Bone-Derived Cells and Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Cryogels
2.3. Characterization
2.4. Biological Analysis
2.4.1. Isolation and Culture of Human Trabecular Bone-Derived Cells (HTB)
2.4.2. Isolation and Culture of Human Bone Marrow Derived-Mesenchymal Stem Cells (BM-MSC)
2.4.3. Cell Seeding and Osteogenic Differentiation
2.4.4. HTB and BM-MSC Metabolic Activity
2.4.5. Histological and Immunohistochemical Analysis
2.4.6. Statistical Analysis
3. Results and Discussion
3.1. Scaffold Characterization
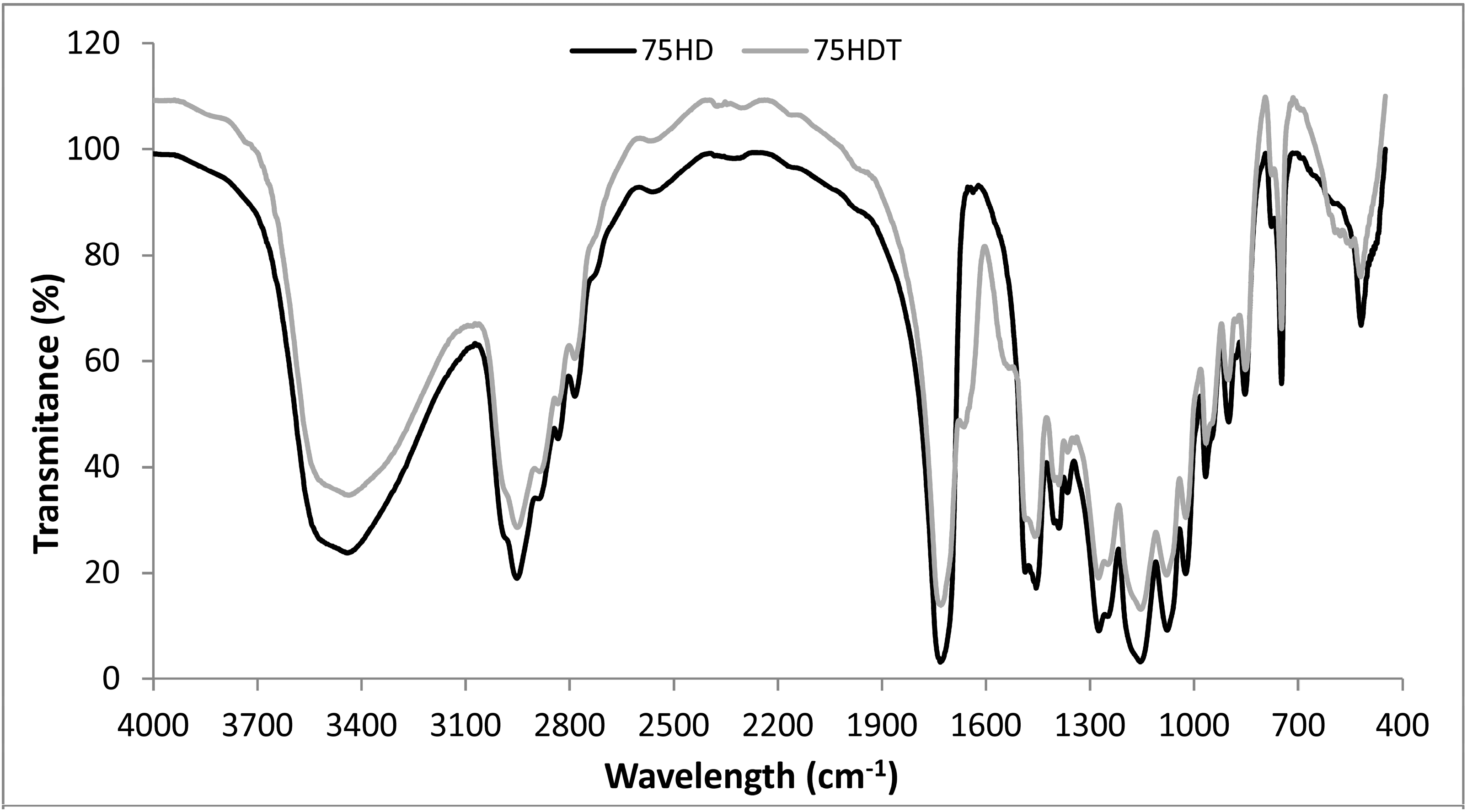
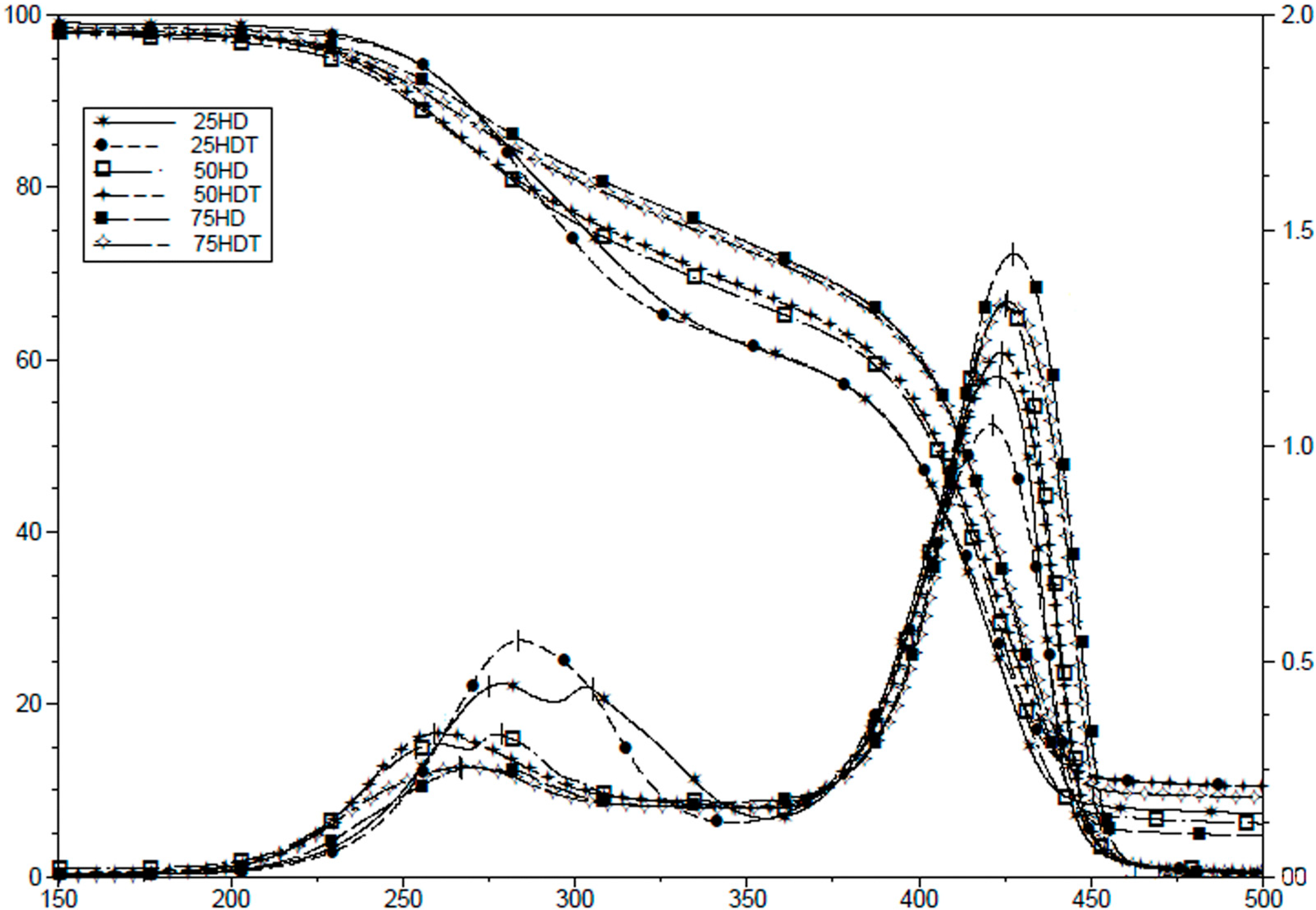
| Sample | Porosity (%) | Modal Size (μm) |
|---|---|---|
| 25 HD | 66.7 (9.5) | 100–200 |
| 25 HDT | 75.2 (5.5) | 100–200 |
| 50 HD | 53.8 (5.7) | 100–200 |
| 50 HDT | 59.0 (7.3) | 400–500 |
| 75 HD | 59.3 (8.2) | 200–300 |
| 75 HDT | 73.4 (5.5) | 300 and 400 |
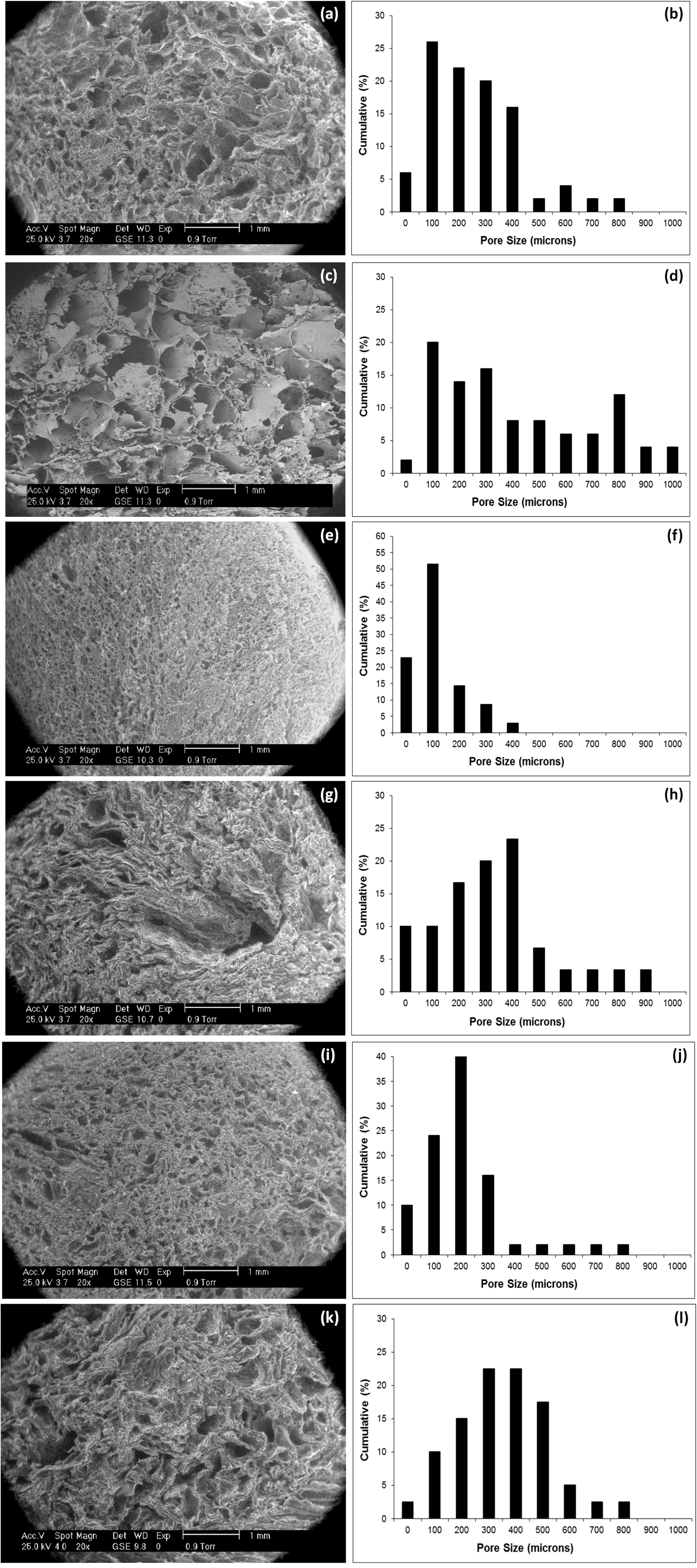
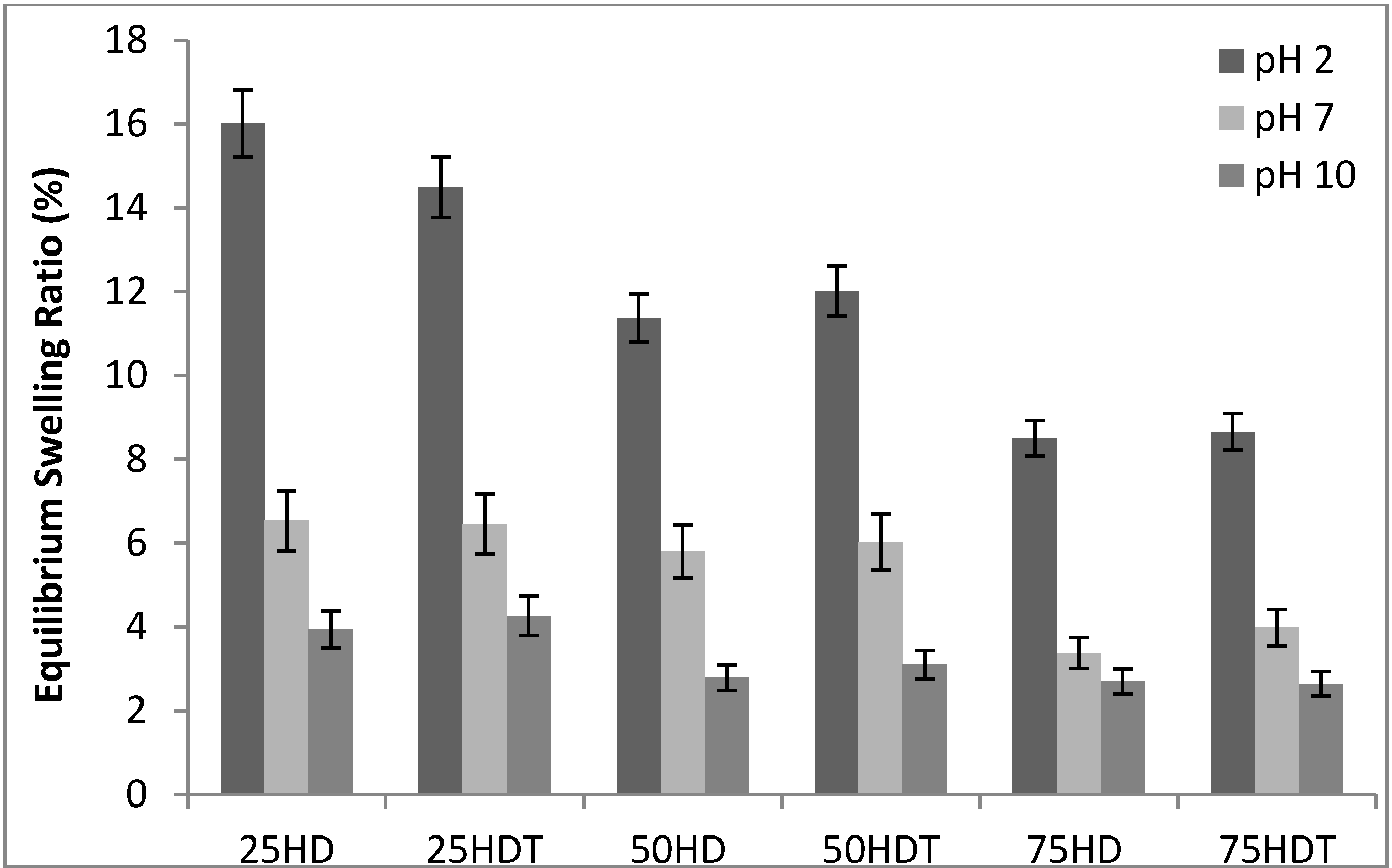

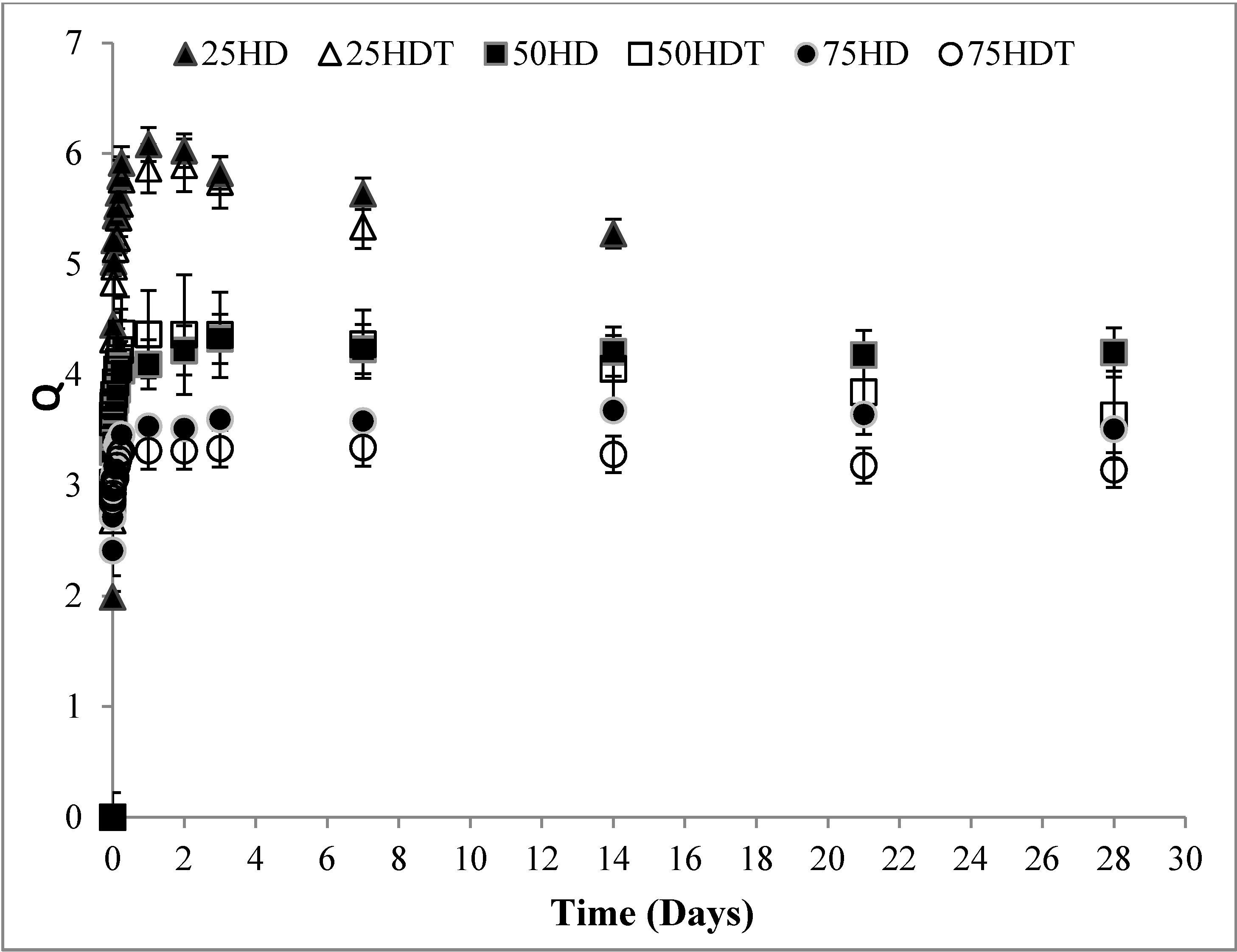
3.2. Biological Analysis
3.2.1. HTB and BM-MSC Metabolic Activity
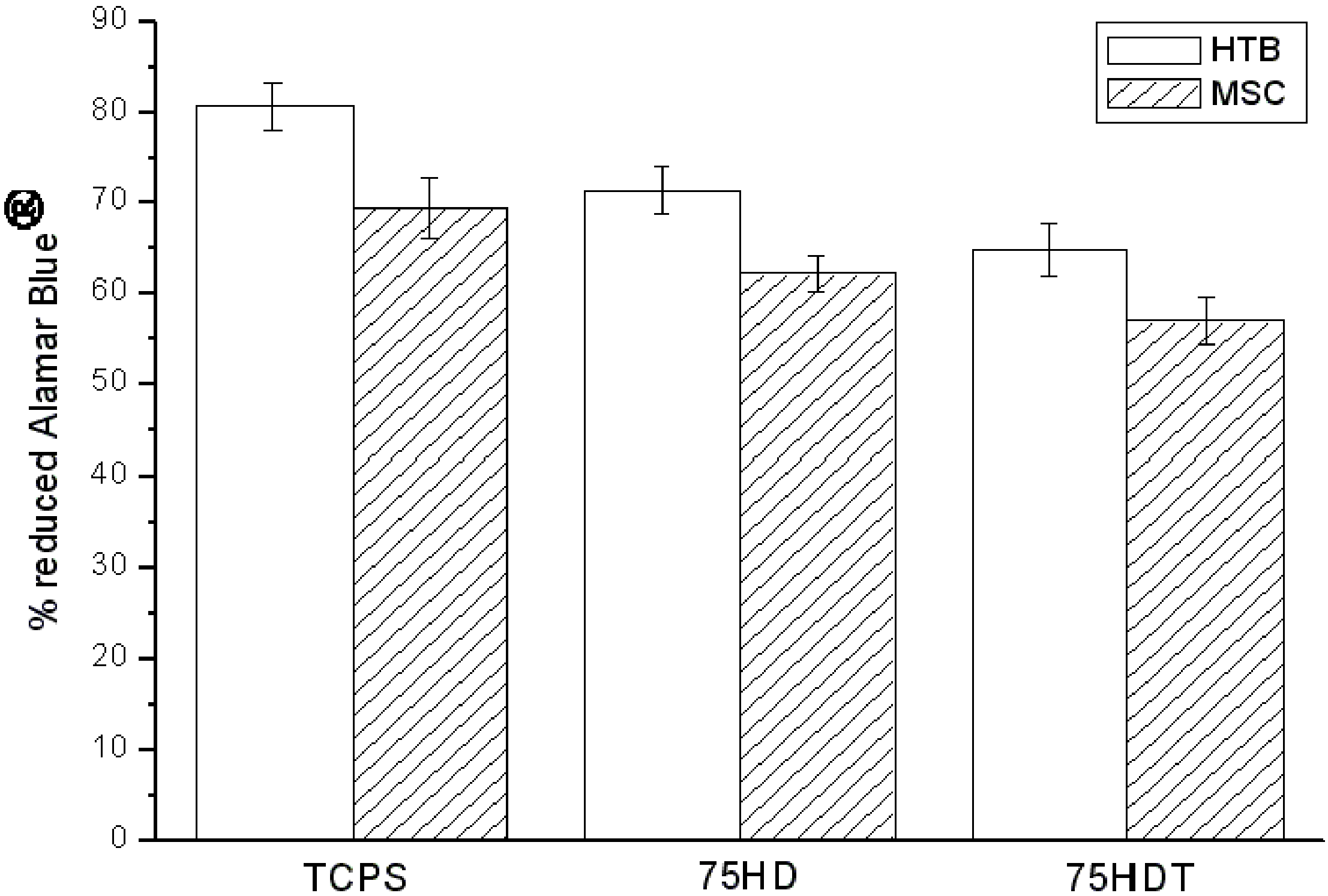
3.2.2. Histological and Immunohistochemical Analysis
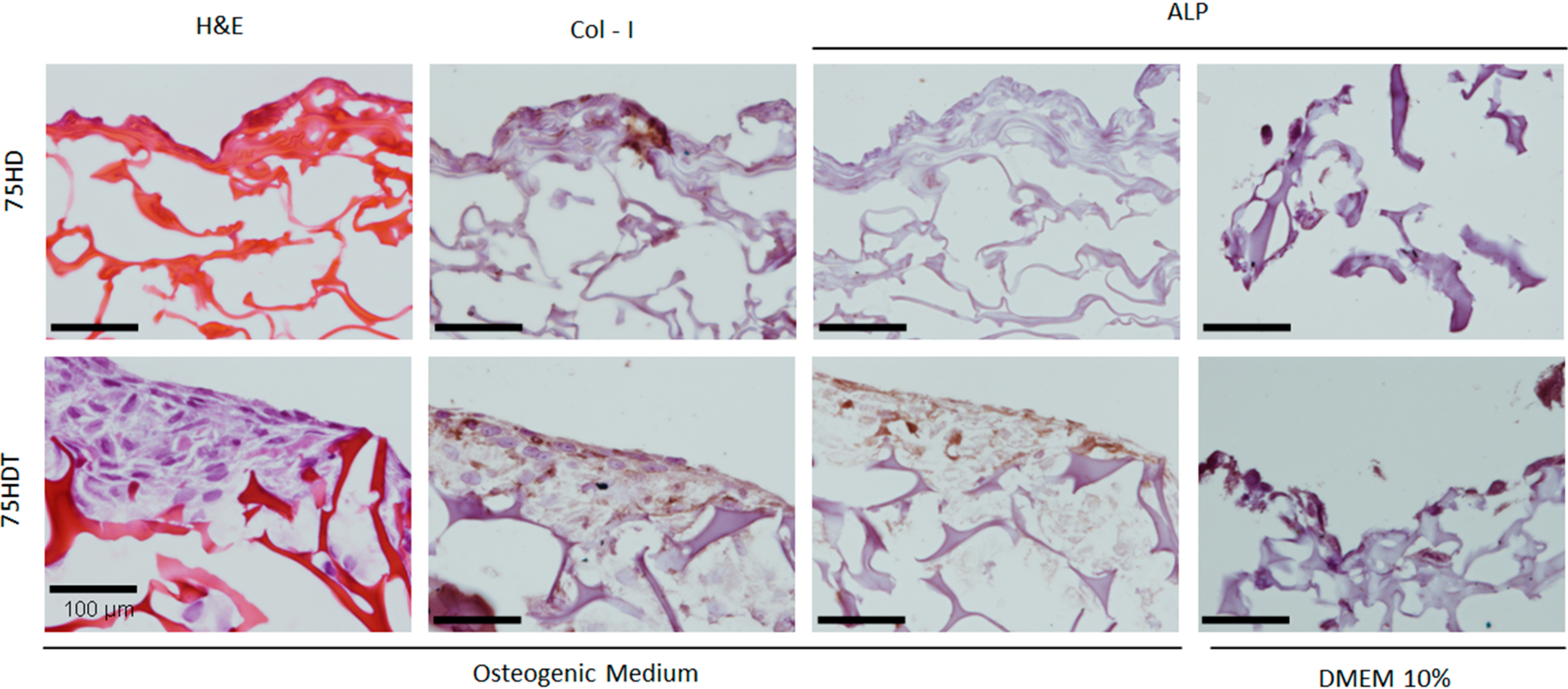
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kirsebom, H.; Aguilar, M.R.; Roman, J.S.; Fernandez, M.; Prieto, M.A.; Bondar, B. Macroporous scaffolds based on chitosan and bioactive molecules. J. Bioact. Compat. Polym. 2007, 22, 621–636. [Google Scholar]
- Dispinar, T.; van Camp, W.; de Cock, L.J.; de Geest, B.G.; du Prez, F.E. Redox-responsive degradable PEG cryogels as potential cell scaffolds in tissue engineering. Macromol. Biosci. 2012, 12, 383–394. [Google Scholar] [PubMed]
- Lozinski, V.I. Cryogels on teh basis of natural and synthetic polymers: Preparation, properties and applications. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar]
- Yao, K.J.; Shen, S.C.; Yun, J.X.; Wang, L.H.; He, X.J.; Yu, X.M. Preparation of polyacrylamide-based supermacroporous monolithic cryogel beds under freezing-temperature variation conditions. Chem. Eng. Sci. 2006, 61, 6701–7608. [Google Scholar]
- Kirsebom, H.; Rata, G.; Topgaard, D.; Mattiasson, B.; Galaev, I.Y. Mechanism of Cryopolymerization: Diffusion-controlled polymerization in a nonfrozen microphase. An NMR study. Macromolecules 2009, 42, 5208–5214. [Google Scholar]
- Arvidsson, P.; Plieva, F.M.; Lozinsky, V.I.; Galaev, I.Y.; Mattiasson, B. Direct chromatographic capture of enzyme from crude homogenate using immobilized metal affinity chromatography on a continuous supermacroporous adsorbent. J. Chromatogr. A 2003, 986, 275–290. [Google Scholar] [PubMed]
- Kumar, A.; Bansal, V.; Nandakumar, K.S.; Galaev, I.Y.; Roychoudhury, P.K.; Holmdahl, R.; Mattiasson, B. Integrated bioprocess for the production and isolation of urokinase from animal cell culture using supermacroporous cryogel matrices. Biotechnol. Bioeng. 2006, 93, 636–646. [Google Scholar] [PubMed]
- Le Noir, M.; Plieva, F.; Hey, T.; Guieysse, B.; Mattiasson, B. Macroporous molecularly imprinted polymer/cryogel composite systems for the removal of endocrine disrupting trace contaminants. J. Chromatogr. A 2007, 1154, 158–164. [Google Scholar]
- Tripathi, A.; Kumar, A. Multi-featured macroporous agarose-alginate cryogel: Synthesis and characterization for bioengineering applications. Macromol. Biosci. 2011, 11, 22–35. [Google Scholar] [PubMed]
- Bolgen, N.; Plieva, F.; Galaev, I.Y.; Mattiasson, B.; Piskin, E. Cryogelation for preparation of novel biodegradable tissue-engineering scaffolds. J. Biomater. Sci. Polym. Ed. 2007, 18, 1165–1179. [Google Scholar] [PubMed]
- Kirsebom, H.; Elowsson, L.; Berillo, D.; Cozzi, S.; Inci, I.; Piskin, E.; Galaev, I.Y.; Mattiasson, B. Enzyme-catalyzed crosslinking in a partly frozen state: A new way to produce supermacroporous protein structures. Macromol. Biosci. 2013, 13, 67–76. [Google Scholar] [PubMed]
- Rodriguez-Lorenzo, L.M.; Saldana, L.; Benito-Garzon, L.; García-Carrodeguas, R.; de Aza, S.; Vilaboa, N.; Román, J.S. Feasibility of ceramic-polymer composite cryogels as scaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 2012, 6, 421–433. [Google Scholar] [PubMed]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Synthesis and hydration properties of pH-sensitive p(HEMA)-based hydrogels containing 3-(trimethoxysilyl)propyl methacrylate. Biomacromolecules 2003, 4, 497–503. [Google Scholar] [PubMed]
- Cherng, J.Y.; vandeWetering, P.; Talsma, H.; Crommelin, D.J.A.; Hennink, W.E. Effect of size and serum proteins on transfection efficiency of poly((2-dimethylamino)ethyl methacrylate)-plasmid nanoparticles. Pharm. Res. 1996, 13, 1038–1042. [Google Scholar] [PubMed]
- Maji, S.; Mitschang, F.; Chen, L.N.; Jin, Q.; Wang, Y.X.; Agarwal, S. Functional poly(dimethyl aminoethyl methacrylate) by combination of radical ring-opening polymerization and click chemistry for biomedical applications. Macromol. Chem. Phys. 2012, 213, 1643–1654. [Google Scholar]
- Aguilar, M.R.; Gallardo, A.; Lechuga, L.M.; Calle, A.; San Roman, J. Modulation of proteins adsorption onto the surface of chitosan complexed with anionic copolymers. real time analysis by surface plasmon resonance. Macromol. Biosci. 2004, 4, 631–638. [Google Scholar] [PubMed]
- Bosetti, M.; Boccafoschi, F.; Calarco, A.; Leigheb, M.; Gatti, S.; Piffanelli, V.; Peluso, G.; Cannas, M. Behaviour of human mesenchymal stem cells on a polyelectrolyte-modified HEMA hydrogel for silk-based ligament tissue engineering. J. Biomater. Sci. Polym. Ed. 2008, 19, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Volkmer, T.M.; Lengler, F.; Barreiro, O.; Sousa, V.C.; dos Santos, L.A. Novel method for the obtainment of nanostructured calcium phosphate cements: Synthesis, mechanical strength and cytotoxicity. Powder Technol. 2013, 235, 599–605. [Google Scholar]
- Gallagher, J.A. Human osteoblast culture. Methods Mol. Med. 2003, 80, 3–18. [Google Scholar] [PubMed]
- Richler, C.; Yaffe, D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev. Biol. 1970, 23, 1–22. [Google Scholar] [PubMed]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997, 64, 295–312. [Google Scholar] [PubMed]
- Anderson, J.M.; Vines, J.B.; Patterson, J.L.; Chen, H.; Javed, A.; Jun, H.W. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta Biomater. 2011, 7, 675–682. [Google Scholar] [PubMed]
- Hollander, A.P.; Hatton, P.V. Biopolymer Methods in Tissue Engineering Methods in Molecular Biology; Humana Press: New York, NY, USA, 2006; pp. 147–157. [Google Scholar]
- Magalhaes, J.; Sousa, R.A.; Mano, J.F.; Reis, R.L.; Blanco, F.J.; San Roman, J. Synthesis and characterization of sensitive hydrogels based on semi-interpenetrated networks of poly 2-ethyl-(2-pyrrolidone) methacrylate and hyaluronic acid. J. Biomed. Mater. Res. Part A 2013, 101A, 157–166. [Google Scholar]
- Roy, D.; Guthrie, J.T.; Perrier, S. Synthesis of natural-synthetic hybrid materials from cellulose via the RAFT process. Soft Matter 2008, 4, 145–155. [Google Scholar]
- Fahami, A.; Nasiri-Tabrizi, B.; Ebrahimi-Kahrizsangi, R. Synthesis of calcium phosphate-based composite nanopowders by mechanochemical process and subsequent thermal treatment. Ceram. Int. 2012, 38, 6729–6738. [Google Scholar]
- Jain, E.; Srivastava, A.; Kumar, A. Macroporous interpenetrating cryogel network of poly(acrylonitrile) and gelatin for biomedical applications. J. Mater. Sci. Mater. Med. 2009, 20, 173–179. [Google Scholar]
- Dainiak, M.B.; Allan, I.U.; Savina, I.N.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.V.; Jungvid, H.; Galaev, I.Y. Gelatin-fibrinogen cryogel dermal matrices for wound repair: Preparation, optimisation and in vitro study. Biomaterials 2010, 31, 67–76. [Google Scholar] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [PubMed]
- You, J.O.; Auguste, D.T. The effect of swelling and cationic character on gene transfection by pH-sensitive nanocarriers. Biomaterials 2010, 31, 6859–6866. [Google Scholar] [PubMed]
- Munoz-Bonilla, A.; Fernandez-Garcia, M.; Haddleton, D.M. Synthesis and aqueous solution properties of stimuli-responsive triblock copolymers. Soft Matter 2007, 3, 725–731. [Google Scholar]
- Sui, X.; Yuan, J.; Zhou, M.; Zhang, J.; Yang, H.; Yuan, W.; Wei, Y.; Pan, C. Synthesis of cellulose-graft-poly(N,N-dimethylamino-2-ethyl methacrylate) copolymers via homogeneous ATRP and their aggregates in aqueous media. Biomacromolecules 2008, 9, 2615–2620. [Google Scholar] [PubMed]
- Chen, Y.; Yi, M. Swelling kinetics and stimuli-responsiveness of poly(DMAEMA) hydrogels prepared by UV-irradiation. Radiat. Phys. Chem. 2001, 61, 65–68. [Google Scholar]
- Grandi, G.; Heitz, C.; Santos, L.A.; Silva, M.L.; Filho, M.S.; Pagnocelli, R.M.; Silva, D.N. Comparative histomorphometric analysis between α-TCP and β-TCPHA granules in the bone repair of rat calvaria. Mater. Res. 2011, 14, 11–16. [Google Scholar]
- Gross, K.A.; Rodríguez-Lorenzo, L.M. Biodegradable composite scaffolds with an interconnected spherical network for bone tissue engineering. Biomaterials 2004, 25, 4955–4962. [Google Scholar] [PubMed]
- Rodriguez-Lorenzo, L.M.; Garcia-Carrodeguas, R.; Rodriguez, M.A.; de Aza, S.; Jiménez, J.; López-Bravo, A.; Fernandez, M.; San Román, J. Synthesis, characterization, bioactivity and biocompatibility of nanostructured materials based on the wollastonite-poly(ethylmethacrylate-co-vinylpyrrolidone) system. J. Biomed. Mater. Res. Part A 2009, 88A, 53–64. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkmer, T.; Magalhães, J.; Sousa, V.; Santos, L.A.; Burguera, E.F.; Blanco, F.J.; Román, J.S.; Rodríguez-Lorenzo, L.M. 2-(Dimethylamino)ethyl Methacrylate/(2-Hydroxyethyl) Methacrylate/α-Tricalcium Phosphate Cryogels for Bone Repair, Preparation and Evaluation of the Biological Response of Human Trabecular Bone-Derived Cells and Mesenchymal Stem Cells. Polymers 2014, 6, 2510-2525. https://doi.org/10.3390/polym6102510
Volkmer T, Magalhães J, Sousa V, Santos LA, Burguera EF, Blanco FJ, Román JS, Rodríguez-Lorenzo LM. 2-(Dimethylamino)ethyl Methacrylate/(2-Hydroxyethyl) Methacrylate/α-Tricalcium Phosphate Cryogels for Bone Repair, Preparation and Evaluation of the Biological Response of Human Trabecular Bone-Derived Cells and Mesenchymal Stem Cells. Polymers. 2014; 6(10):2510-2525. https://doi.org/10.3390/polym6102510
Chicago/Turabian StyleVolkmer, Tiago, Joana Magalhães, Vania Sousa, Luis A. Santos, Elena F. Burguera, Francisco J. Blanco, Julio San Román, and Luis M. Rodríguez-Lorenzo. 2014. "2-(Dimethylamino)ethyl Methacrylate/(2-Hydroxyethyl) Methacrylate/α-Tricalcium Phosphate Cryogels for Bone Repair, Preparation and Evaluation of the Biological Response of Human Trabecular Bone-Derived Cells and Mesenchymal Stem Cells" Polymers 6, no. 10: 2510-2525. https://doi.org/10.3390/polym6102510
APA StyleVolkmer, T., Magalhães, J., Sousa, V., Santos, L. A., Burguera, E. F., Blanco, F. J., Román, J. S., & Rodríguez-Lorenzo, L. M. (2014). 2-(Dimethylamino)ethyl Methacrylate/(2-Hydroxyethyl) Methacrylate/α-Tricalcium Phosphate Cryogels for Bone Repair, Preparation and Evaluation of the Biological Response of Human Trabecular Bone-Derived Cells and Mesenchymal Stem Cells. Polymers, 6(10), 2510-2525. https://doi.org/10.3390/polym6102510





