Embryonic Stem Cells Maintain an Undifferentiated State on Dendrimer-Immobilized Surface with d-Glucose Display
Abstract
: In serial passaging cultures of mouse embryonic stem (ES) cells, we employed a dendrimer-immobilized substrate that displayed D-glucose as a terminal ligand. The D-glucose-displaying dendrimer (GLU/D) surface caused the ES cells to form loosely attached spherical colonies, while those on a gelatin-coated surface formed flatter colonies that were firmly attached to the surface. Despite the morphological similarities between the colonies on the GLU/D surface and aggregates on a conventional bacteriological dish, immunostaining and RT-PCR analyses revealed the maintenance of cells within the spherical colonies on the GLU/D surface in an undifferentiated state with very low expressions of primitive endoderm markers. On the bacteriological dish, however, the cells within the aggregates showed a different cellular state with partial differentiation into the primitive endoderm lineage, and the expression level increased gradually along with the number of passages. These results indicate that the GLU/D surface can be a potential tool for controlling the ES cell morphology and then govern their self-renewal and fate.1. Introduction
The mouse embryonic stem (ES) cells can be derived from 2-cell stage embryos to hatched blastocysts. Also the single blastomere can be used to establish mouse ES cells [1]. Depending upon the molecular and physicochemical environment, the pluripotent cells can either remain in an undifferentiated state or give rise to three primary germ layers, ectoderm, endoderm and mesoderm. In contrast to the cells located in the inner cell mass, mouse ES cell lines remain pluripotent in vitro and self-renew in the presence of leukemia inhibitory factor (LIF). For many years ES cells were thought to be a homogeneous cell population. However, recently this concept has been challenged by several groups. Cui et al. [2] demonstrated the spatial distribution of adhesion molecules within ES cell colonies and Toyooka et al. [3] proved the existence of cell subpopulations in culture of mouse ES cells. In fact, ES cells are commuting between metastable states from the inner cell mass to the epiblast stage. These reversible states are associated with distinct potential of differentiation [3–5]. This is thus a highly dynamic self-renewing cell population which responds to the environmental clues, such as growth factors in the culture medium or secreted elements within the ES cell colonies, and signals arising from adhesion to the substrate (i.e., extracellular matrices, ECM) and stiffness of the substrate to maintain their pluripotency or to differentiate [6].
While much effort has been focused on identifying soluble factors to control ES cell fate, investigators recently have begun to appreciate the potential importance of insoluble signals such as cell adhesion, cell shape, mechanical forces and substrate rigidity, in stem cell differentiation [7–9]. So far, many researchers have paid attention to stimulation of receptors on the cytoplasmic membrane of ES cells through interactions with solid surfaces. For example, culture surfaces that can enhance cellular functions, such as ES cell growth, migration and differentiation, have been achieved through the incorporation of tethered adhesive ligands including growth factors and cytokines [10–13].
In our recent paper [14], we introduced our strategies to design a culture surface using a dendrimer substrate to regulate the cell morphology and function. The defined polyamidoamine dendrimer structure with the large number of terminal amino groups gives great flexibility in the design variables, including the ligand species presented on the terminal groups, dendrimer size and ligand density, which makes these polymers suitable for use as biocompatible nanometer-sized capsules in gene- or drug-delivery systems, as well as in scaffolds for cell culturing. In addition, we proposed an in vitro culture system using a synthesized dendrimer having D-glucose as a functional ligand for enrichment of undifferentiated ES cells by serial passaging on the surface [15]. Given these findings, in the current work, we examined the morphological observation and gene expression analyses for colonies of ES cells formed on a D-glucose-displaying dendrimer surface in terms of maintaining pluripotency of ES cells.
2. Materials and Methods
2.1. Preparation of GLU/D Surface
A conventional polystyrene surface of a 6-well culture plate (surface area: 9.6 cm2, Nunclon Delta Flask, Nalge Nunc, Roskilde, Denmark) was used as a starter material for preparing the D-glucose-displaying dendrimer substrate. The surface bearing 4th-generation dendrimer with D-glucose display was created by the following reactions under sterile conditions as outlined in Figure 1.
In step 1, to display a hydroxyl group on the starter surface, an aqueous solution of 50 nmol/mL potassium tert-butoxide (tert-BuOK) was poured into each well, followed by 1 h incubation at ambient temperature. The well was then washed three times with sterilized water. In step 2, an aqueous solution of 360 μmol/mL glutaraldehyde was introduced into the well and allowed to stand for 1 h, followed by washing with a large amount of water. In step 3, the well was treated with 360 μmol/mL tris(2-aminoethyl) amine solution (pH 9.0) for 1 h to produce a dendron structure and then rinsed with water. Steps 2 and 3 were repeated until the 4th-generation surface was synthesized. Thereafter, to display D-glucose as a terminal ligand, 0.5 μmol/mL D-glucose solution was added to the well and allowed to stand for 2 h. Then a 0.5 μmol/mL sodium borohydride solution was poured into the well and left to stand for 24 h, and the well was washed with water, yielding a 4th-generation surface with D-glucose display. When a 25-cm2 T-flask (Nunclon Delta Flask; Nalge Nunc, Denmark) was used, the procedures for displaying D-glucose on the surface were the same as described above, except that the amounts of reagents changed in proportion to the treated surface area. The plates or flasks with prepared surfaces were stored at 4 °C under sterile conditions until use for 2 weeks or less.
2.2. ES Cell Cultures
A feeder-free murine germline-competent ES cell line, EB3, was routinely maintained on a surface with 0.1% gelatin coating in Glasgow minimum essential medium (GMEM) with 1,000 U/mL LIF. The density of seeding cells was fixed at 5.0 × 103 cells/cm2. Passages 14–20 of undifferentiated ES cells were used in all the experiments.
Cell passaging cultures were performed in GMEM in the presence of LIF on the GLU/D surface and a conventional bacteriological dish as a control, according to the previously reported procedure [15].
2.3. Analyses
RNA extraction, cDNA synthesis and RT-PCR analysis were performed, as described previously [16]. The primer sequences employed for the RT-PCR analysis are indicated in Table 1.
For the immunostaining analysis, the cells grown on the GLU/D surface and conventional bacteriological dish at passages 1 and 4 were embedded with an OCT compound (Sakura Finetech, Tokyo, Japan) and were frozen in liquid nitrogen. The samples were sliced at a thickness of 7 μm and were fixed with buffered 4% paraformaldehyde. Blocking of non-specific proteins was performed using 3% bovine serum albumin in phosphate-buffered saline (PBS). For co-immunostaining with anti-Nanog (RCAB0001P; ReproCELL, Yokohama, Japan) and anti-Gata-4 antibodies (sc-1237; Santa Cruz Biotechnology, Santa Cruz, CA, USA), the specimens were incubated overnight at 4 °C with a mixture of these primary antibodies. After washing with 0.8% Tween 20 in PBS, the specimens were first incubated with Alexa-Fluor-568-conjugated donkey anti-goat IgG antibody (Molecular Probes, Eugene, OR, USA) at room temperature for 1 h. After washing with 0.8% Tween 20 in PBS, the specimens were incubated with Alexa-Fluor-488-conjugated goat anti-rabbit IgG antibody (Molecular Probes) at room temperature for 1 hour. After washing, they were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO, USA). Fluorescence images were obtained using an Olympus IX70 fluorescence microscope.
The alkaline phosphatase (ALP) activity was detected using a commercially available analytical kit (cat. no. 86R-1KT; Sigma-Aldrich) according to the manufacturer's recommendation. The cells seeded in a 6-well plate were subjected to colony observation for ALP activity analysis after 5 day culture.
3. Results and Discussion
Based on the mechanisms of cell stimulation for signaling regulation, many strategies have been proposed for maintaining the undifferentiated state and pluripotent ability of ES cells during in vitro cultures. An example of this includes exogenous stimulation via signaling molecules by introducing them into medium or onto the culture substrate. Some studies have reported that the exogenous soluble regulators such as LIF can function effectively by means of arraying them in direct contact with the targets for signaling receptor on the cytoplasmic membrane [17]. Alternative strategies employ intercellular signaling through regulating Rho family GTPase activity in relation to the undifferentiated state and pluripotent ability of ES cells. As some of those intracellular signaling pathways are known to crosstalk with Rho family GTPases in mammalian cell lines [18], it is possible that the E-cadherin-mediated cell-cell adhesions in ES cells are also under the control of pluripotency regulators through modulating the Rho family GTPase signaling pathway. Harb et al. have demonstrated that endogeous Rho family GTPases are required for the maintenance of cell-cell contacts in ES cells [19]. It was demonstrated that the Rho family GTPases play critical roles in the regulation of primary cell-cell interactions through which coherent colony structures are assembled.
3.1. ES Cells Make Appreciable Formation of Undifferentiated Spherical Colonies on GLU/D Surface
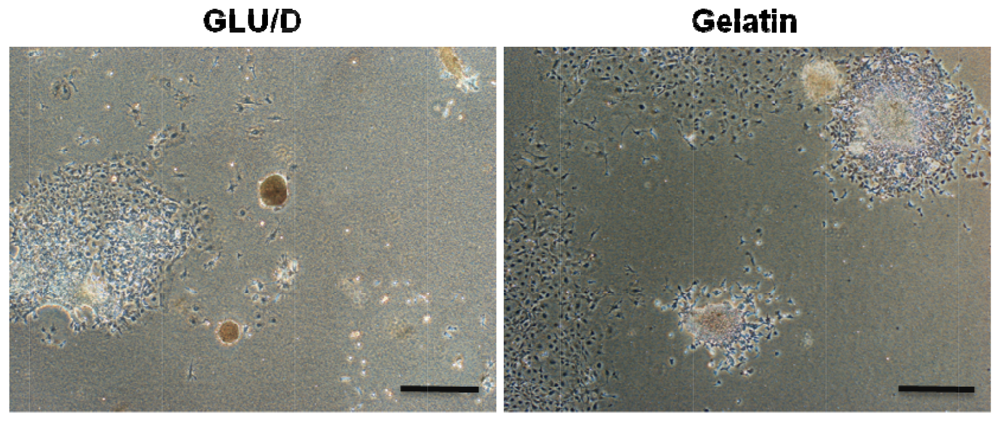
ES cells self-renew as coherent colonies in which cells maintain tight cell-cell contact. A tightly integrated colony formation has been considered to be one of the essential criteria to define the undifferentiated state of ES cells [2]. These phenomena of ES colony formations seem to be concordant with our previous results obtained in the culture of mouse ES cells on the GLU/D surface. The time-lapse observation revealed that the cells on the GLU/D surface actively migrated and showed morphological fluctuations with temporal stretching and contracting, compared to those growing on the conventional gelatin-coated surface. The ES cells on the GLU/D surface developed growing colonies and yielded three-dimensional structures. The colonies continued to grow until the cells became very densely packed. However, the cells on the gelatin-coated surface formed flatter colonies with firm attachment to the surface. Interestingly, differentiated ES cells formed colonies on the gelatin-coated surface, suggesting that cell mobility was specific to undifferentiated ES cells. As shown in Figure 2, furthermore, the ES cells grown on the GLU/D surface were readily stained for ALP, although the ES cells on the gelatin-coated surface were not densely stained for ALP, which indicates partial differentiation. This may be caused by cell spreading from colonies or the deformation of colonies. Although the mechanism underlying spherical colony formation on the GLU/D surface is still unclear, one possibility is that this surface permits moderate activation of Rho family GTPases associated with promoting formation of spherical colonies by E-cadherin-mediated cell-cell contacts. The results obtained in the present study indicate that the formation of spherical colonies on the GLU/D surface will vary in relation to cell-cell communication, suggesting that the cell-cell contacts are important for maintaining the undifferentiated state and pluripotent ability of ES cells.
3.2. ES Cells Grown on GLU/D Surface Maintain an Undifferentiated State and Pluripotent Ability during Serial Passages
When ES cells are allowed to aggregate in the absence of LIF, they form a structure known as the embryoid body (EB), which contains all three germ layers. On the other hand, when EBs are formed in the presence of LIF (denoted here as cell aggregates), the cell aggregation induces Nanog repression and primitive endoderm (expressing Coup-tf1 and Gata4 marker genes) differentiation at the peripheral layer of the cell aggregates [20]. However, recently some reports have shown that ES cells maintain their pluripotent characteristics when cultured in suspension culture as aggregates [21,22]. These studies suggested that when the cells are cultured under appropriate conditions, the aggregation of ES cells does not always lead to differentiation.
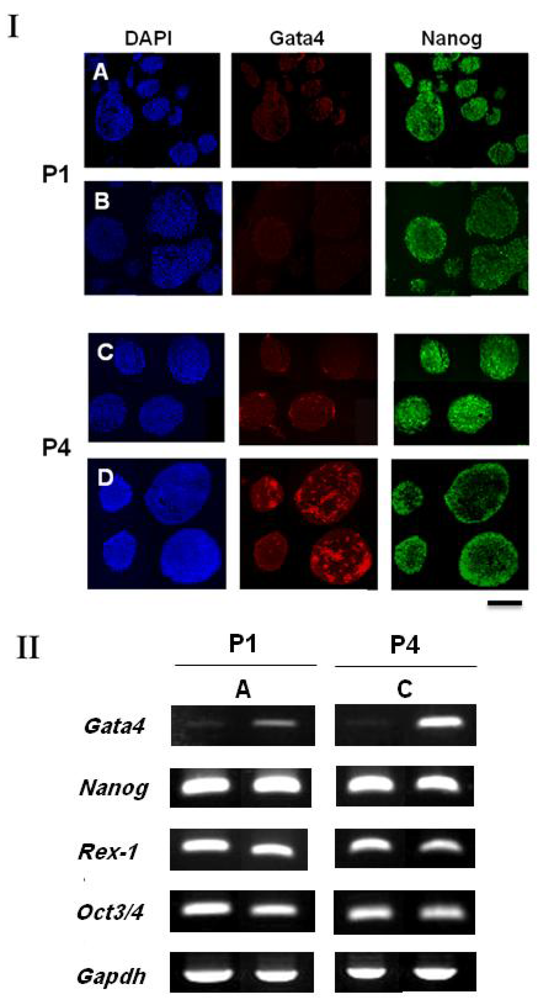
Here, while the spherical colonies appearing on the GLU/D surface in the present study were morphologically similar to floating (non-attached) cell aggregates, growing under a LIF-existing culture condition, the undifferentiated state of ES cells within each aggregate was found to be different. We conducted the suspension culture of ES cells using a bacteriological dish to yield floating cell aggregates. The undifferentiated states of the spherical colony cells grown on the GLU/D surface and the bacteriological dish at passages 1 and 4 were then compared by immunostaining and RT-PCR analysis. Immunostaining results demonstrated that both ES cells were similar with respect to the expression of Gata4 and Nanog at passage 1 [Figure 3(I)(A,B)]. Concerning the cell aggregates at passage 4 on the dish, however, Gata4 was expressed not only in the outermost layer but also inside of the cell aggregates, thus showing the accumulation of primitive endoderm cells. Gata4 expression was not predominately evident for the spherical colony cells on the GLU/D surface [Figure 3(I)(C,D)]. Furthermore, as seen in Figure 3(II), the RT-PCR results for the cell aggregates grown on the bacteriological dish revealed that passaging caused a notable increase in the expression level of Gata4. In contrast, the expression level of Rex-1, an ES cell undifferentiated-state marker regulated by Nanog [23], decreased. These results indicate that the cells within the spherical colonies on the GLU/D surface could be in an undifferentiated state with a very low expression of primitive endoderm, in contrast to the cells within the cell aggregates in bacteriological dish with partial differentiation into the primitive endoderm lineage.
Consequently, it is most likely that the maintenance of undifferentiated state and pluripotent ability within the spherical colonies is closely related to intracellular signaling pathways, such as the Rho family GTPase pathways, which in turn promotes cadherin-mediated cell-cell contact, as previously shown for human mesenchymal stem cells cultured on the GLU/D surface [24]. Taken together, the present findings suggest that the dendrimer surface with D-glucose display offers a model of designing a substrate, based on interactions between the microenvironment and cells, as a tool for maintaining proper undifferentiated state of ES cells in an ex vivo stem cell culture system.
4. Conclusions
We demonstrated that the GLU/D surface caused mouse ES cells to form loosely attached spherical colonies with morphologies similar to the cell aggregates formed in the presence of LIF. However, the immunostaining and RT-PCR results revealed the maintenance of these spherical colony cells within the spherical colonies on the GLU/D surface in an undifferentiated state with a very low expression of primitive endoderm markers, being distinct from the cells within the cell aggregates that showed partial differentiation into the primitive endoderm lineage. These results indicate that the GLU/D surface can be a potential tool for controlling the ES cell morphology and then govern their self-renewal and fate.

| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| Gata4 | gcctgtatgtaatgcctgcg | ccgagcaggaatttgaagagg |
| Nanog | tgccaggaagcagaagatgcggac | cactggtttttctgccaccgcttg |
| Rex-1 | attttctggtgcacaccgga | ccaccttcagcatttcttcc |
| Oct3/4 | ggcgttctctttggaaaggtgttc | ctcgaaccacatccttctct |
| Gapdh | accacagtccatgccatcac | tccaccaccctgttgctgta |
Acknowledgments
This research was supported by the Research Fellowship for Young Scientists, from the Japan Society for the Promotion of Science (JSPS), and the Grant-in-aid for Scientific Research (No. 17360398) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Lee, K.H.; Chuang, C.K.; Guo, S.F.; Tu, C.F. Simple and efficient derivation of mouse embryonic stem cell lines using differentiation inhibitors and proliferation stimulators. Stem Cells Dev. 2011. in press. [Google Scholar]
- Cui, L.; Johkura, K.; Yue, F.; Ogiwara, N.; Okouchi, Y.; Asanuma, K.; Sasaki, K. Spatial distribution and initial changes of SSEA-1 and other cell adhesion-related molecules on mouse embryonic stem cells before and during differentiation. J. Histochem. Cytochem. 2004, 52, 1447–1457. [Google Scholar]
- Toyooka, Y.; Shimosato, D.; Murakami, K.; Takahashi, K.; Niwa, H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 2008, 135, 909–918. [Google Scholar]
- Hayashi, K.; Lopes, S.M.; Tang, F.; Surani, M.A. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 2008, 3, 391–401. [Google Scholar]
- Pelton, T.A.; Sharma, S.; Schulz, T.C.; Rathjen, J.; Rathjen, P.D. Transient pluripotent cell populations during primitive ectoderm formation: Correlation of in vivo and in vitro pluripotent cell development. J. Cell Sci. 2002, 115, 329–339. [Google Scholar]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar]
- Ruiz, S.A.; Chen, C.S. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 2008, 26, 2921–2927. [Google Scholar]
- Alberti, K.; Davey, R.E.; Onishi, K.; George, S.; Salchert, K.; Seib, F.P.; Bornhäuser, M.; Pompe, T.; Nagy, A.; Werner, C.; Zandstra, P.W. Functional immobilization of signaling proteins enables control of stem cell fate. Nat. Methods 2008, 5, 645–650. [Google Scholar]
- Nagaoka, M.; Koshimizu, U.; Yuasa, S.; Hattori, F.; Chen, H.; Tanaka, T.; Okabe, M.; Fukuda, K.; Akaike, T. E-cadherin-coated plates maintain pluripotent ES cells without colony formation. PLoS One 2006. [Google Scholar] [CrossRef]
- Doran, M.R.; Frith, J.E.; Prowse, A.B.; Fitzpatrick, J.; Wolvetang, E.J.; Munro, T.P.; Gray, P.P.; Cooper-White, J.J. Defined high protein content surfaces for stem cell culture. Biomaterials 2010, 31, 5137–5142. [Google Scholar]
- Lam, H.J.; Patel, S.; Wang, A.; Chu, J.; Li, S. In vitro regulation of neural differentiation and axon growth by growth factors and bioactive nanofibers. Tissue Eng. Part A 2010, 16, 2641–2648. [Google Scholar]
- Kim, M-H.; Kino-oka, M.; Taya, M. Designing culture surfaces based on cell anchoring mechanisms to regulate cell morphologies and functions. Biotechnol. Adv. 2010, 28, 7–16. [Google Scholar]
- Mashayekhan, S.; Kim, M.-H.; Miyazaki, S.; Tashiro, F.; Kino-oka, M.; Taya, M.; Miyazaki, J.-I. Enrichment of undifferentiated mouse embryonic stem cells on a culture surface with a glucose displaying dendrimer. Biomaterials 2008, 29, 4236–4243. [Google Scholar]
- Moritoh, Y.; Yamato, E.; Yasui, Y.; Miyazaki, S.; Miyazaki, J. Analysis of insulin producing cells during in vitro differentiation from feeder-free embryonic stem cells. Diabetes 2003, 52, 1163–1168. [Google Scholar]
- Nagaoka, M.; Hagiwara, Y.; Takemura, K.; Murakami, Y.; Li, J.; Duncan, S.A.; Akaike, T. Design of the artificial acellular feeder layer for the efficient propagation of mouse embryonic stem cells. J. Biol. Chem. 2008, 283, 26468–26476. [Google Scholar]
- Karnoub, A.E.; Symons, M.; Campbell, S.L.; Der, C.J. Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res. Treat. 2004, 84, 61–71. [Google Scholar]
- Harb, N.; Archer, T.K.; Sato, N. The Rho-Rock-myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS One 2008. [Google Scholar] [CrossRef]
- Hamazaki, T.; Oka, M.; Yamanaka, S.; Terada, N. Aggregation of embryonic stem cells induces Nanog repression and primitive endoderm differentiation. J. Cell Sci. 2004, 117, 5681–5686. [Google Scholar]
- Cormier, J.T.; Zur Nieden, N.I.; Rancourt, D.E.; Kallos, M.S. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006, 12, 3233–3245. [Google Scholar]
- Zur Nieden, N.I.; Cormier, J.T.; Rancourt, D.E.; Kallos, M.S. Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J. Biotechnol. 2007, 129, 421–432. [Google Scholar]
- Shi, W.; Wang, H.; Pan, G.; Geng, Y.; Guo, Y.; Pei, D. Regulation of the pluripotency marker Rex-1 by Nanog and Sox2. J. Biol. Chem. 2006, 281, 23319–23325. [Google Scholar]
- Kim, M-H.; Kino-oka, M.; Maruyama, N.; Saito, A.; Sawa, Y.; Taya, M. Cardiomyogenic induction of human mesenchymal stem cells by altered Rho family GTPase expression on dendrimer-immobilized surface with D-glucose display. Biomaterials 2010, 31, 7666–7677. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mashayekhan, S.; Kim, M.-H.; Kino-oka, M.; Miyazaki, J.-i.; Taya, M. Embryonic Stem Cells Maintain an Undifferentiated State on Dendrimer-Immobilized Surface with d-Glucose Display. Polymers 2011, 3, 2078-2087. https://doi.org/10.3390/polym3042078
Mashayekhan S, Kim M-H, Kino-oka M, Miyazaki J-i, Taya M. Embryonic Stem Cells Maintain an Undifferentiated State on Dendrimer-Immobilized Surface with d-Glucose Display. Polymers. 2011; 3(4):2078-2087. https://doi.org/10.3390/polym3042078
Chicago/Turabian StyleMashayekhan, Shohreh, Mee-Hae Kim, Masahiro Kino-oka, Jun-ichi Miyazaki, and Masahito Taya. 2011. "Embryonic Stem Cells Maintain an Undifferentiated State on Dendrimer-Immobilized Surface with d-Glucose Display" Polymers 3, no. 4: 2078-2087. https://doi.org/10.3390/polym3042078
APA StyleMashayekhan, S., Kim, M.-H., Kino-oka, M., Miyazaki, J.-i., & Taya, M. (2011). Embryonic Stem Cells Maintain an Undifferentiated State on Dendrimer-Immobilized Surface with d-Glucose Display. Polymers, 3(4), 2078-2087. https://doi.org/10.3390/polym3042078




