Hybrid Magnetic Hydrogel: A Potential System for Controlled Drug Delivery by Means of Alternating Magnetic Fields
Abstract
:1. Introduction
2. Results and Discussion
2.1. Functionalized Nanoparticles (NP-NH2)

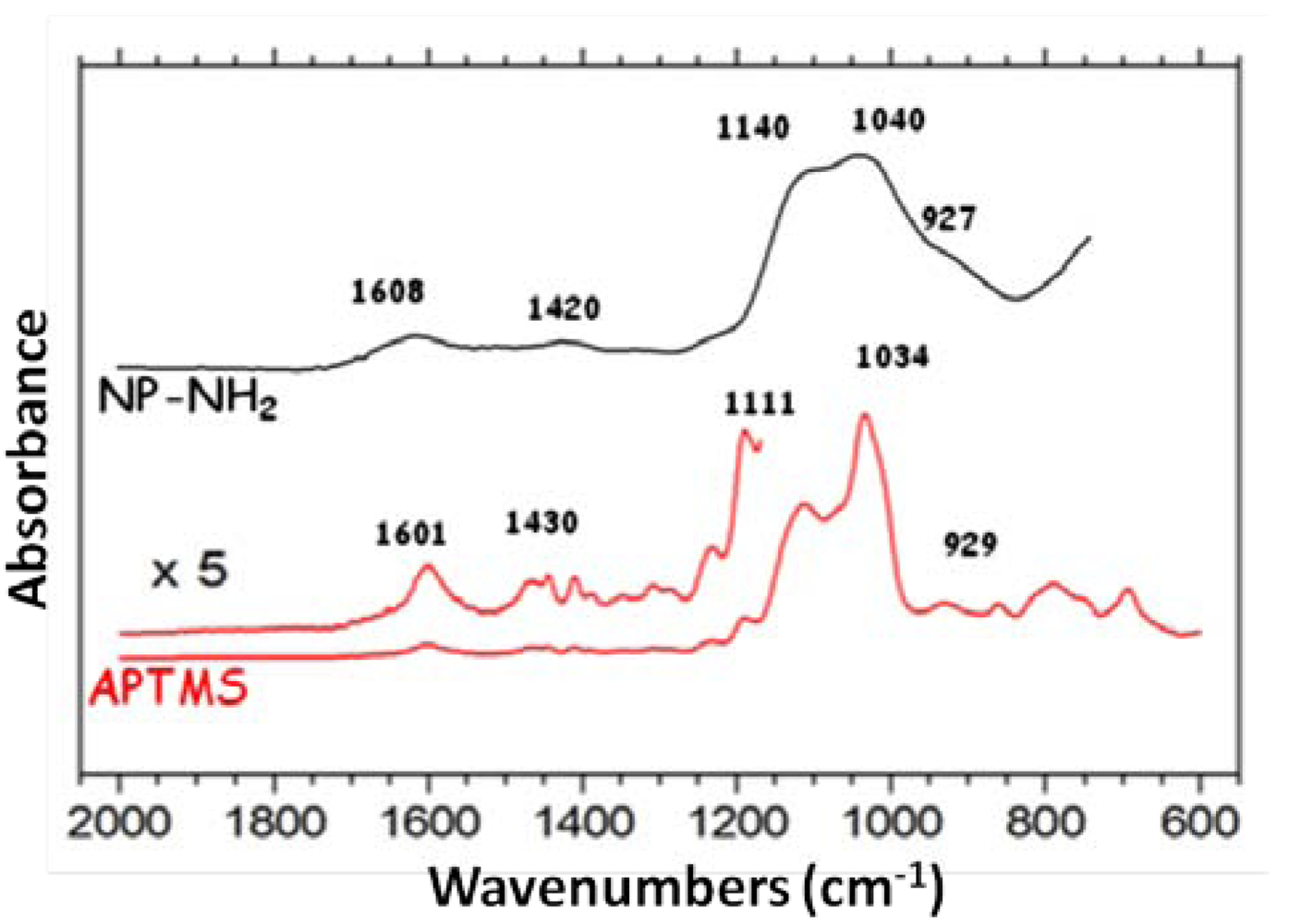
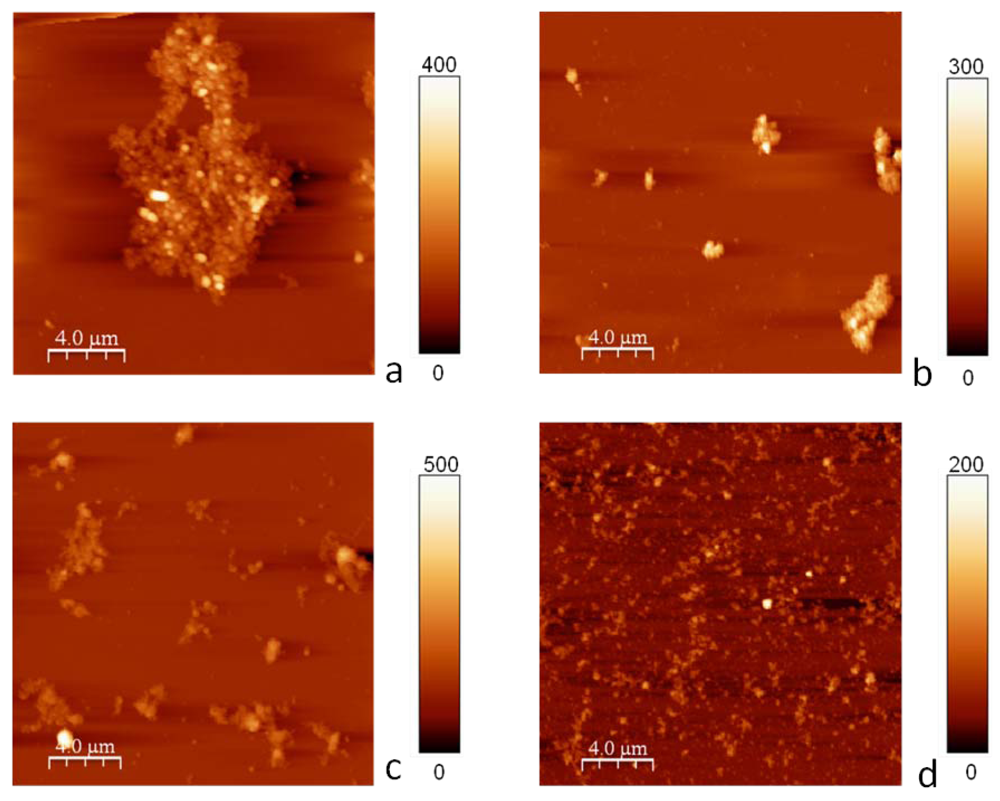
2.2. Sonication of NP-NH2

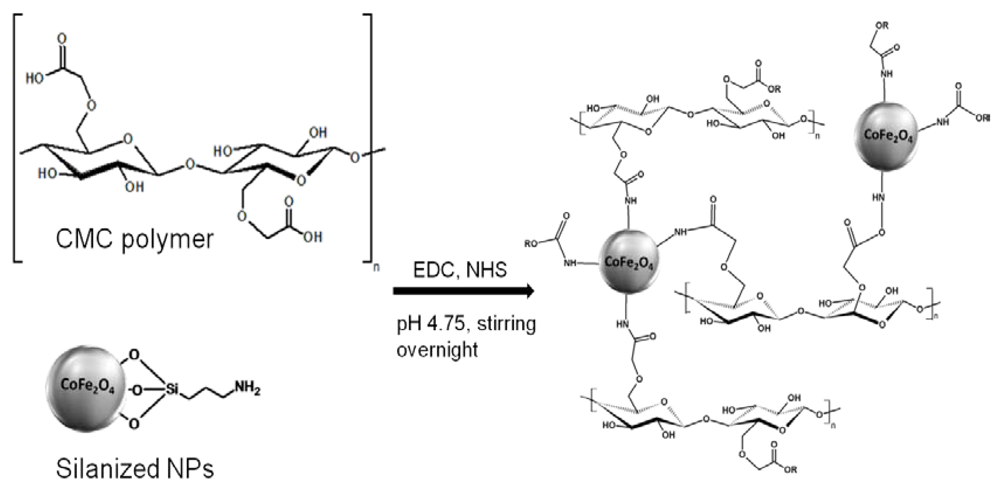
2.3 Hybrid Hydrogel (CMC-NP) Synthesis

| Sample | G' | G" |
|---|---|---|
| CMC-DAP | 550 ± 100 | 60 ± 30 |
| CMC-NP | 3,000 ± 300 | 90 ± 20 |
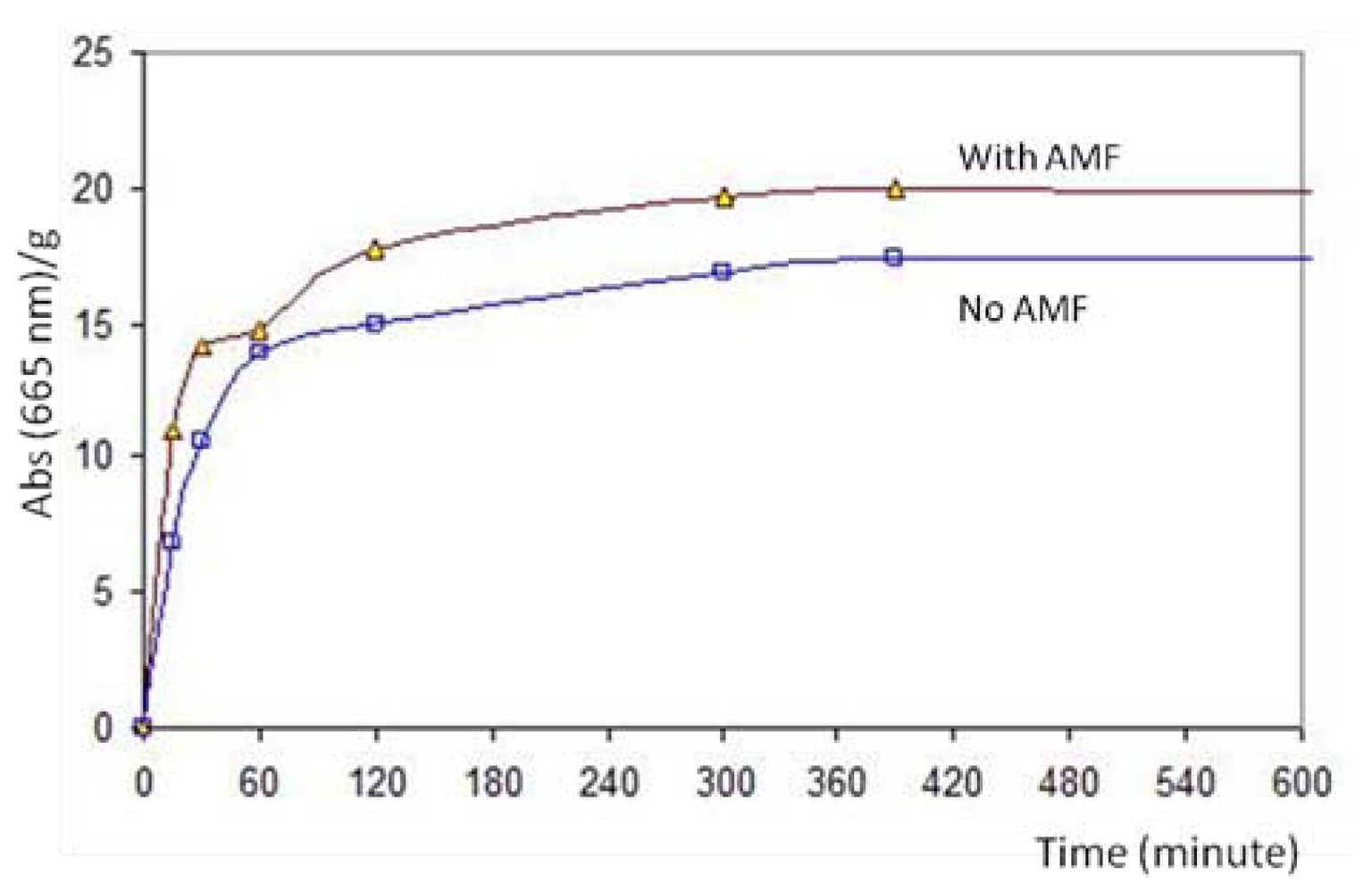
2.4. Drug Loading and Drug Release by Means of AMF
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Functionalization of the CoFe2O4 NPs (NP-NH2)
3.2.2. Preparation of the Hybrid Hydrogel (CMC-NP)
3.2.3. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR/FT-IR)
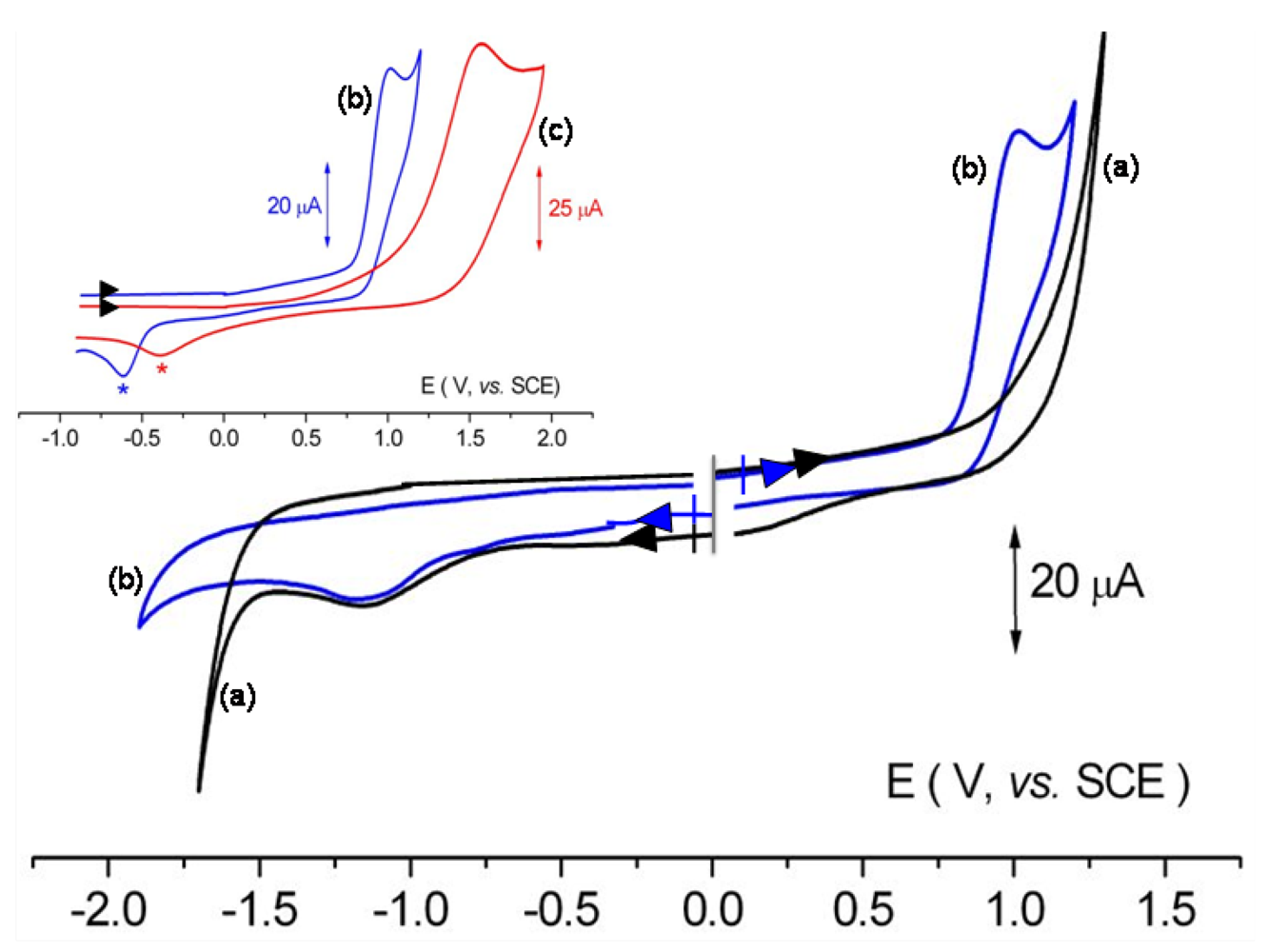
3.2.4. Electrochemical Measurements
3.2.5. Atomic Force Microscopy (AFM)
3.2.6. Rheological Characterization (CMC-NP)
3.2.7. Drug Loading and Drug Release by AMF
4. Conclusions
Acknowledgments
References
- Barbucci, R.; Giardino, R.; de Cagna, M.; Golini, L; Pasqui, D. Inter-Penetrating Hydrogels (IPHs) as a new class of injectable polysaccharide hydrogels with thixotropic nature and interesting mechanical and biological properties. Soft Matter 2010, 6, 3524–3532. [Google Scholar]
- Peppas, N.A. Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Schexnailder, P.J.; Schmidt, G. Nanocomposite hydrogels. Colloid Polym. Sci. 2009, 287, 1–11. [Google Scholar]
- Narayana Reddy, N.; Varaprasad, K.; Subba Reddy, G.V.; Reddy, K.M.S.; Mohan Reddy, K.M.; Mohana Raju, K. Evaluation of blood compatibility and drug release studies of gelatin based magnetic hydrogel nanocomposites. Colloids Surf. A physicochem. Eng. Asp. 2011, 385, 20–27. [Google Scholar]
- Kim, J.; Chun, C.; Kimd, B.; Hong, J.; Cho, J.K. Thermosensitive/magnetic poly(organophosphazene) hydrogel as a long-term magnetic resonance contrast platform. Biomaterials 2012, 33, 218–222. [Google Scholar]
- Meenach, A.; Hilt, Z.; Anderson, W. Poly(ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy. Acta Biomater. 2010, 6, 1039–1046. [Google Scholar]
- Gaihre, B.; Khil, S.; Lee, R.; Kim, Y. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. Int. J. Pharm. 2009, 365, 180–189. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Chomoucka, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar]
- Mahmoudi, M.; Simchi, A.; Hafeli, O.U. Superparamagnetic iron oxide nanoparticles with rigid cross-linked polyethylene glycol fumarate coating for application in imaging and drug delivery. J. Phys. Chem. C 2009, 113, 8124–8131. [Google Scholar]
- Satarkar, S.; Hilt, Z. Hydrogel nanocomposites as remote-controlled biomaterials. Acta Biomater. 2008, 4, 11–16. [Google Scholar] [CrossRef]
- Satarkar, S.; Hilt, Z. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release 2008, 130, 246–251. [Google Scholar]
- Barbucci, R.; Pasqui, D.; Giani, G.; de Cagna, M.; Fini, M.; Giardino, R.; Atrei, A. A novel strategy for engineering hydrogels with ferromagnetic nanoparticles as crosslinkers of the polymer chains. Potential applications as a targeted drug delivery system. Soft Matter 2011, 7, 5558–5565. [Google Scholar]
- Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, Y. Controlled pulsatile drug release from a ferrogelby a high-frequency magnetic field. Macromolecules 2007, 40, 6786–6788. [Google Scholar]
- Brulé, S.; Levy, M.; Wilhelm, C.; Letourneur, D.; Gazeau, F.; Ménager, C.; Le Visage, C. Doxorubicin release triggered by alginate embedded magnetic nanoheaters: A combined therapy. Adv. Mater. 2011, 23, 787–790. [Google Scholar]
- Liang, Y.; Zhang, L.M.; Jiang, W.; Lei, W. Embedding magnetic nanoparticles into polysaccharide-based hydrogels for magnetically assisted bioseparation. Chem. Phys. Chem. 2007, 8, 2367–2372. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Gao, Q.; Chen, J.; Ren, B.; Liu, X.; Tong, Z. Facile fabrication of well-defined hydrogel beads with magnetic nanocomposite shells. Int. J. Pharm. 2009, 376, 92–98. [Google Scholar]
- Pasqui, D.; Atrei, A.; Giani, G.; de Cagna, M.; Barbucci, R. Metal oxide nanoparticles as cross-linkers in polymeric hybrid hydrogels. Mater. Lett. 2011, 65, 392–395. [Google Scholar]
- Shin, K.; Cho, Y.; Choi, J.; Kim, K. Facile synthesis of silver-deposited silanized magnetite nanoparticles and their application for catalytic reduction of nitrophenols. Appl. Catal. A Gen. 2012. In press.. [Google Scholar]
- Berghoute, Y.; Mendonc, M.H.; Hamdani, M.; Pereira, M.I.S. Electrochemical behaviour of FexCo3−xO4 with (x = 0, 1, 2 and 3) oxides thin film electrodes in alkaline medium. J. Appl. Electrochem. 2009, 39, 2469–2479. [Google Scholar]
- Laouini, E.; Douch, J.; Hamdani, M.; Berghoute, Y.; Mendonc, M.H.; Pereira, M.I.S.; Singht, R.N. Cathodic behaviour of CoFe2O4 spinel electrodes in alkaline medium. J. Appl. Electrochem. 2011, 41, 731–740. [Google Scholar]
- Choi, Y.J.; Tzy-Jiun, U.; Luo, M. Sol-gel chemistry of an aqueous precursor solution for YBCO thin films. J. Sol-Gel. Sci. Technol. 2009, 51, 124–133. [Google Scholar] [CrossRef]
- Hernando, J.; Pourrostami, T.; Garrido, J.A.; Williams, O.A.; Gruen, D.M.; Kromka, A.; Steinmüller, D.; Stutzmann, M. Immobilization of horseradish peroxidase via an amino silane on oxidized ultrananocrystalline diamond. Diamond Relat. Mater. 2007, 16, 138–143. [Google Scholar] [CrossRef]
- Wang, M.; Liechti, K.M.; Wang, Q.; White, J.M. Self-assembled silane monolayers: Fabrication with nanoscale uniformity. Langmuir 2005, 21, 1848–1857. [Google Scholar]
- Moon, J.H.; Shin, J.W.; Kim, S.Y.; Park, J.W. Formation of uniform aminosilane thin layers: An imine formation to measure relative surface density of the amine group. Langmuir 1996, 12, 4621–4624. [Google Scholar] [CrossRef]
- Pallandre, A.; Glinel, K.; Jonas, A.M.; Nysten, B. Binary nanopatterned surfaces prepared from siane monolayers. Nanoletters 2004, 4, 365–371. [Google Scholar]
- Kurth, D.G.; Bein, T. Surface reactions on thin layers of silane coupling agents. Langmuir 1993, 9, 2965–2973. [Google Scholar]
- Xu, R. Progress in nanoparticles characterization: Sizing and zeta potential measurement. Particuology 2008, 6, 112–115. [Google Scholar]
- Rodríguez-Arco, L.; López-López, M.; González-Caballero, F.; Durán, J. Steric repulsion as a way to achieve the required stability for the preparation of ionic liquid-based ferrofluids. J. Colloid Interf. Sci. 2011, 357, 252–254. [Google Scholar]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.; Reichel, C.; Coester, C.; Tuomi, T.; Rehbergand, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2584664/ (accessed on 28 April 2012).
- Zhang, X.; Yin, L.; Tang, M.; Pu, Y. Optimized method for preparation of TiO2 nanoparticles dispersion for biological study. J. Nanosci. Nanotechnol. 2010, 10, 5213–5219. [Google Scholar]
- Vargas, A.F.; Orozco, V.H.; Rault, F.; Giraud, S.; Devaux, E.; Lopez, B.L. Influence of fiber-like nanofillers on the rheological, mechanical, thermal and fire properties of polypropylene: An application to multifilament yarn. Compos. Part. A Appl. Sci. Manuf. 2010, 41, 1797–1806. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. Rep. 2006, 53, 73–197. [Google Scholar]
- Hu, X.; Zhou, J.; Zhang, N.; Tan, H.; Gao, C. Preparation and properties of an injectable scaffold of poly(lactic-co-glycolic acid) microparticles/chitosan hydrogel. J. Mech. Behav. 2008, 1, 352–359. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Giani, G.; Fedi, S.; Barbucci, R. Hybrid Magnetic Hydrogel: A Potential System for Controlled Drug Delivery by Means of Alternating Magnetic Fields. Polymers 2012, 4, 1157-1169. https://doi.org/10.3390/polym4021157
Giani G, Fedi S, Barbucci R. Hybrid Magnetic Hydrogel: A Potential System for Controlled Drug Delivery by Means of Alternating Magnetic Fields. Polymers. 2012; 4(2):1157-1169. https://doi.org/10.3390/polym4021157
Chicago/Turabian StyleGiani, Gabriele, Serena Fedi, and Rolando Barbucci. 2012. "Hybrid Magnetic Hydrogel: A Potential System for Controlled Drug Delivery by Means of Alternating Magnetic Fields" Polymers 4, no. 2: 1157-1169. https://doi.org/10.3390/polym4021157
APA StyleGiani, G., Fedi, S., & Barbucci, R. (2012). Hybrid Magnetic Hydrogel: A Potential System for Controlled Drug Delivery by Means of Alternating Magnetic Fields. Polymers, 4(2), 1157-1169. https://doi.org/10.3390/polym4021157




