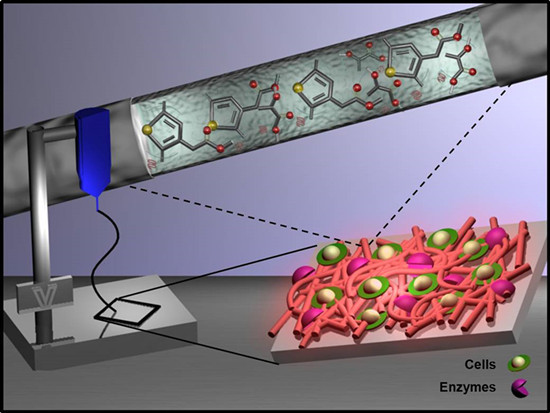
Nanomembranes and Nanofibers from Biodegradable Conducting Polymers
Abstract
:1. Introduction
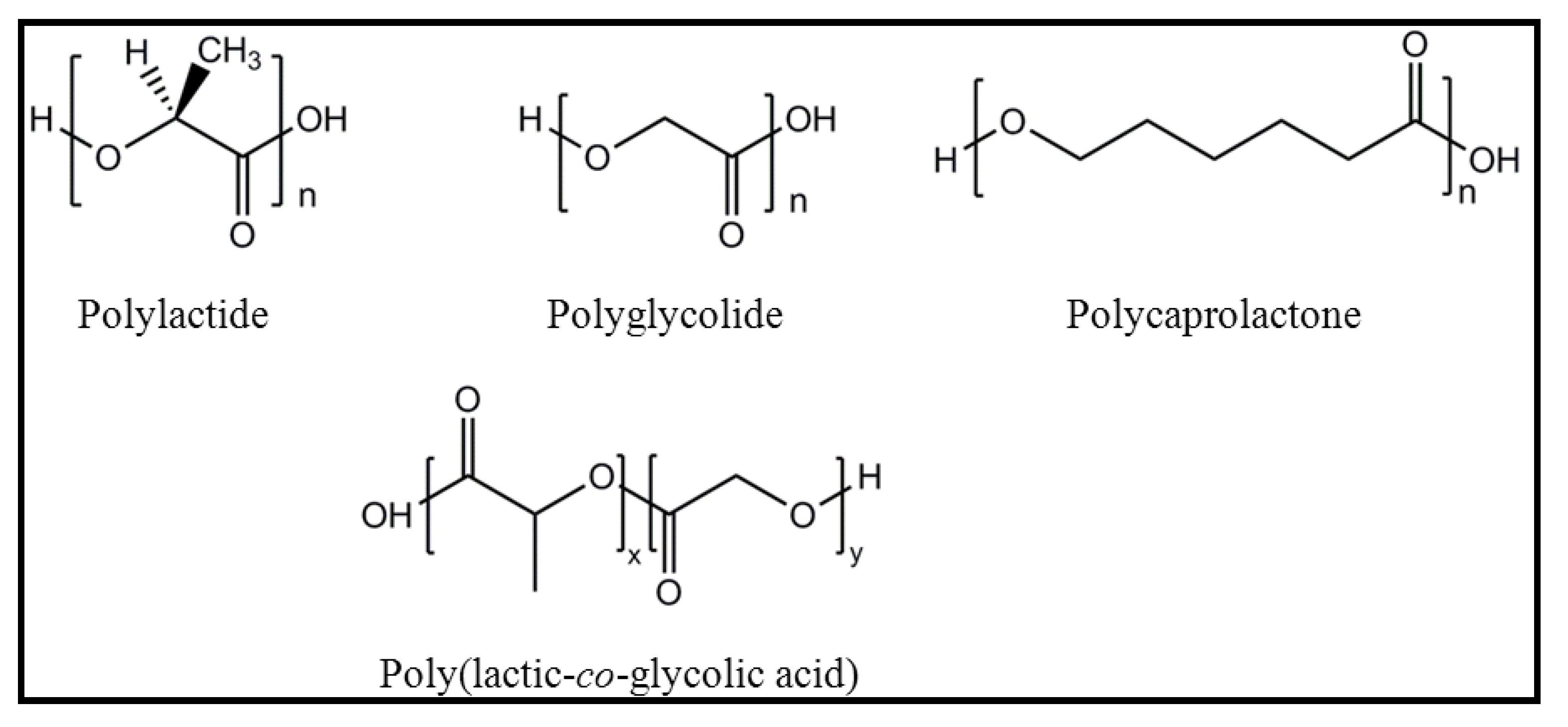
2. Base Materials
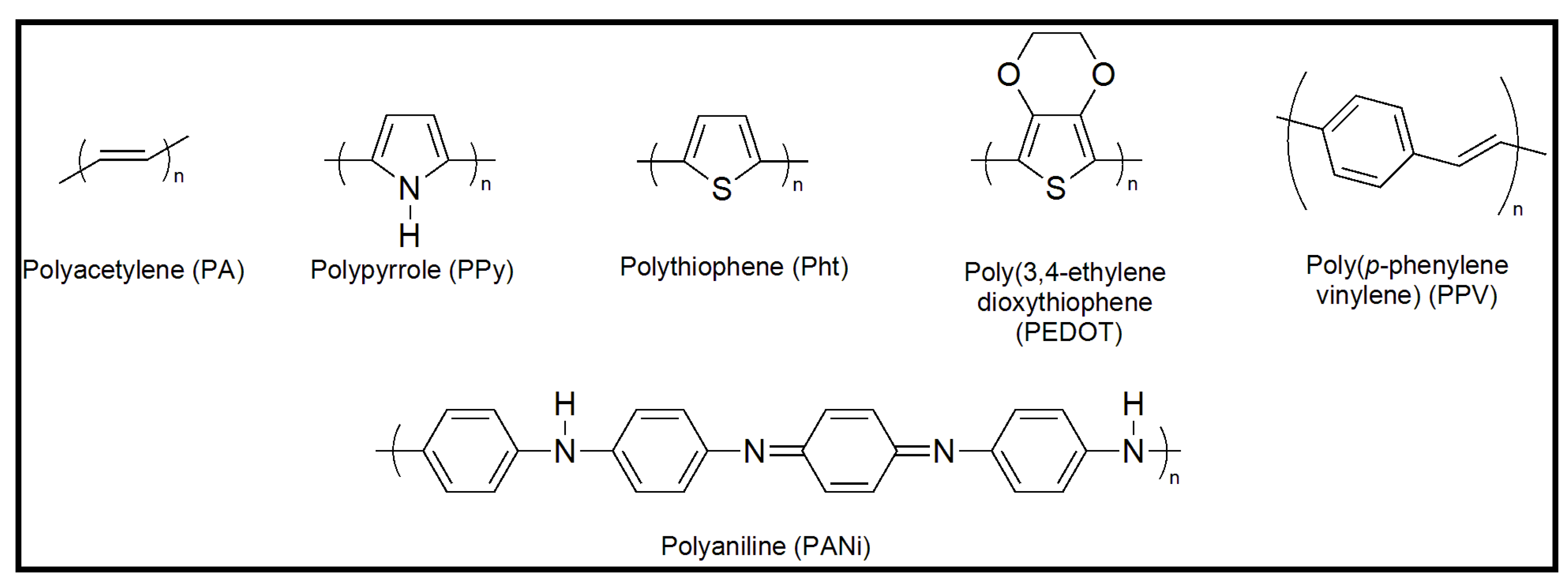
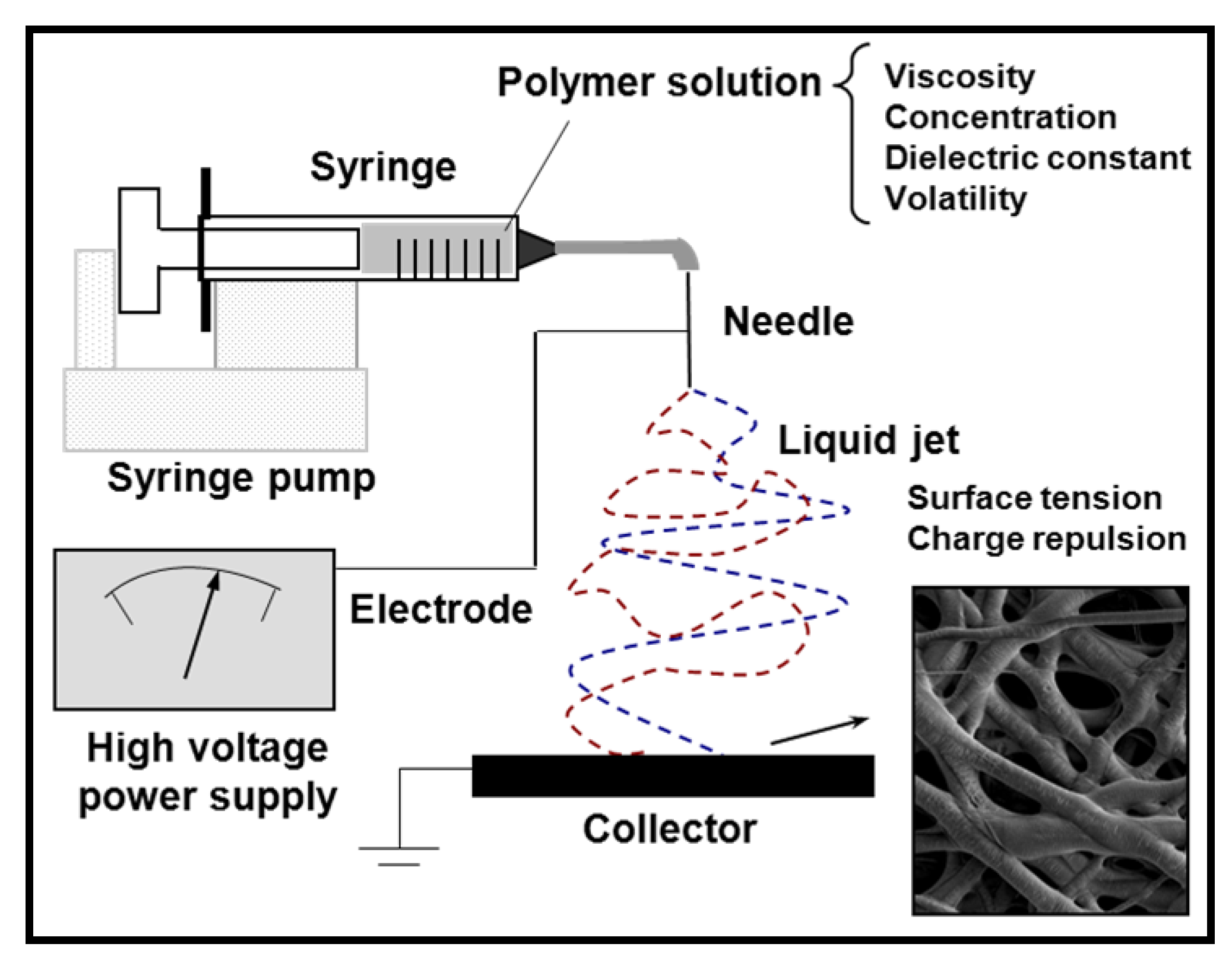
3. Preparation of Nanofibers and Nanomembranes: Electrospinning and Spin Coating
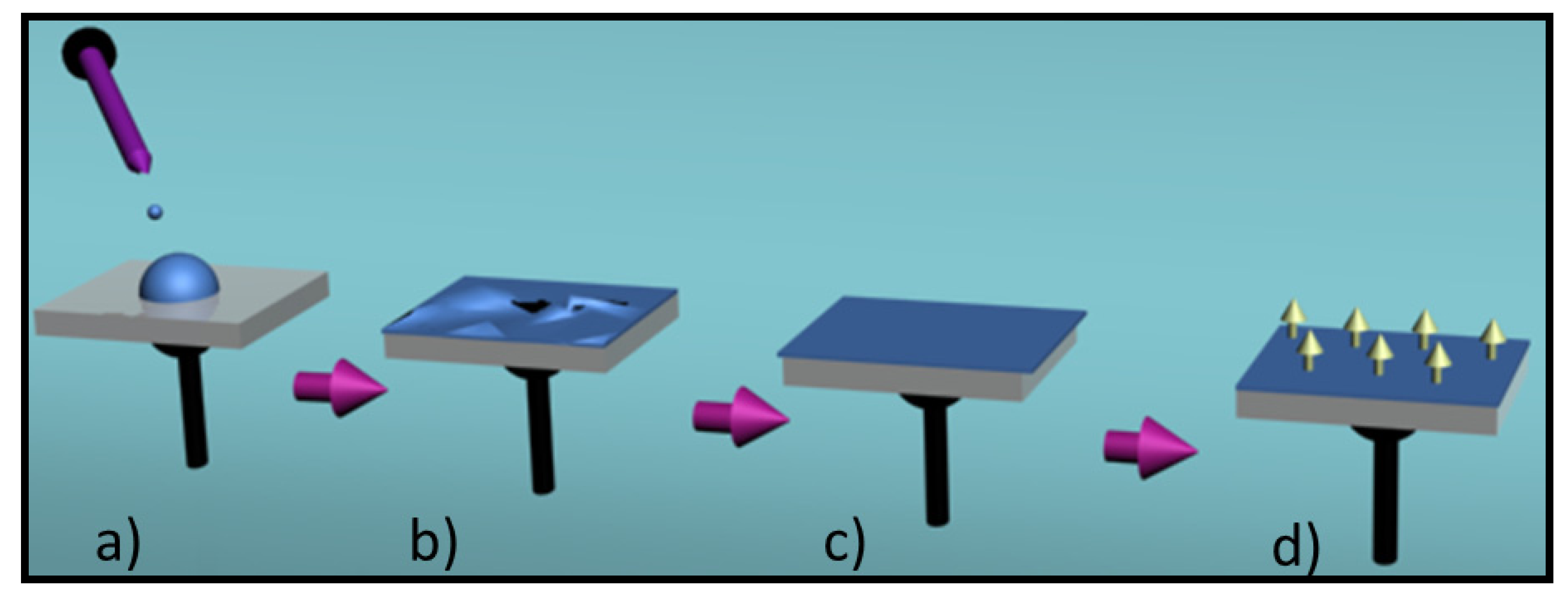
4. Conducting and Biodegradable Systems Based on Aniline Units
4.1. Development of Novel Biodegradable Samples Having Aniline Units

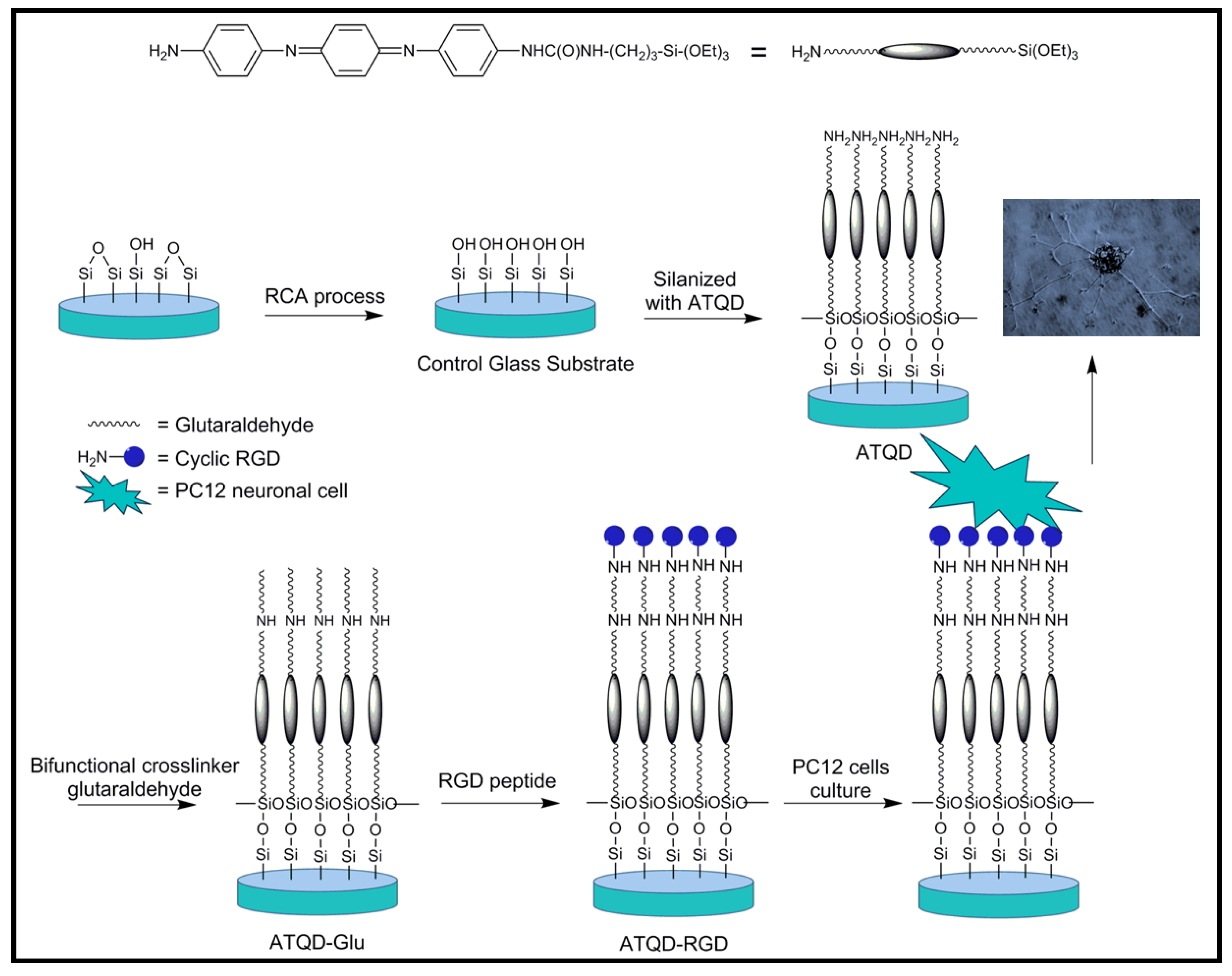
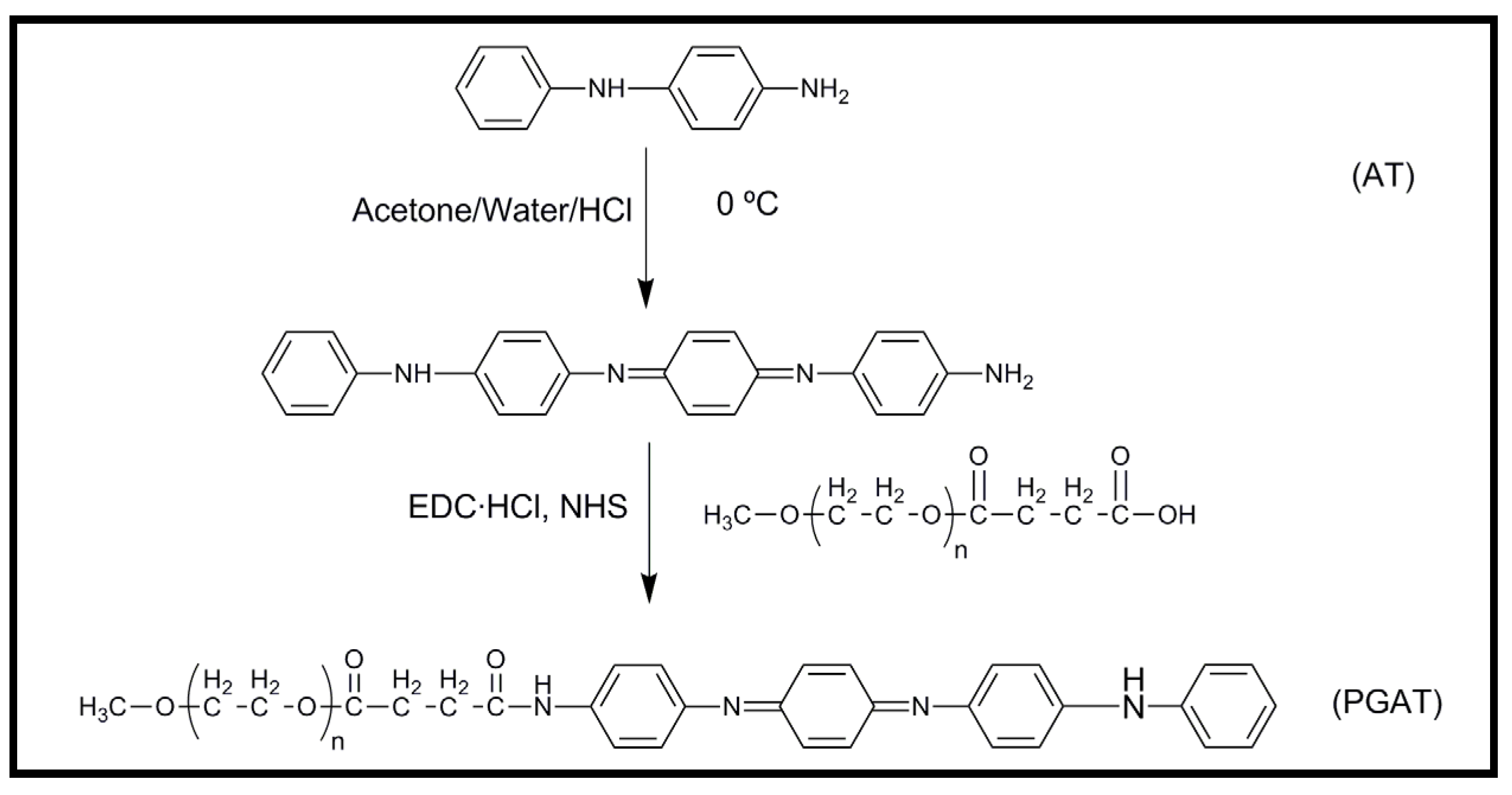
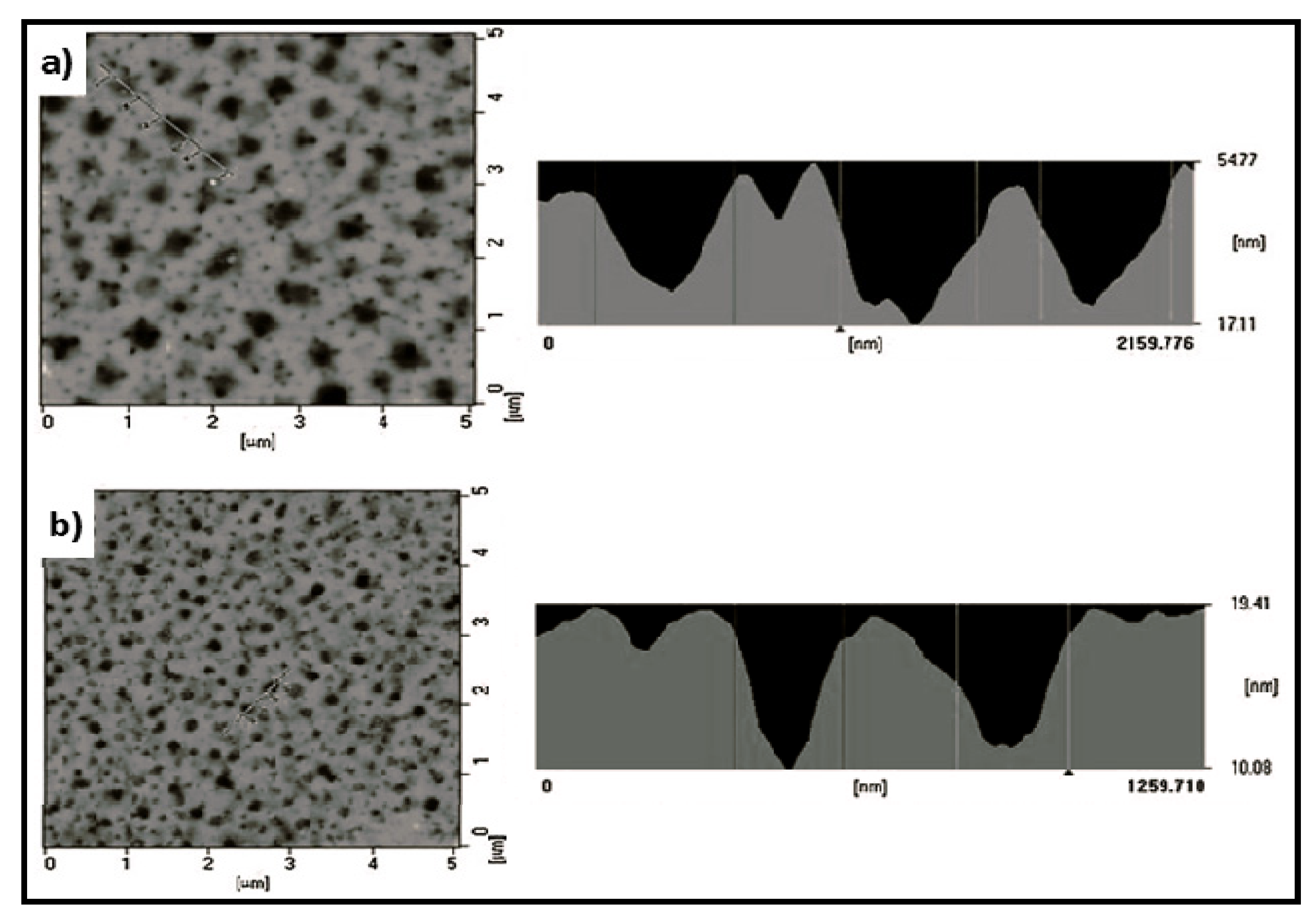

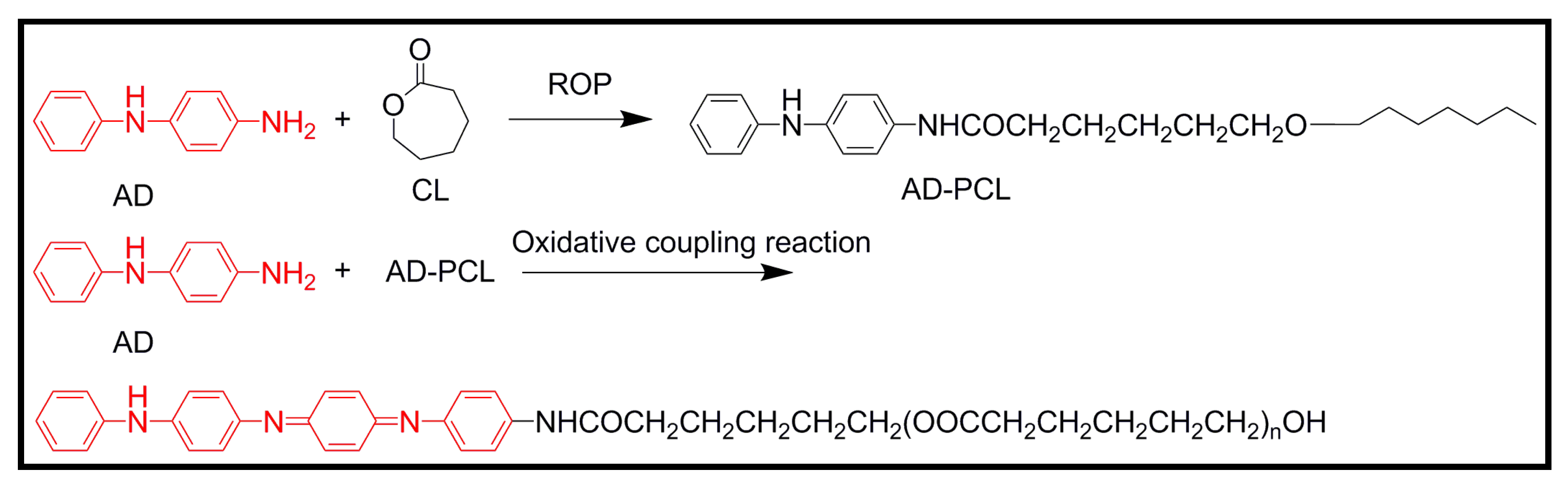
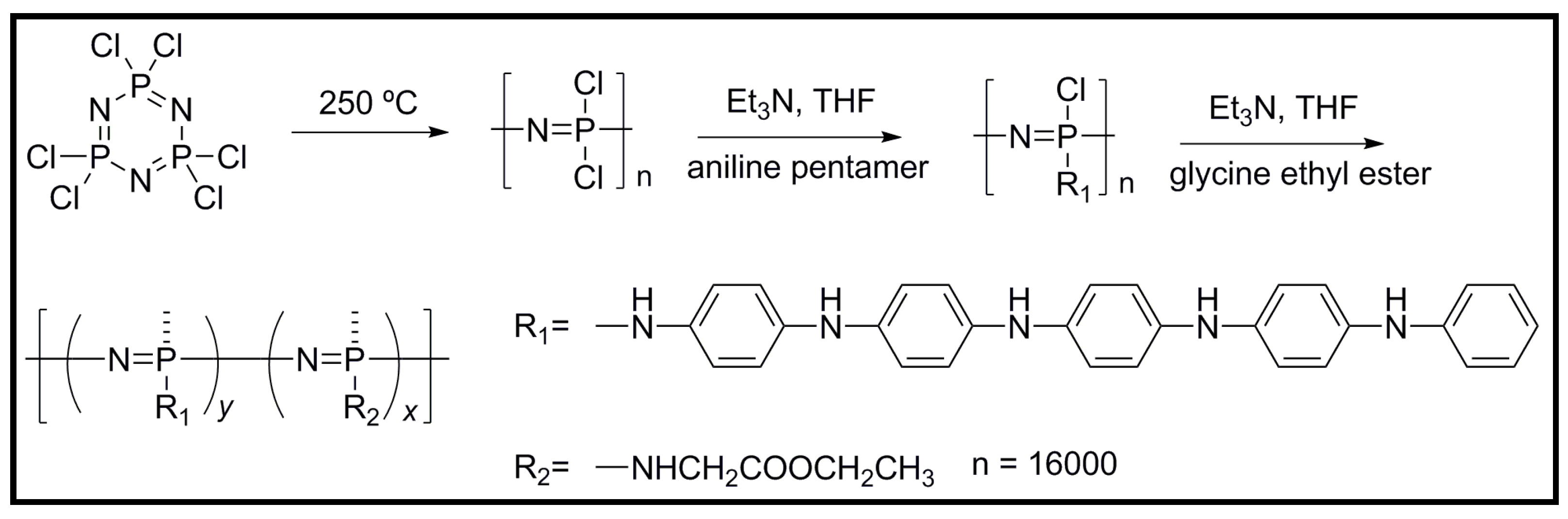
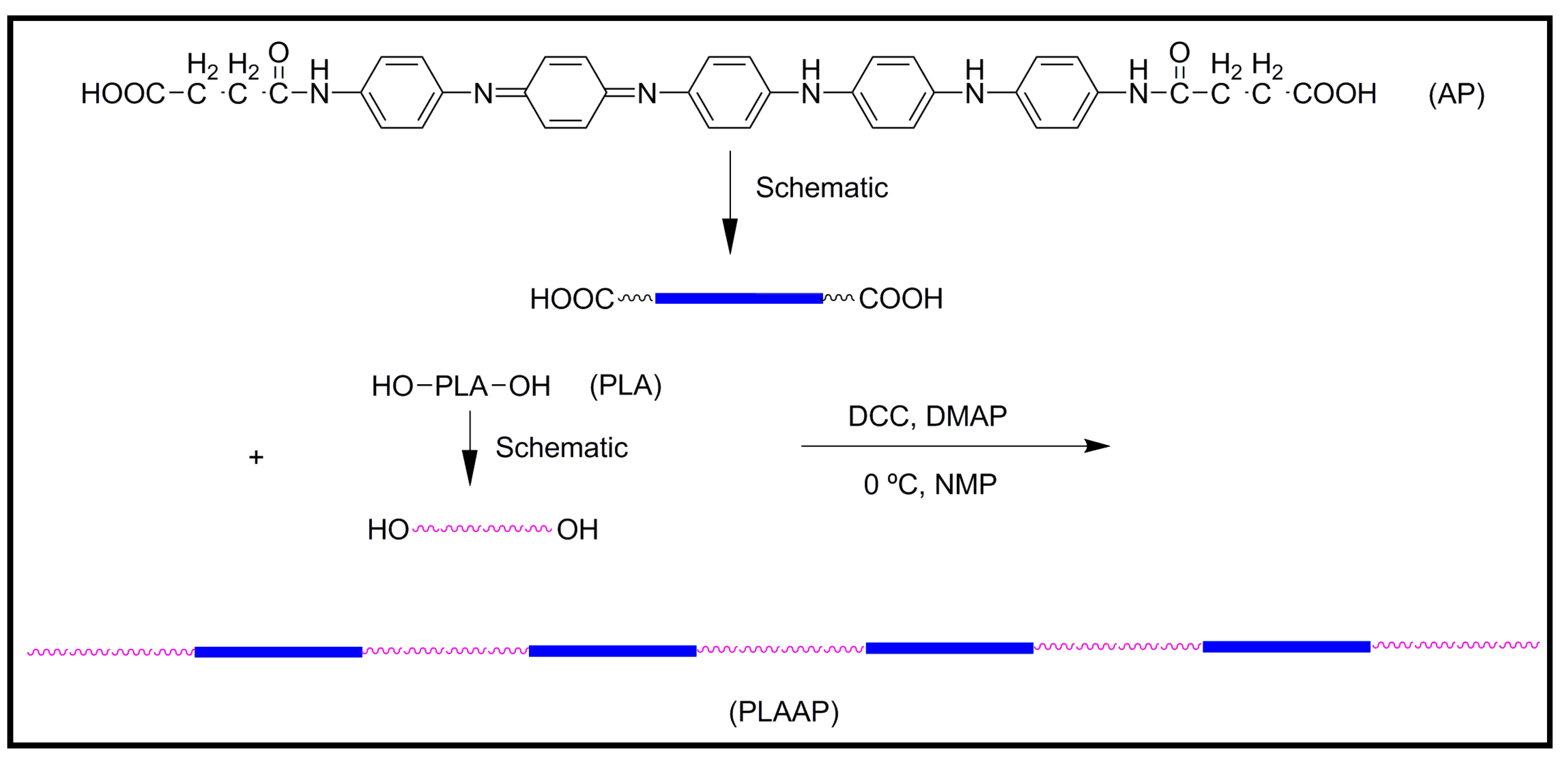
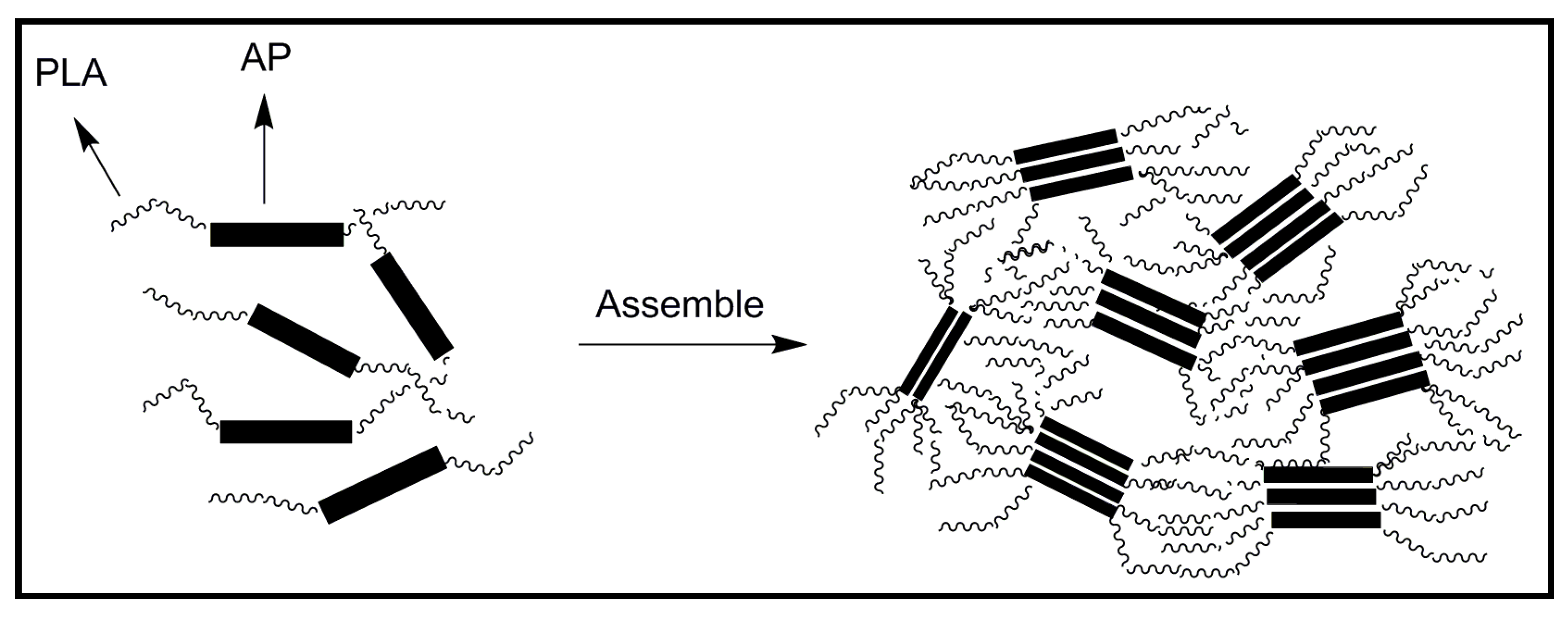
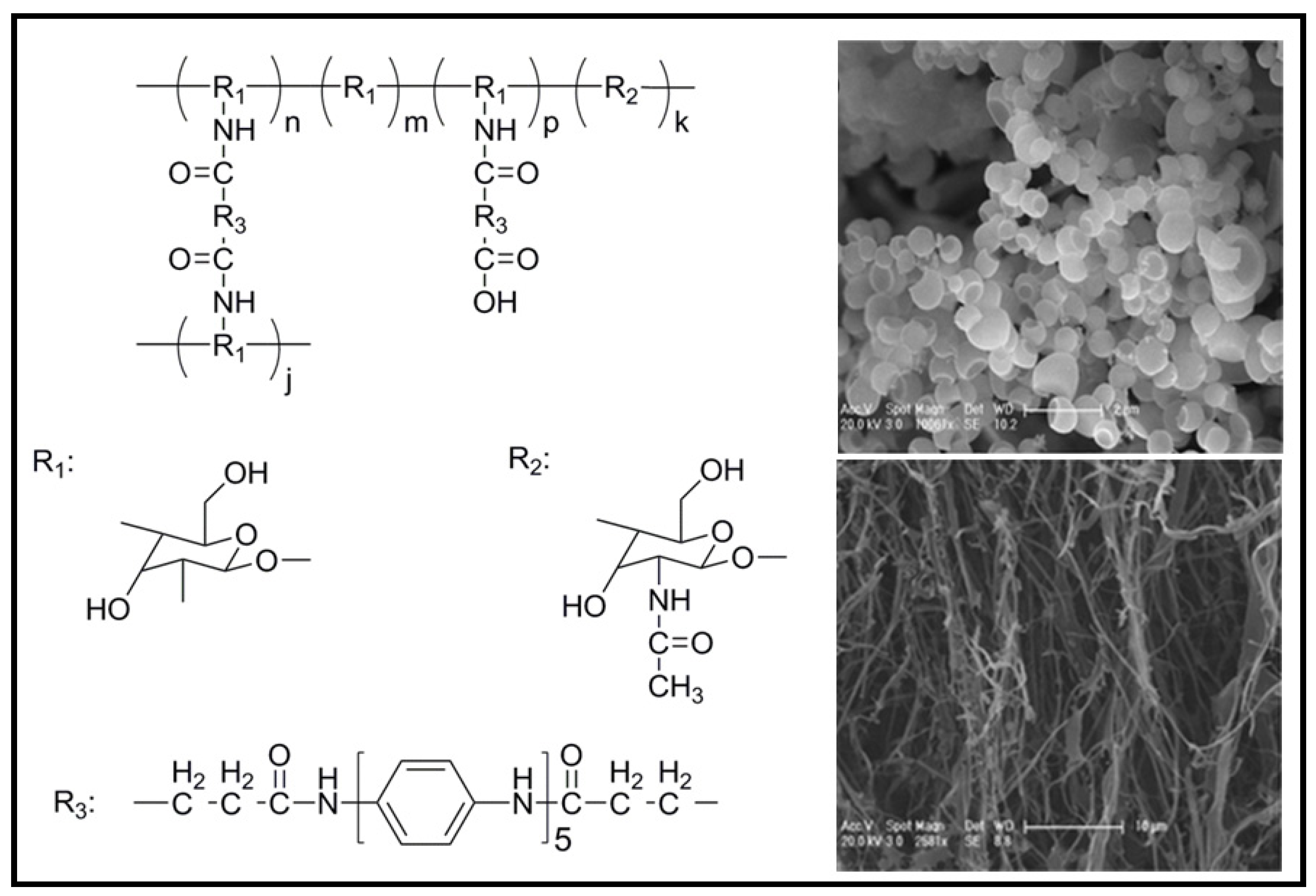

4.2. Biodegradable Scaffolds Constituted by Nanofibers of Polyaniline
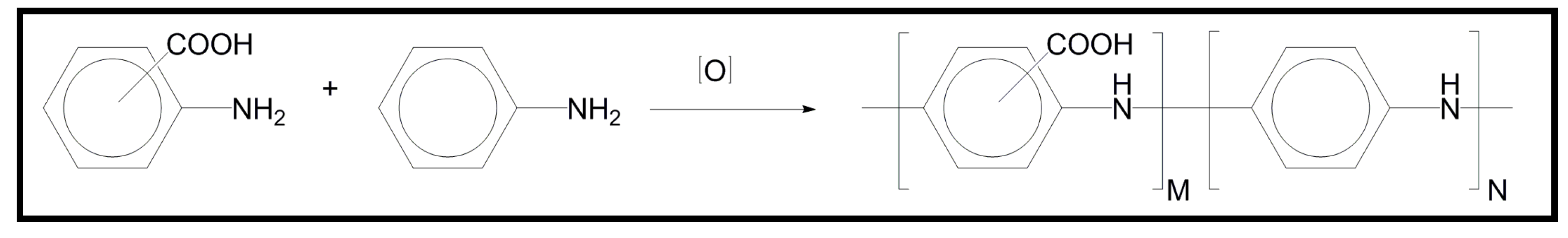
5. Conducting and Biodegradable Systems Related with Polypyrrole
5.1. Development of Novel Biodegradable Polymes and Blends Based on Pyrrole Units


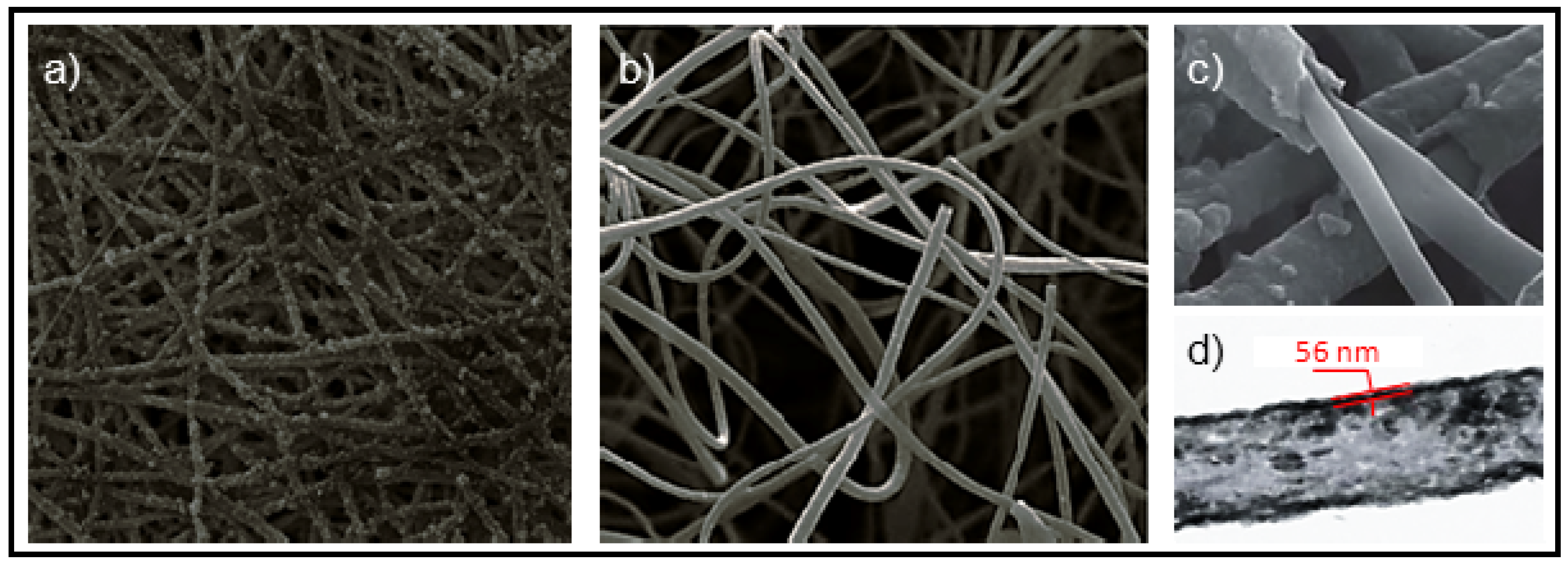
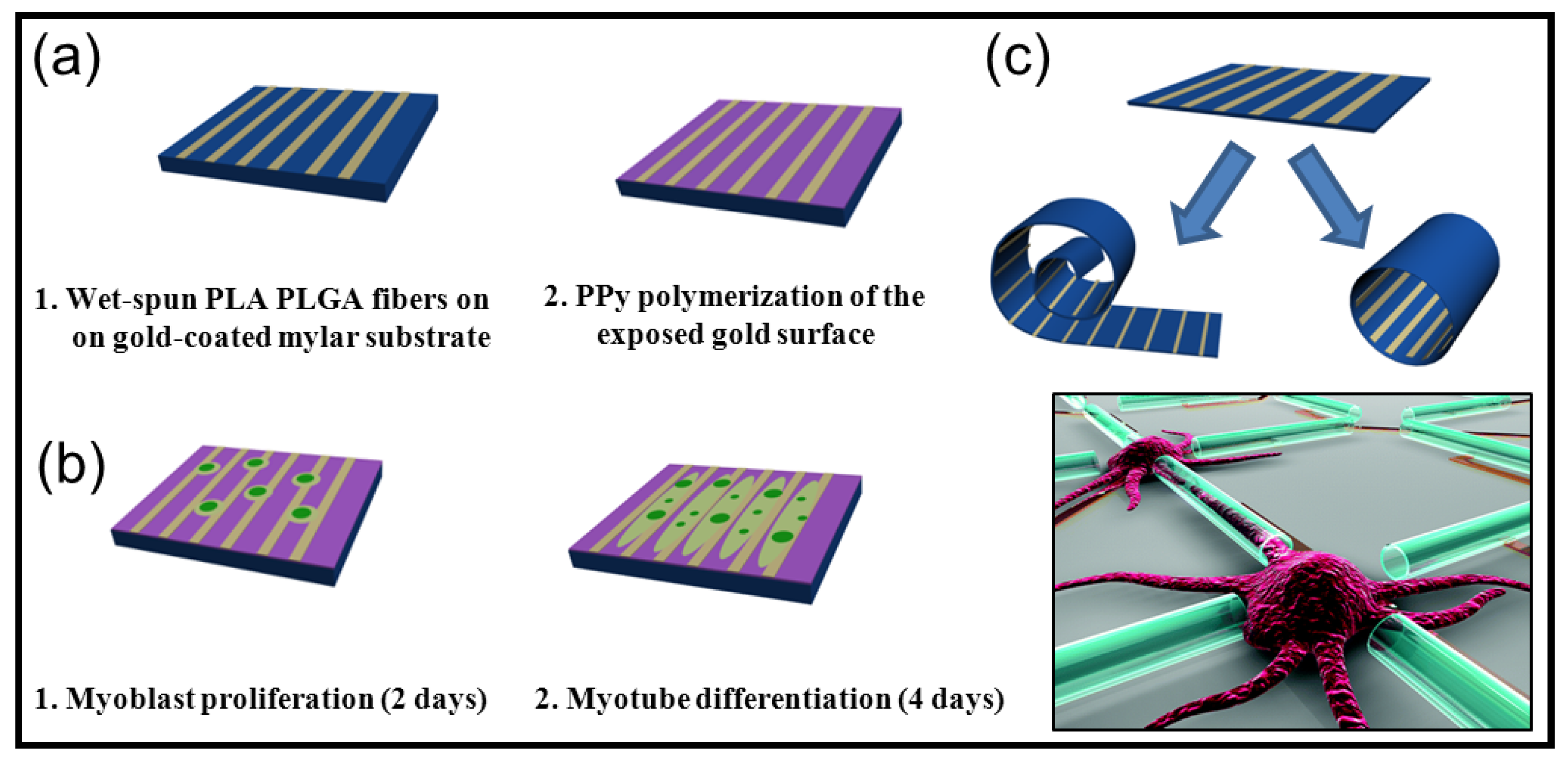
5.2. Biodegradable Scaffolds Constituted by Nanofibers that Incorporated Polypyrrole
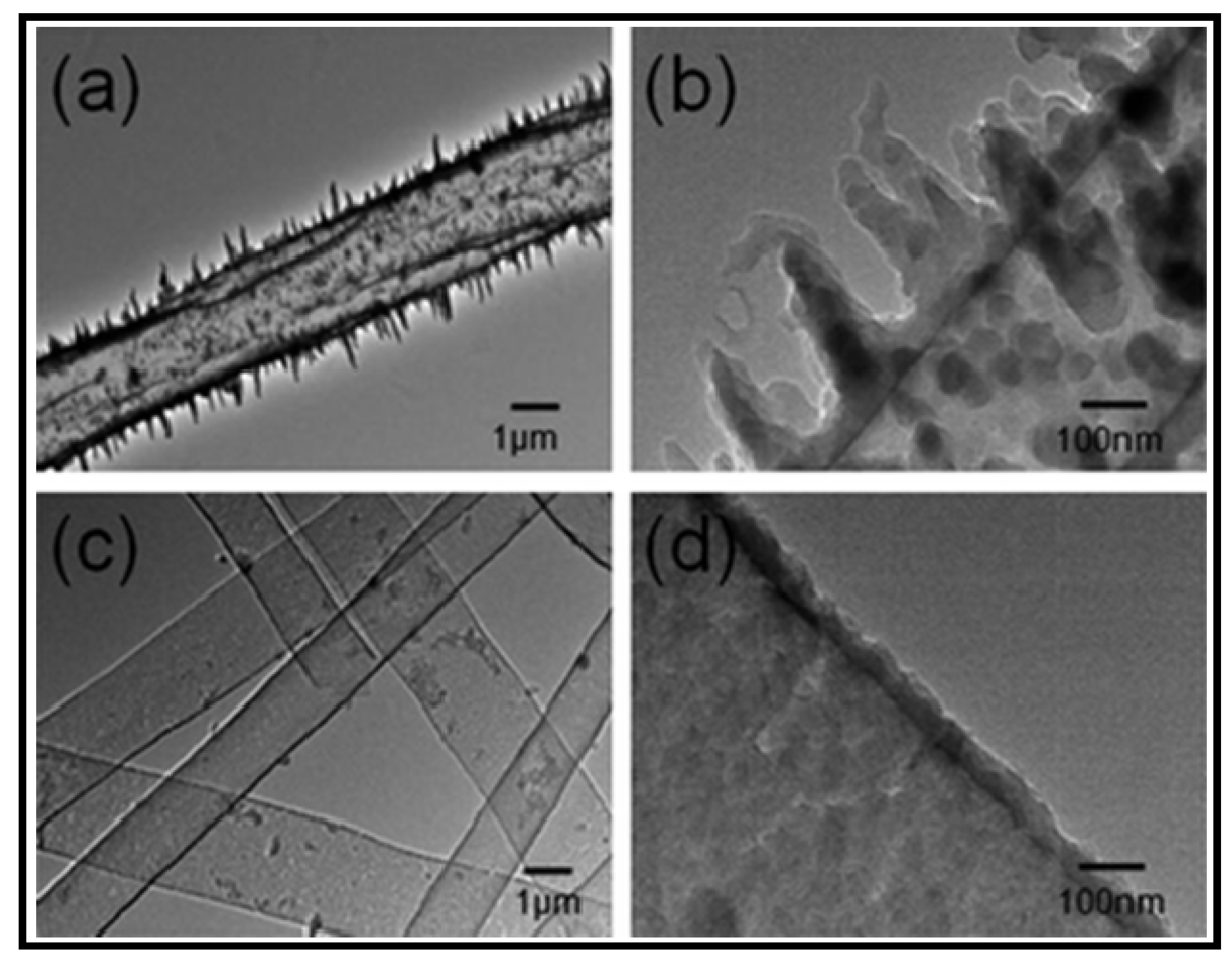
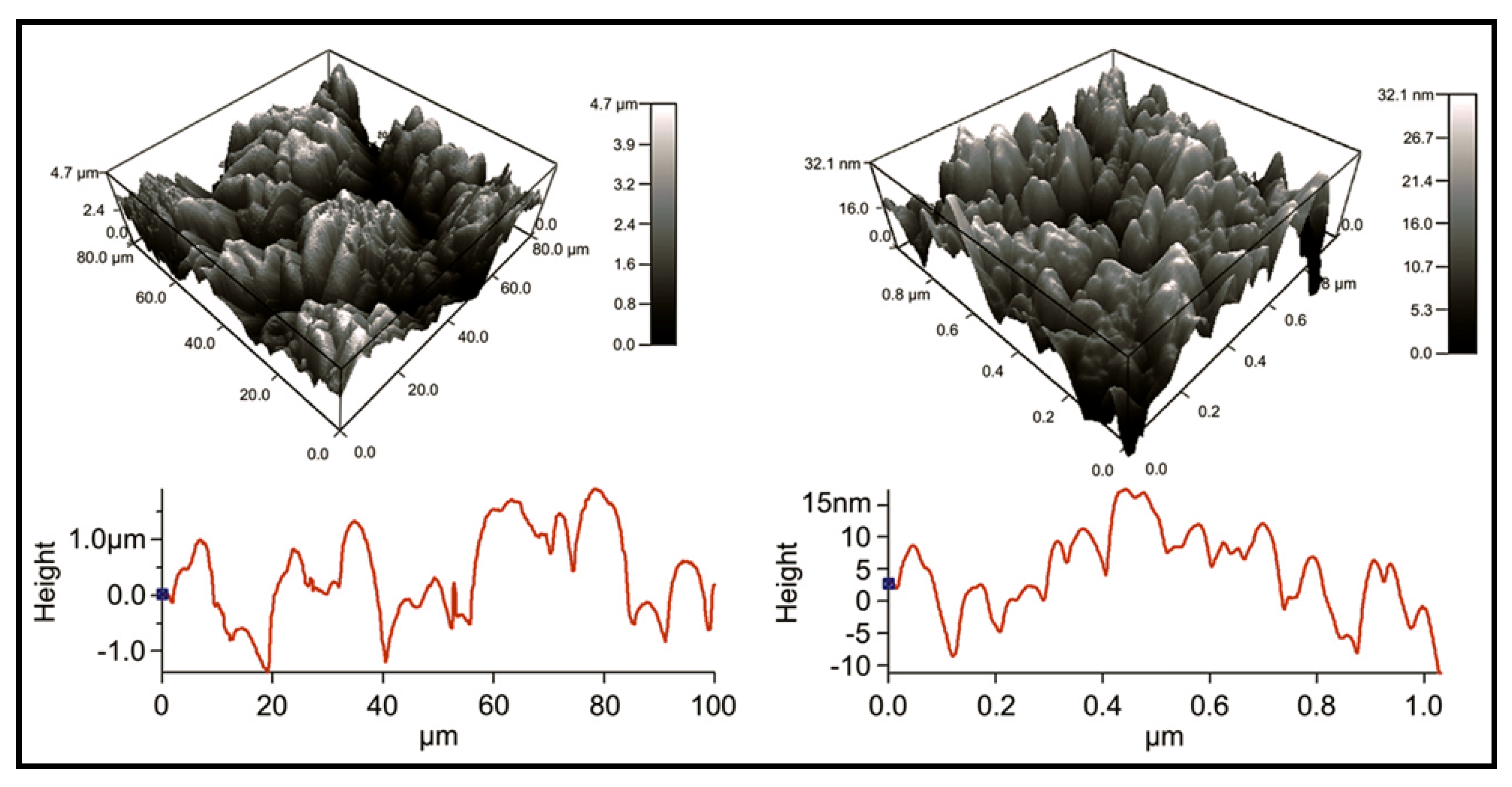
5.3. Biodegradable Nanomembranes that Incorporated Conducting Polypyrrole
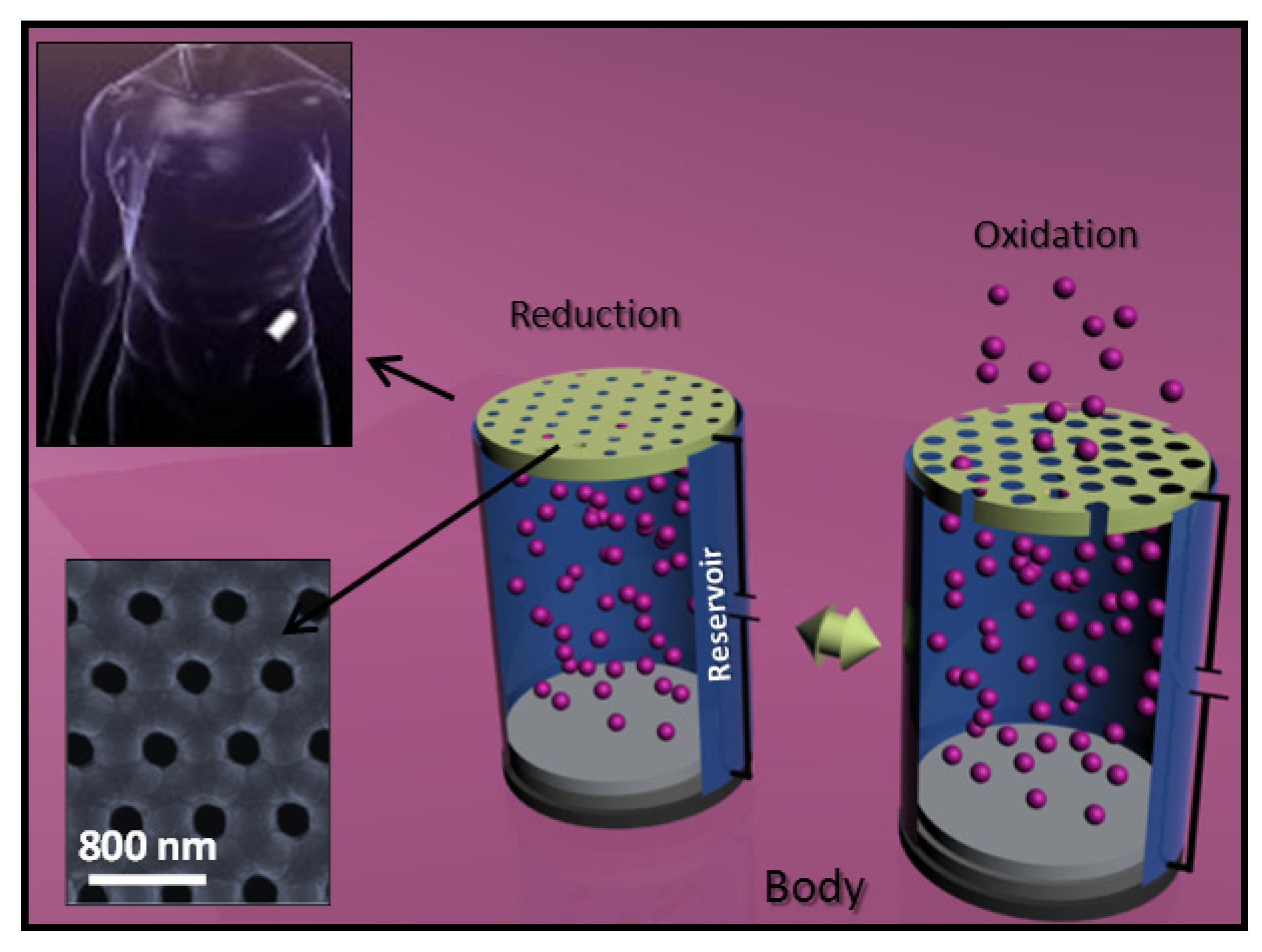
6. Conducting and Biodegradable Systems Related with Polythiophenes
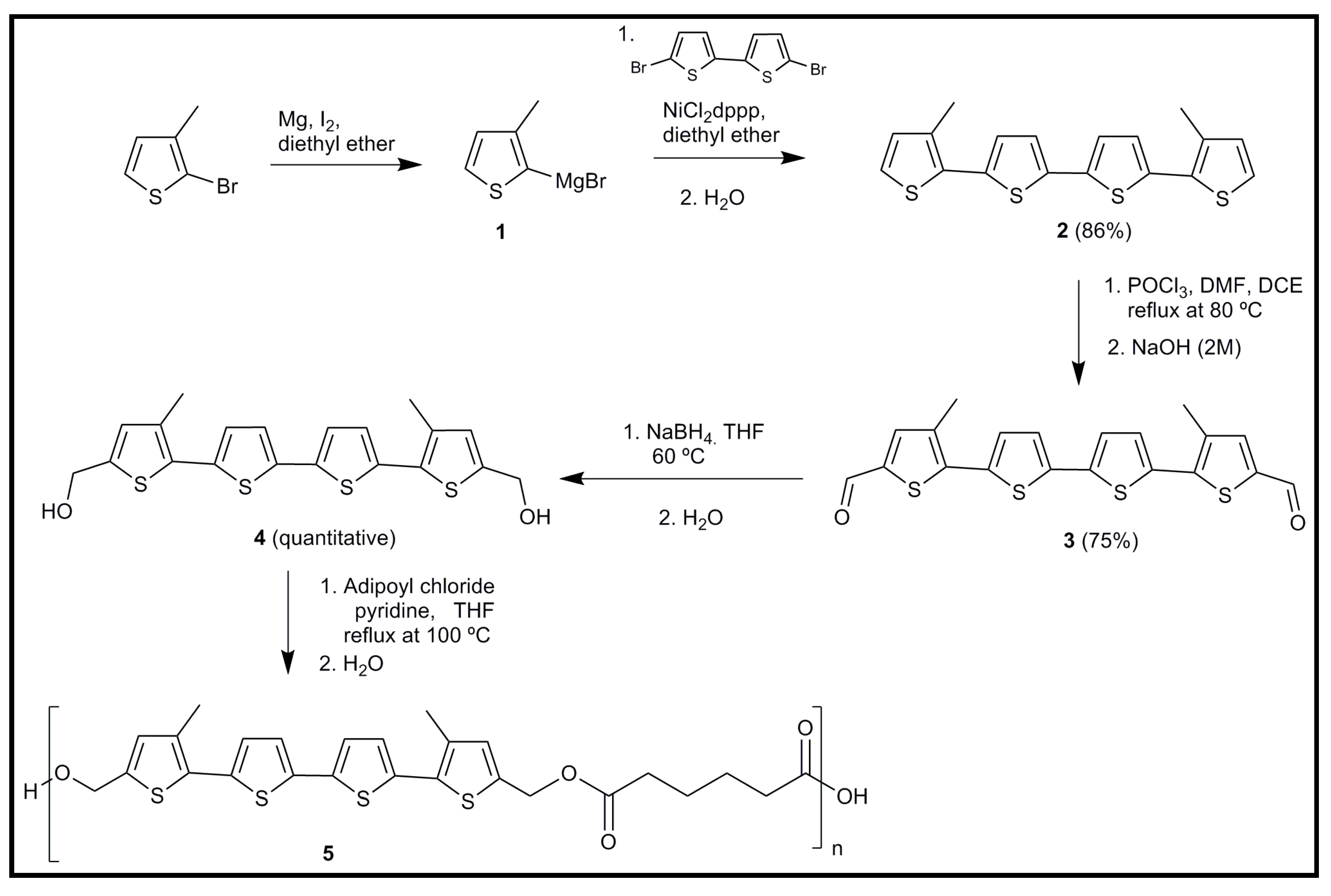
6.1. Development of Novel Biodegradable Samples Having Thiophenes
6.2. Applications of Biodegradable Constructs Based on Electrospun Nanofibers of Thiophene Derivatives
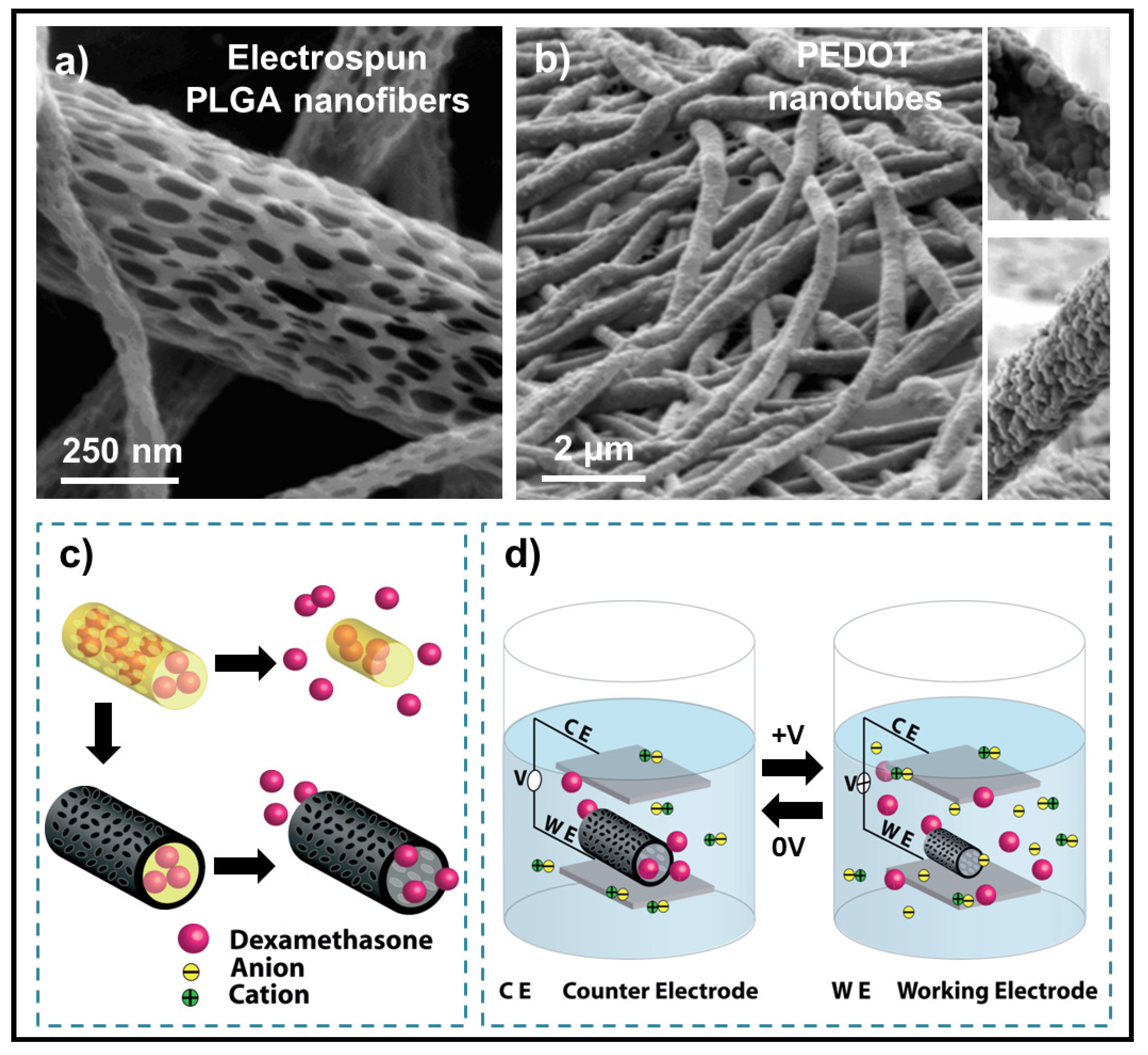
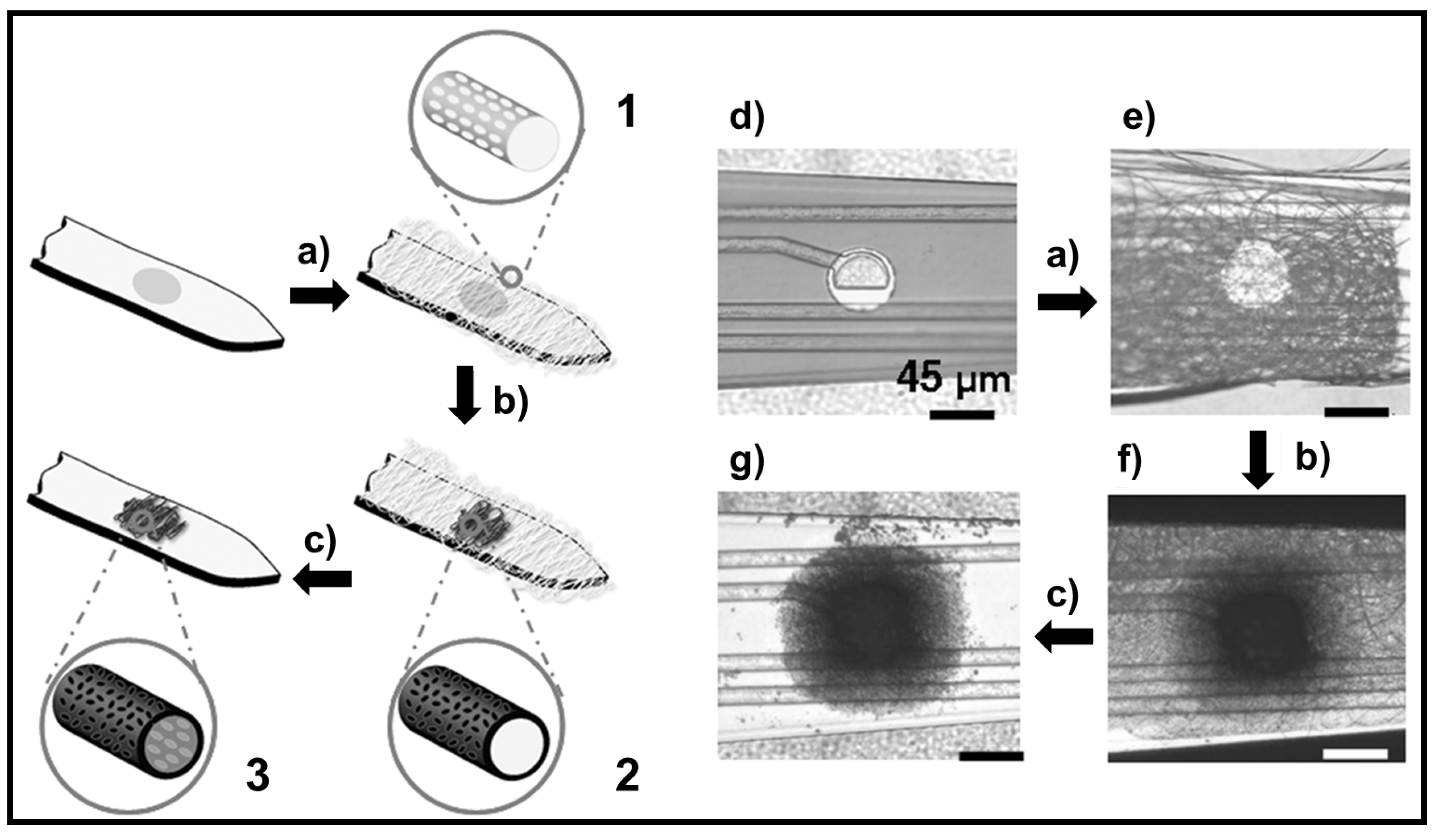
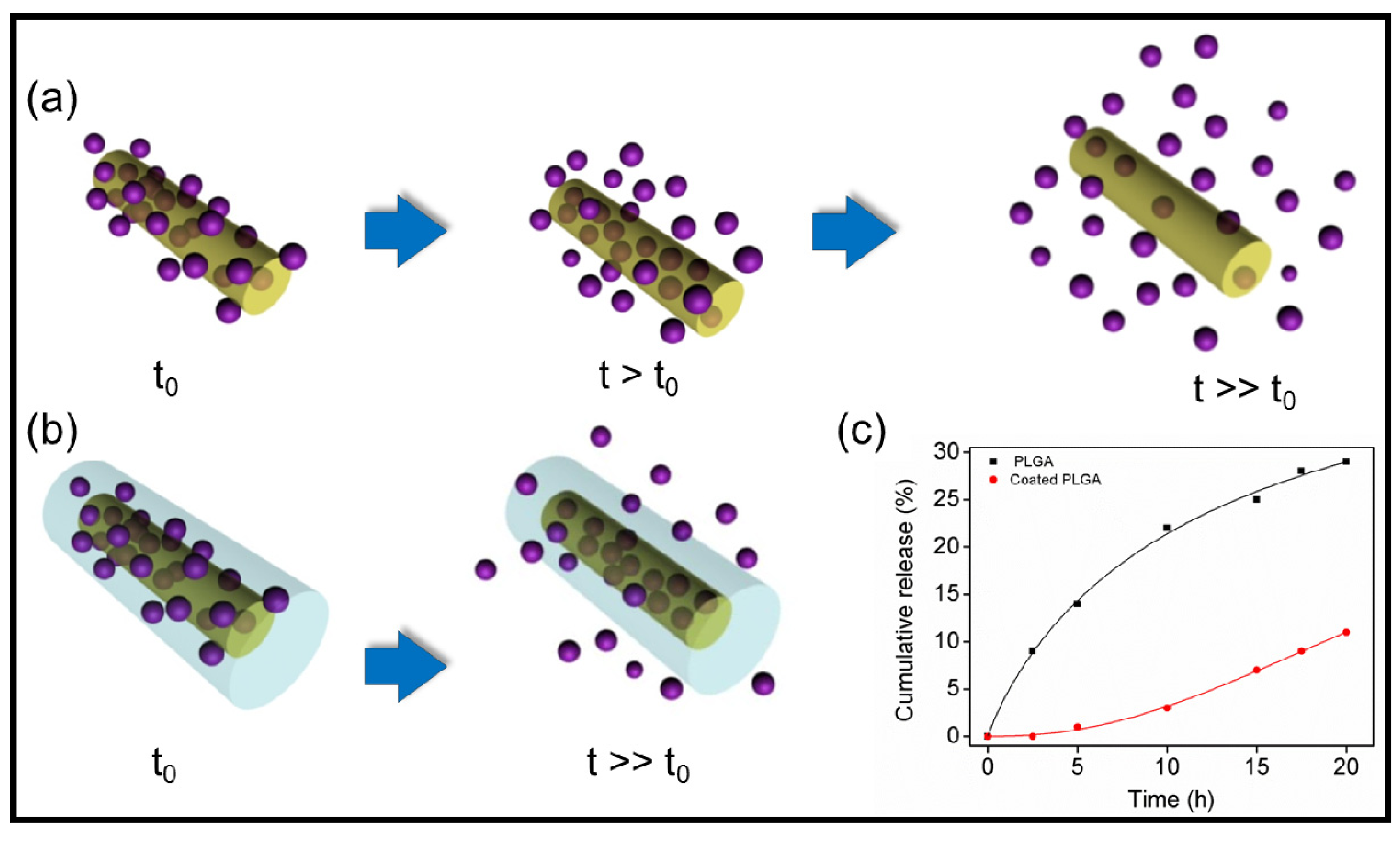
6.3. Applications of Biodegradable Constructs Based on Films and Nanomembranes of Thiophene Derivatives
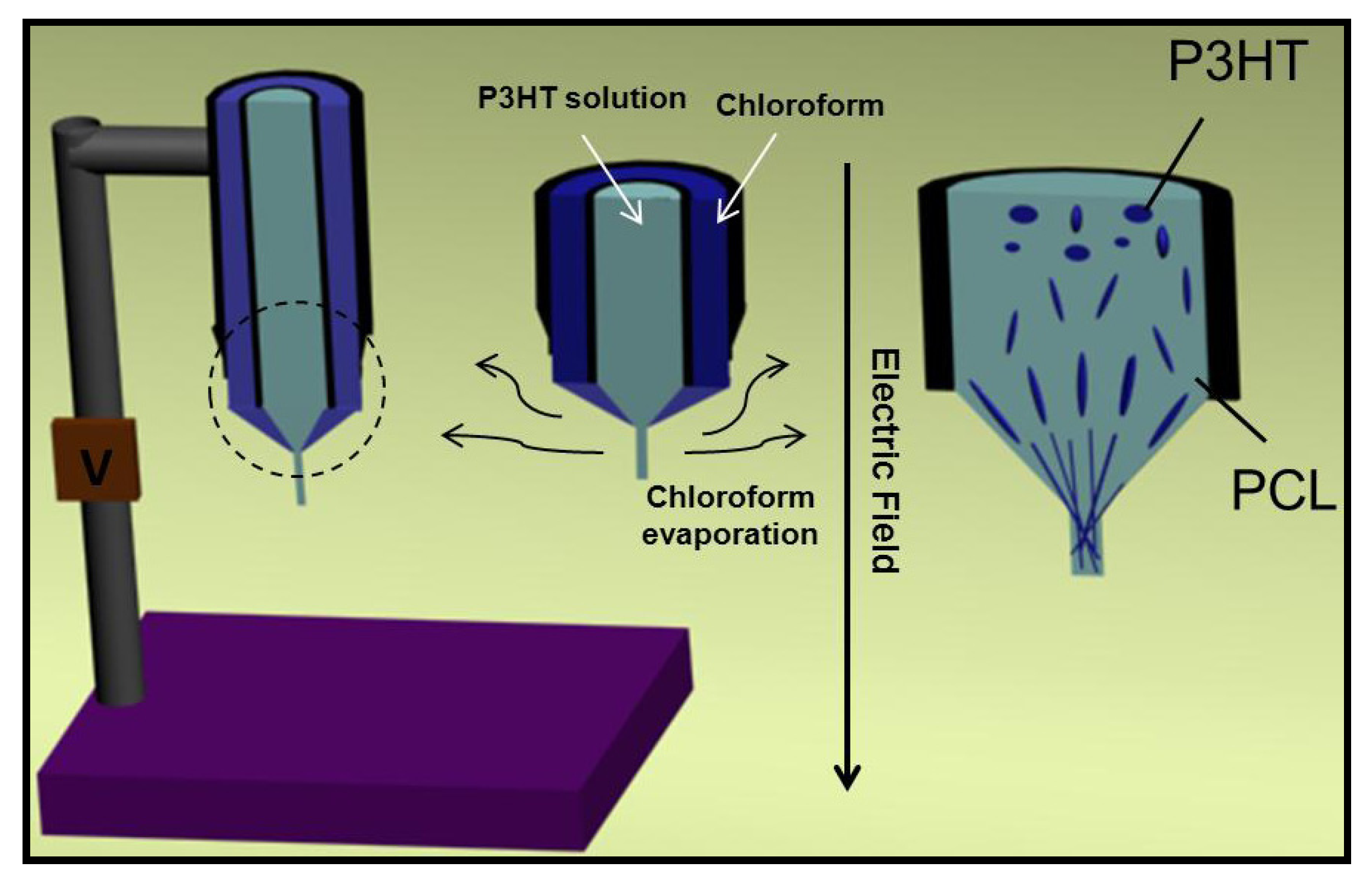
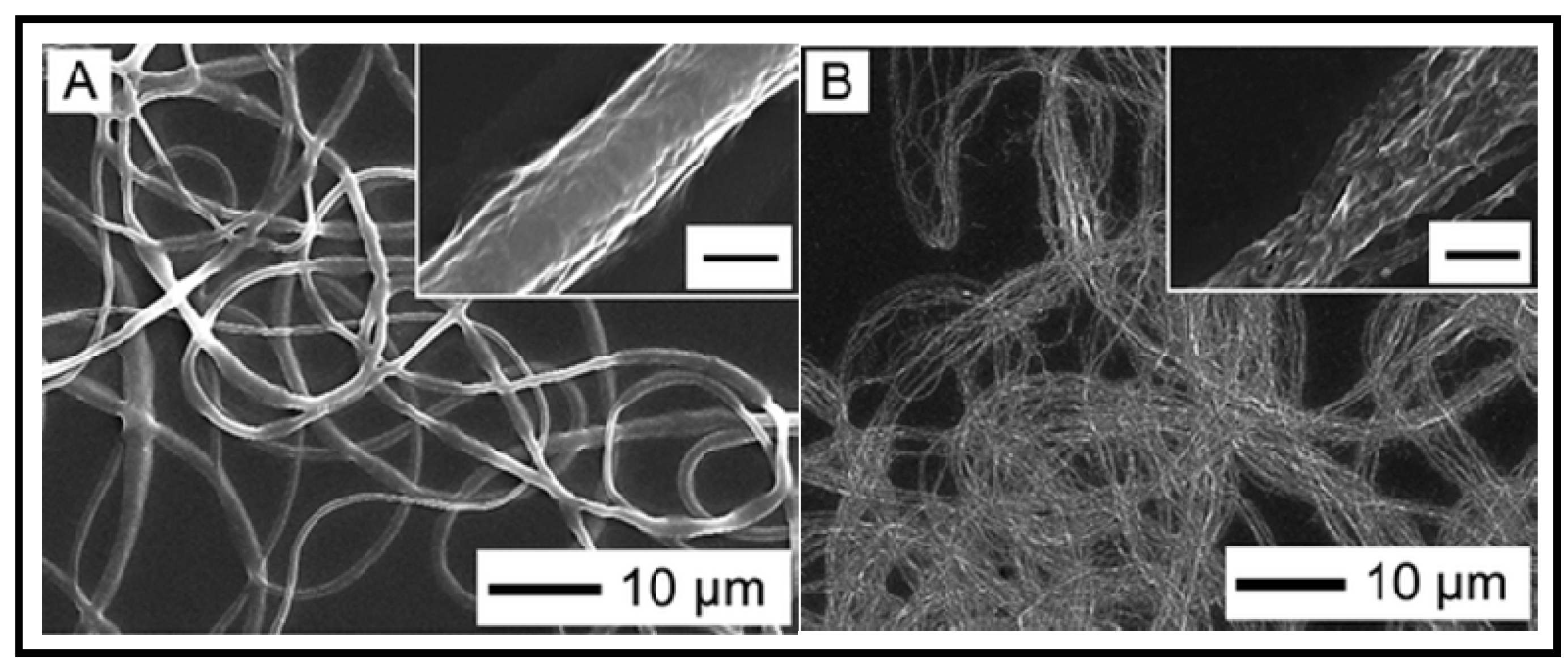
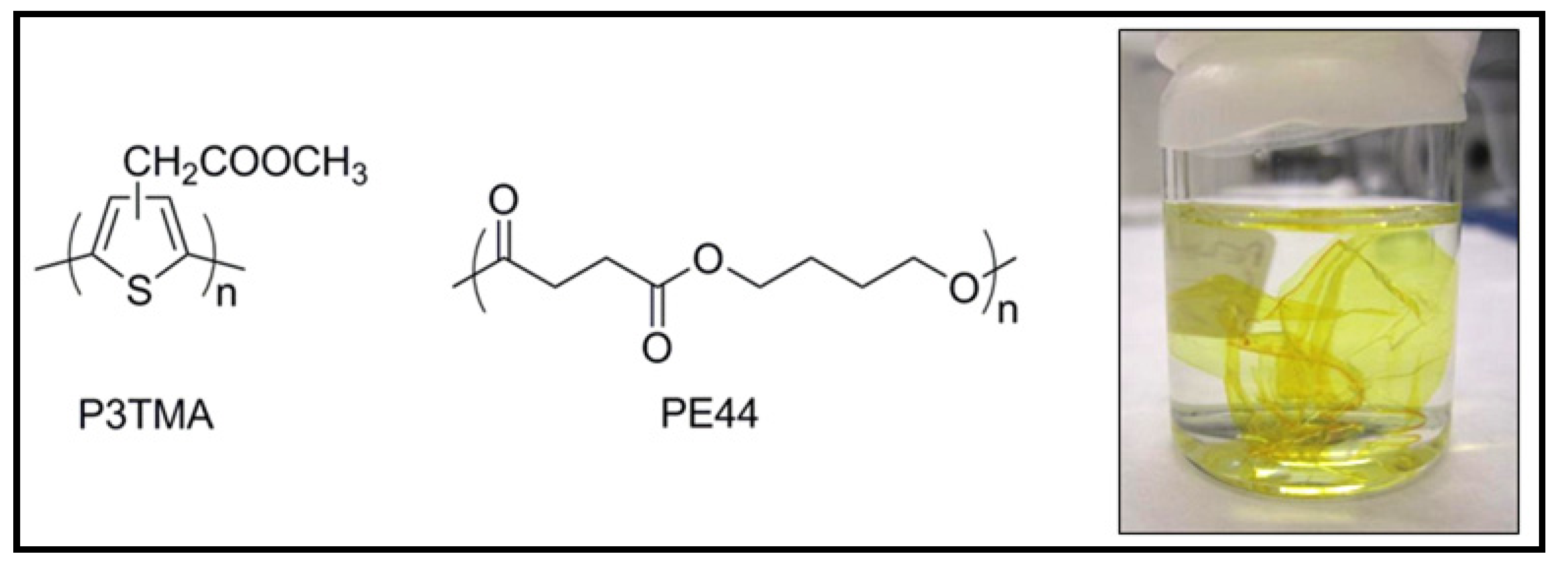
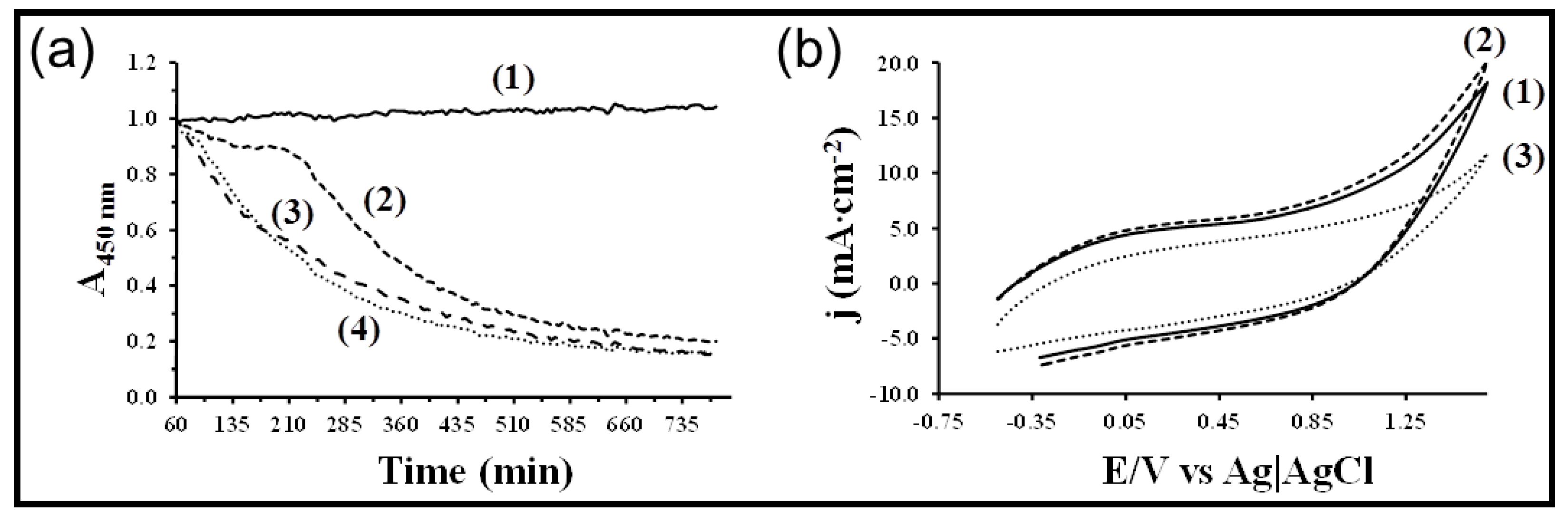
7. Other Conducting and Biodegradable Systems
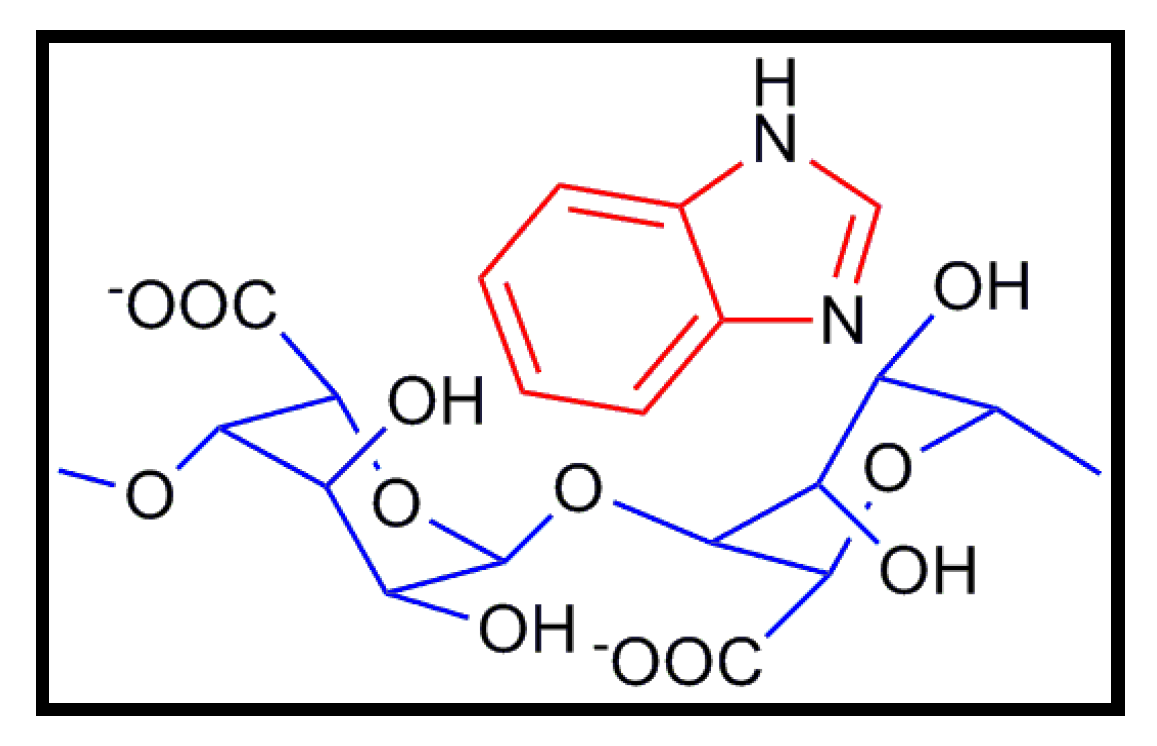
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tiwari, A.; Tiwari, A. Bioengineered Nanomaterials; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [Green Version]
- Tiwari, A.; Ramalingam, M.; Kobayashiu, H.; Turner, A.P.F. Biomedical Materials and Diagnostic Devices; Wiley-Scrivener Publishing LLC: Beverly, MA, USA, 2012. [Google Scholar] [Green Version]
- Tiwari, A.; Mishra, A.K.; Kobayashi, H.; Turner, A.P.F. Intelligent Nanomaterials; Wiley-Scrivener Publishing LLC: Salem, MA, USA, 2012. [Google Scholar] [Green Version]
- Tiwari, A.; Tiwari, A. Nanomaterials in Drug Delivery, Imaging and Tissue Engineering; Wiley-Scrivener Publishing LLC: Salem, MA, USA, 2013. [Google Scholar] [Green Version]
- Ramalinga, M.; Tiwari, A.; Ramakrishna, S.; Kobayashi, H. Integrated Biomaterials for Biomedical Technology; Wiley-Scrivener Publishing LLC: Beverly, MA, USA, 2012. [Google Scholar] [Green Version]
- Sharma, P.K.; Dutta, R.K.; Pandey, A.C. Advances in multifunctional magnetic nanoparticles. Adv. Mater. Lett. 2011, 2, 246–263. [Google Scholar] [CrossRef]
- Tiwari, A. Recent Developments in Bio-Nanocomposites for Biomedical Applications; Nova Science Publishers Inc.: New York, NY, USA, 2011. [Google Scholar] [Green Version]
- Sharma, C.; Gautam, S.; Dinda, A.K.; Mishra, N.C. Cartilage tissue engineering: Current Scenario and Challenges. Adv. Mater. Lett. 2011, 2, 90–99. [Google Scholar]
- Ramalingama, M.; Tiwari, A. Spatially controlled cell growth using patterned biomaterials. Adv. Mater. Lett. 2010, 1, 179–187. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ndreu, A.; Piras, A.M.; Nikkola, L.; Sindelar, T.; Ylikauppila, H.; Harlin, A.; Chiellini, E.; Hasirci, V.; Redl, H. Biodegradable nanomats produced by electrospinning: Expanding multifunctionality and potential for tissue engineering. J. Nanosci. Nanotechnol. 2007, 7, 862–882. [Google Scholar] [CrossRef]
- Teo, W.E.; He, W.; Ramakrishna, S. Electrospun scaffold tailored for tissue-specific extracellular matrix. Biotechnol. J. 2006, 1, 918–929. [Google Scholar] [CrossRef]
- Moroni, L.; de Wijn, J.R.; van Blitterswijk, C.A. Integrating novel technologies to fabricate smart scaffolds. J. Biomater. Sci. Polym. E 2008, 19, 543–572. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliver. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef]
- Jiang, C.; Markutsya, S.; Pikus, Y.; Tsukruk, V.V. Freely suspended nanocomposite membranes as highly sensitive sensors. Nat. Mater. 2004, 3, 721–728. [Google Scholar] [CrossRef]
- Vendamme, R.; Onoue, S.Y.; Nakao, A.; Kunitake, T. Robust free-standing nanomembranes of organic/inorganic interpenetrating networks. Nat. Mater. 2006, 5, 494–501. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ahn, J.-H.; Choi, W.M.; Kim, H.-S.; Kim, T.-H.; Song, J.; Huang, Y.Y.; Liu, Z.; Lu, C.; Rogers, J.A. Stretchable and foldable silicon integrated circuits. Science 2008, 320, 507–511. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Park, S.I.; Xiong, Y.J.; Kim, R.H.; Elvikis, P.; Meitl, M.; Kim, D.H.; Wu, J.; Yoon, J.; Yu, C.J.; Liu, Z.J.; et al. Printed assemblies of inorganic light-emitting diodes for deformable and semitransparent displays. Science 2009, 325, 977–981. [Google Scholar] [CrossRef]
- Tiwaki, A.; Kumar, R.; Prabaharan, M.; Pandey, R.R.; Kumasi, P.; Chaturvedi, A.; Mishra, A.K. Nanofibrous polyaniline thin film prepared by plasma-induced polymerization technique for detection of NO2 gas. Polym. Adv. Technol. 2010, 21, 615–620. [Google Scholar] [CrossRef]
- Norris, I.D.; Shaker, M.M.; Ko, F.K.; MacDiarmid, A.G. Electrostatic fabrication of ultrafine conducting fibers: Polyaniline/polyethylene oxide blends. Synth. Met. 2000, 114, 109–114. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Jones, W.E.; Norris, I.D.; Gao, J.; Johnson, A.T.; Pinto, N.J.; Hone, J.; Han, B.; Ko, F.K.; Okuzaki, H.; et al. Electrostatically-generated nanofibers of electronic polymers. Synth. Met. 2001, 119, 27–30. [Google Scholar] [CrossRef]
- Ali, Y.; Kumar, V.; Sonkawade, R.G.; Dhaliwal, A.S. Fabrication of polyaniline nanofibers by chronopotentiometry. Adv. Mater. Lett. 2012, 3, 388–392. [Google Scholar]
- Ali, Y.; Kumar, V.; Dhaliwal, R.G.; Sonkawade, R.G. Surface modification of polyaniline nanofiber using silver nanoparticles to enhance sensing properties. Adv. Mater. Lett. 2013, 4, 368–372. [Google Scholar]
- Wei, Y.; Lelkes, P.I.; MacDiarmid, A.G.; Guterman, E.; Cheng, S.; Palouian, K. Contemporary Topics in Advanced Polymer Science and Technology; Peking University Press: Beijing, China, 2004; pp. 430–436. [Google Scholar] [Green Version]
- Bidez, P.; Li, S.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. E 2006, 17, 199–212. [Google Scholar] [CrossRef]
- Kotwal, A.; Schmidt, C.E. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials 2001, 22, 1055–1064. [Google Scholar] [CrossRef]
- Valentini, R.F. Nerve Guidance Channels in the Biomedical Engineering HandBook, 2nd ed.; Bronzino, J.D., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2000. [Google Scholar] [Green Version]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Dzenis, Y. Spinning continuous fibers for nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kotaki, M.; Zhang, Y.; Mo, X.; Ramakrishna, S. Recent advances in polymer nanofibers. J. Nanosci. Nanotechnol. 2004, 4, 52–65. [Google Scholar]
- Dhakate, S.R.; Singla, B.; Uppal, M.; Mathur, R.B. Effect of processing parameters on morphology and thermal properties of polycarbonate nanofibers. Adv. Mater. Lett. 2010, 1, 200–204. [Google Scholar]
- Sharma, S. Ferroelectric nanofibers: Principle, processing and applications. Adv. Mater. Lett. 2013, 4, 522–533. [Google Scholar]
- Dersch, R.; Steinhart, M.; Boudriot, U.; Greiner, A.; Wendorff, J.H. Nanoprocessing of polymers: Applications in medicine, sensors, catalysis, photonics. Polym. Adv. Technol. 2005, 16, 276–282. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Process. Tech. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar]
- Terada, D.; Kobayashi, H.; Zhang, K.; Tiwari, A.; Yoshikawa, C.; Hanagata, N. Transient charge-masking effect of applied voltage on electrospinning of pure chitosan nanofibers from aqueous solutions. Sci. Technol. Adv. Mater. 2012, 13, 1–9. [Google Scholar]
- Tiwari, A.; Terada, D.; Yoshikawa, C.; Kobayashi, H. An enzyme-free highly glucose-specific assay using self-assembled aminobenzene boronic acid upon polyelectrolytes electrospun nanofibers-mat. Talanta 2010, 82, 1725–1732. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.Y.; Mylonakis, A.; Han, J.J.; MacDiarmid, A.G.; Chen, X.S.; Lelkes, P.I.; Wei, Y. Electroactive oligoaniline-containing self-assembled monolayers for tissue engineering applications. Biomacromolecules 2007, 8, 3025–3034. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Zhuang, X.; Zhang, P.; Chen, X.; Wei, Y.; Wang, X. Preparation and characterization of biodegradable and electroactive polymer blend materials based on mPEG/tetraaniline and PLLA. Macromol. Biosci. 2011, 11, 806–813. [Google Scholar] [CrossRef]
- Wang, Q.; He, W.; Huang, J.; Liu, S.; Wu, G.; Teng, W.; Wang, Q.; Don, Y. Synthesis of water soluble, biodegradable, and electroactive polysaccharide crosslinker with aldehyde and carboxylic groups for biomedical applications. Macromol. Biosci. 2011, 11, 362–372. [Google Scholar] [CrossRef]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Universal two-step approach to degradable and electroactive block copolymers and networks from combined ring-opening polymerization and post-functionalization via oxidative coupling reactions. Macromolecules 2011, 44, 5227–5236. [Google Scholar] [CrossRef]
- Zhang, Q.-S.; Yan, Y.-H.; Li, S.-P.; Feng, T. Synthesis of a novel biodegradable and electroactive polyphosphazene for biomedical application. Biomed. Mater. 2009, 4. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Y.; Li, S.; Feng, T. The synthesis and characterization of a novel biodegradable and electroactive polyphosphazene for nerve regeneration. Mater. Sci. Eng. C 2010, 30, 160–166. [Google Scholar] [CrossRef]
- Huang, L.; Zhuang, X.; Hu, J.; Lang, L.; Zhang, P.; Wang, Y.; Chen, X.; Wei, Y.; Jing, X. Synthesis of biodegradable and electroactive multiblock polylactide and aniline pentamer copolymer for tissue engineering applications. Biomacromolecules 2008, 9, 850–858. [Google Scholar] [CrossRef]
- Huang, L.; Hu, J.; Lang, L.; Wang, X.; Zhang, P.; Jing, X.; Wang, X.; Chen, X.; Lelkes, P.I.; MacDiarmid, A.G.; et al. Synthesis and characterization of electroactive and biodegradable ABA block copolymer of polylactide and aniline pentamer. Biomaterials 2007, 28, 1741–1751. [Google Scholar] [CrossRef]
- Hu, J.; Huang, L.H.; Zhuang, X.L.; Zhang, P.B.; Lang, L.; Chen, X.S.; Wei, Y.; Jing, X.B. Electroactive aniline pentamer cross-linking chitosan for stimulation growth of electrically sensitive cells. Biomacromolecules 2008, 9, 2637–2644. [Google Scholar] [CrossRef]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Enhanced electrical conductivity by macromolecular architecture: Hyperbranched electroactive and degradable block copolymers based on poly(ε-caprolactone) and aniline pentamer. Macromolecules 2010, 43, 4472–4480. [Google Scholar] [CrossRef]
- Guo, B.; Sun, Y.; Finne-Wistrand, A.; Mustafa, K.; Albertsson, A.-C. Electroactive porous tubular scaffolds with degradability and non-cytotoxicity for neural tissue regeneration. Acta Biomater. 2012, 8, 144–153. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef]
- Picciani, P.H.S.; Medeiros, E.S.; Pan, Z.; Orts, W.J.; Mattoso, L.H.C.; Soares, B.G. Development of conducting polyaniline/poly(lactic acid) nanofibers by electrospinning. J. Appl. Polym. Sci. 2009, 112, 744–753. [Google Scholar] [CrossRef]
- McKeon, K.D.; Lewis, A.; Freeman, J.W. Electrospun poly(d,l-Lactide) and polyaniline scaffold characterization. J. Appl. Polym. Sci. 2010, 115, 1566–1572. [Google Scholar] [CrossRef]
- Borriello, A.; Guarino, V.; Schiavo, L.; Alvarez-Perez, M.A.; Ambrosio, L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. Mater. Sci. Eng. C 2011, 22, 1053–1062. [Google Scholar]
- Gizdavic-Nikolaidis, M.; Ray, S.; Bennett, J.R.; Easteal, A.J.; Cooney, R.P. Electrospun functionalized polyaniline copolymer-based nanofibers with potential application in tissue engineering. Macromol. Biosci. 2010, 10, 1424–1431. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, Y.; Hattori, S.; Terada, D.; Sharma, A.K.; Turner, A.P.F.; Kobayashi, H. Influence of poly(N-isopropylacrylamide)-CNT-polyaniline three dimensional electrospun microfabric scaffolds on cell growth and viability. Biopolymers 2013, 99, 334–341. [Google Scholar] [CrossRef]
- Sharma, Y.; Tiwari, A.; Hattori, S.; Terada, D.; Sharma, A.K.; Ramalingan, M.; Kobayashi, H. Fabrication of conducting electrospun nanofibers scaffold for three-dimensional cells culture. Int. J. Biol. Macromol. 2012, 51, 627–631. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Grapenson, S.; Jakob, A. Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties. Polymer 2006, 47, 1597–1603. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite utgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C.E. Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications. Adv. Funct. Mater. 2002, 12, 33–37. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Rivers, T.J. Biodegradable, Electrically Conducting Polymer for Tissue Engineering Applications. U.S. Patent 6,696,575, 23 February 2004. [Google Scholar]
- Wang, S.; Lu, L.; Gruetzmacher, J.A.; Currier, B.L.; Yaszemski, M.J. Synthesis and characterizations of biodegradable and crosslinkable poly(epsilon-caprolactone fumarate), poly(ethylene glycol fumarate), and their amphiphilic copolymer. Biomaterials 2006, 27, 832–841. [Google Scholar] [CrossRef]
- Runge, M.B.; Dadsetan, M.; Baltrusaitis, J.; Knight, A.M.; Ruesink, T.; Lazcano, E.; Lu, L.; Windebank, A.J.; Yaszemsk, M.J. The development of electrically conductive polycaprolactone fumarate-polypyrrole composite materials for nerve regeneration. Biomaterials 2010, 31, 5916–5926. [Google Scholar] [CrossRef]
- Jabbari, E.; Wang, S.; Lu, L.; Gruetzmacher, J.A.; Ameenuddin, S.; Hefferan, T.E.; Currier, B.L.; Windebank, A.J.; Yaszemski, M.J. Synthesis, material properties, and biocompatibility of a novel self-cross-linkable poly(caprolactone fumarate) as an injectable tissue engineering scaffold. Biomacromolecules 2005, 6, 2503–2511. [Google Scholar] [CrossRef]
- Durgam, H.; Sapp, S.; Deister, C.; Khaing, Z.; Chang, E.; Luebben, S.; Schmidt, C.E. Novel degradable copolymers of polypyrrole support cell proliferation and enhance neurite out-growth with electrical stimulation. J. Biomater. Sci. Polym. E 2010, 21, 1265–1282. [Google Scholar] [CrossRef]
- Shi, G.; Rouabhia, M.; Wang, Z.; Dao, L.H.; Zhang, Z. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials 2004, 25, 2477–2488. [Google Scholar] [CrossRef]
- Cen, L.; Neoh, K.G.; Kang, E.T. Surface functionalization of polypurrole film with glucose oxidase and viologen. Biosens. Bioelectron. 2003, 18, 363–374. [Google Scholar] [CrossRef]
- Wang, Z.; Roberge, C.; Wan, Y.; Dao, L.H.; Guidoin, R.; Zhang, Z. A biodegradable electrical bioconductor made of polypyrrole nanoparticle/poly(d,l-Lactide) composite a preliminary in vitro biostability study. J. Biomed. Mater. Res. A 2003, 66, 738–746. [Google Scholar]
- Zhang, Z.; Rouabhia, M.; Wang, Z.; Roberge, C.; Shi, G.; Roche, P.; Li, J.; Dao, L.H. Electrically conductive biodegradable polymer composite for nerve regeneration: Electricity-stimulated neurite outgrowth and axon regeneration. Artif. Organs 2007, 31, 13–22. [Google Scholar] [CrossRef]
- Boutry, C.M.; Müller, M.; Hierold, C. Junctions between metals and blends of conducting and biodegradable polymers (PLLA-PPy and PCL-PPy). Mater. Sci. Eng. C 2012, 32, 1610–1620. [Google Scholar] [CrossRef]
- Boutry, C.M.; Gerber-Hörler, I.; Hierold, C. Electrically conducting biodegradable polymer composites (polylactide-polypyrrole and polycaprolactone-polypyrrole) for passive resonant circuits. Polym. Eng. Sci. 2013, 53, 1196–1208. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-Lactic-co-Glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-Lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Tian, T.; Deng, J.; Xie, Z.; Zhao, Y.; Feng, Z.; Kang, X.; Gu, Z. Polypyrrole hollow fiber for solid phase extraction. Analyst 2012, 137, 1846–1852. [Google Scholar]
- Kang, T.S.; Lee, S.W.; Joo, J.; Lee, J.Y. Electrically conducting polypyrrole fibers spun by electrospinning. Synth. Met. 2005, 153, 61–64. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J. Biomed. Mater. Res. A 2011, 99, 376–385. [Google Scholar]
- Kuhn, H.H.; Child, A.D. Electrically Conducting Textiles. Handbook of Conducting Polymers, 2nd ed.; Skotheim, T.A., Elsenaumer, R.L., Reynolds, J.R., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 993–1104. [Google Scholar]
- Nair, S.; Natarajan, S.; Kim, S.H. Fabrication of electrically conducting polypyrrole-poly(ethylene oxide) composite nanofibers. Macromol. Rapid Commun. 2005, 26, 1599–1603. [Google Scholar] [CrossRef]
- Xie, J.; MacEwan, M.R.; Willerth, S.M.; Li, X.; Moran, D.W.; Sakiyama-Elbert, S.E.; Xia, Y. Conductive core-sheath nanofibers and their potential application in neural tissue engineering. Adv. Funct. Mater. 2009, 19, 2312–2318. [Google Scholar]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, S.; Zhang, X.; Feng, W.; Yuan, X. Preparation and properties of electrospun poly(ε-caprolactone)/polypyrrole membranes. Acta Polym. Sin. 2010, 1, 1094–1099. [Google Scholar]
- Wang, Y.Z.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.; Roca, M.I.; Martinez, J.G.; Meseguer-Olmo, L.; Cenis, J.L.; Moraleda, J.M.; Otero, T.F. Fabrication of conductive electrospun silk fibroin scaffolds by coating with poypyrrole for biomedical applications. Biochemistry 2012, 85, 36–43. [Google Scholar]
- Jin, L.; Wang, T.; Feng, Z.-Q.; Zhu, M.; Leach, M.K.; Naim, Y.I.; Jiang, Q. Fabrication and characterization of a novel polypyrrole fibrous scaffold designed for 3D cell culture. J. Mater. Chem. 2012, 22, 18321–18326. [Google Scholar]
- Mohammadi, A.; Ameli, A.; Alizadeh, N. Headspace solid-phase microextraction using a dodecylsulfate-doped polypyrrole film coupled to ion mobility spectrometry for the simultaneous determination of atrazine and ametryn in soil and water samples. Talanta 2009, 78, 1107–1114. [Google Scholar] [CrossRef]
- Li, X.; Zhong, M.; Chen, J. Electrodeposited polyaniline as a fiber coating for solid-phase microextraction of organochlorine pesticides from water. J. Sep. Sci. 2008, 31, 2839–2845. [Google Scholar]
- Li, M.; Wei, Z.; Jiang, L. Polypyrrole nanofiber arrays synthesized by a biphasic electrochemical strategy. J. Mater. Sci. 2008, 18, 2276–2280. [Google Scholar]
- Yang, S.Y.; Yang, J.-A.; Kim, E.-S.; Jeon, G.; Oh, E.J.; Choi, K.Y.; Hahn, S.K.; Kim, J.K. Single-file diffusion of protein drugs through cylindrical nanochannels. ACS Nano 2010, 4, 3817–3822. [Google Scholar] [CrossRef]
- Jeon, G.; Yang, S.Y.; Byun, J.; Kim, J.K. Electrically actuable smart nanoporous membrane for pulsatile drug release. Nano Lett. 2011, 11, 1284–1288. [Google Scholar] [CrossRef]
- Smela, E.; Gadegaard, N. Surprising volume change in PPy(DBS): An atomic force microscopy study. Adv. Mater. 1999, 11, 953–957. [Google Scholar] [CrossRef]
- Smela, E.; Gadegaard, N. Volume change in polypyrrole studied by atomic force microscopy. J. Phys. Chem. B 2001, 105, 9395–9405. [Google Scholar] [CrossRef]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Ge, D.; Ru, X.; Hong, S.; Jiang, S.; Tu, J.; Wang, J.; Zhang, A.; Ji, S.; Linkov, V.; Ren, B.; et al. Coating metals on cellulose–polypyrrole composites: A new route to self-powered drug delivery system. Electrochem. Commun. 2010, 12, 1367–1370. [Google Scholar] [CrossRef]
- Collier, J.H.; Camp, J.P.; Hudson, T.W.; Schmidt, C.E. Synthesis and characterization of polypyrrole–hyaluronic acid composite biomaterials for tissue. J. Biomed. Mater. Res. A 2000, 50, 574–584. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar]
- Akkouch, A.; Shi, G.; Zhang, Z.; Rouabhia, M. Bioactivating electrically conducting polypyrrole with fibronectin and bovine serum albumin. J. Biomed. Mater. Res. A 2010, 92, 221–231. [Google Scholar]
- Razal, J.; Kita, M.; Quigley, A.F.; Kennedy, E.; Moulton, S.E.; Kapsa, R.M.I.; Clark, G.M.; Wallace, G.G. Wet-spun biodegradable fibers on conducting platforms: Novel architectures for muscle regeneration. Adv. Funct. Mater. 2009, 19, 3381–3388. [Google Scholar] [CrossRef]
- Quigley, A.F.; Razal, J.M.; Thompson, B.C.; Moulton, S.E.; Kita, M.; Kennedy, E.L.; Clark, G.M.; Wallace, G.G.; Lapsa, R.M.I. A conducting-polymer platform with biodegradable fibers for stimulation and guidance of axonal growth. Adv. Mater. 2009, 21, 4393–4397. [Google Scholar] [CrossRef]
- Yu, M.; Huang, Y.; Ballweg, J.; Shin, H.; Huang, M.; Savage, D.E.; Lagally, M.G.; Dent, E.W.; Blick, R.H.; Williams, J.C. Semiconductor nanomembrane tubes: Three-dimensional confinement for controlled neurite outgrowth. ACS Nano 2011, 5, 2447–2457. [Google Scholar] [CrossRef]
- Kim, J.; Seo, Y.B. Electro-active paper actuators. Smart Mater. Struct. 2002, 11, 355–360. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Kim, J.; Deshpande, S.D.; Yun, S.; Li, Q. A comparative study of conductive polypyrrole and polyaniline coatings on electro-active papers. Polym. J. 2006, 38, 659–668. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Deshpande, S.D. Synthesis, characterization and actuation behavior of polyaniline-cellophane based electro-active paper actuator. Polym. Int. 2007, 56, 1530–1536. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Mahadeva, S.K.; Yun, K.; Yang, S.Y.; Maniruzzaman, M. Paper actuators made with cellulose and hybrid materials. Sensors 2010, 10, 1473–1485. [Google Scholar] [CrossRef]
- Guimard, N.K.E.; Sessler, J.L.; Schmidt, C.E. Towards a biocompatible and biodegradable copolymer incorporating electroactive oligothiophene units. Macromolecules 2009, 42, 502–511. [Google Scholar] [CrossRef]
- Baughman, R.H.; Shacklette, R.L.; Elsenbaumer, R.L.; Plichta, E.J.; Becht, C. Conjugated Polymeric Materials: Opprtunities in Electronics, Optoelectronics, and Molecular Electronics; NATO ASI Series E; Brédas, J.L., Chance, R.R., Eds.; Applied Science: Mons, Belgium, 1990; Volume 182, pp. 559–582. [Google Scholar]
- Pei, Q.B.; Inganläs, O. Conjugated polymers and the bending cantilever method: Electrical muscles and smart devices. Adv. Mater. 1992, 4, 277–278. [Google Scholar] [CrossRef]
- Otero, T.F.; Cortes, M.T. Artificial muscles with tactile sensitivity. Adv. Mater. 2003, 15, 279–282. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Murray, P.; Spinks, G.M.; Wallace, G.G. Mechanism of electromechanical actuation in polypyrrole. Synth. Met. 1995, 73, 247–256. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Shaw, S.J.; Wallace, G.G. Pulsed-amperometric detection of urea in blood samples on a conducting polypyrrole-urease biosensor. Anal. Chim. Acta 1997, 341, 155–160. [Google Scholar] [CrossRef]
- Pernaut, J.M.; Reynolds, J.R. Use of conducting electroactive polymers for drug delivery and sensing of bioactive molecules. A redox chemistry approach. J. Phys. Chem. B 2000, 104, 4080–4090. [Google Scholar] [CrossRef]
- Reinhard, C.S.; Radomsky, M.L.; Saltzman, W.M.; Hilton, J.; Brem, H. Polymeric controlled release of dexamethasone in normal rat brain. J. Control. Release 1991, 1683, 331–339. [Google Scholar]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting polymer nanotubes for controlled drug release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef]
- Jager, E.W.H.; Inganäs, O.; Lundström, I. Microrobots for micrometer-size objects in aqueous media: Potential tools for single-cell manipulation. Science 2000, 288, 2335–2338. [Google Scholar] [CrossRef]
- Kipke, D.R.; Shain, W.; Buzsáki, G.; Fetz, E.; Henderson, J.M.; Hetke, J.F.; Schalk, G. Symposium: Advanced neurotechnologies for chronic neural interfaces: New horizons and clinical opportunities. J. Neurosci. 2008, 28, 11830–11838. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Meth. 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef]
- Isaksson, J.; Kjäll, P.; Nilsson, D.; Robinson, N.D.; Berggren, M.; Richter-Dahlfors, A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 2007, 6, 673–679. [Google Scholar] [CrossRef]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef]
- Abidian, M.R.; Ludwig, K.A.; Marzullo, T.C.; Martin, D.C.; Kipke, D.R. Interfacing conducting polymer nanotubes with the central nervous system: Chronic neural recording using poly(3,4-ethylenedioxythiophene) nanotubes. Adv. Mater. 2009, 21, 3764–3770. [Google Scholar] [CrossRef]
- Abidian, M.R.; Martin, D.C. Multifunctional nanobiomaterials for neural interfaces. Adv. Funct. Mater. 2009, 19, 573–585. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.J.; Friend, R.H.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.M.W.; Spiering, A.J.H.; Janssen, R.A.J.; Meijer, E.W. Microstructure–mobility correlation in self-organised, conjugated polymer field-effect transistors. Synthetic Met. 2000, 111–112, 129–132. [Google Scholar] [CrossRef]
- Liu, H.; Reccius, C.H.; Craighead, H.G. Single electrospun regioregular poly(3-hexylthiophene) nanofiber field-effect transistor. Appl. Phys. Lett. 2005, 87, 253106:1–253106:3. [Google Scholar]
- Lee, S.; Moon, G.D.; Jeong, U. Continuous production of uniform poly(3-hexylthiophene) (P3HT) nanofibers by electrospinning and their electrical properties. J. Mater. Chem. 2009, 19, 743–748. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Axially aligned electrically conducting biodegradable nanofibers for neural regeneration. J. Mater. Sci. Mater. M 2012, 23, 1797–1809. [Google Scholar] [CrossRef]
- Armelin, E.; Gomes, A.L.; Pérez-Madrigal, M.M.; Puiggalí, J.; Franco, L.; del Valle, L.J.; Rodríguez-Galán, A.; de Carvalho Campos, J.S.; Ferrer-Anglada, N.; Alemán, C. Biodegradable free-standing nanomembranes of conducting polymer: Polyester blends as bioactive platforms for tissue engineering. J. Mater. Chem. 2012, 22, 585–594. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Armelin, E.; del Valle, L.J.; Estrany, F.; Alemán, C. Bioactive and electroactive response of flexible polythiophene: Polyester nanomembranes for tissue engineering. Polym. Chem. 2012, 3, 979–991. [Google Scholar] [CrossRef]
- Pérez Madrigal, M.M.; Giannotti, M.I.; Oncins, G.; Franco, L.; Armelin, E.; Puiggalí, J.; Sanz, F.; del Valle, L.J.; Alemán, C. Bioactive nanomembranes of semiconductor polythiophene and thermoplastic polyurethane: Thermal, nanostructural and nanomechanical properties. Polym. Chem. 2013, 4, 568–583. [Google Scholar] [CrossRef]
- Teixeira-Dias, B.; del Valle, L.J.; Aradilla, D.; Estrany, F.; Alemán, C. A conducting polymer/protein composite with bactericidal and electroactive properties. Macromol. Mater. Eng. 2012, 297, 427–436. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.P.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef]
- Rachocki, A.; Pogorzelec-Glaser, K.; Pawlaczyk, C.; Tritt-Goc, J. Morphology, molecular dynamics and electric conductivity of carbohydrate polymer film based on algínic acid and benzimidazole. Carbohyd. Res. 2011, 346, 2718–2726. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Llorens, E.; Armelin, E.; Del Mar Pérez-Madrigal, M.; Del Valle, L.J.; Alemán, C.; Puiggalí, J. Nanomembranes and Nanofibers from Biodegradable Conducting Polymers. Polymers 2013, 5, 1115-1157. https://doi.org/10.3390/polym5031115
Llorens E, Armelin E, Del Mar Pérez-Madrigal M, Del Valle LJ, Alemán C, Puiggalí J. Nanomembranes and Nanofibers from Biodegradable Conducting Polymers. Polymers. 2013; 5(3):1115-1157. https://doi.org/10.3390/polym5031115
Chicago/Turabian StyleLlorens, Elena, Elaine Armelin, María Del Mar Pérez-Madrigal, Luís Javier Del Valle, Carlos Alemán, and Jordi Puiggalí. 2013. "Nanomembranes and Nanofibers from Biodegradable Conducting Polymers" Polymers 5, no. 3: 1115-1157. https://doi.org/10.3390/polym5031115
APA StyleLlorens, E., Armelin, E., Del Mar Pérez-Madrigal, M., Del Valle, L. J., Alemán, C., & Puiggalí, J. (2013). Nanomembranes and Nanofibers from Biodegradable Conducting Polymers. Polymers, 5(3), 1115-1157. https://doi.org/10.3390/polym5031115