The Optimal Amount of PAMAM G3 Dendrimer in Polyurethane Matrices Makes Them a Promising Tool for Controlled Drug Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Foam Preparation
2.3. Microscopy
2.4. Density and Porosity Measurements
2.5. Rheological Measurements
2.6. Sterilization Process for PU–PAMAM Matrices and RF Foams
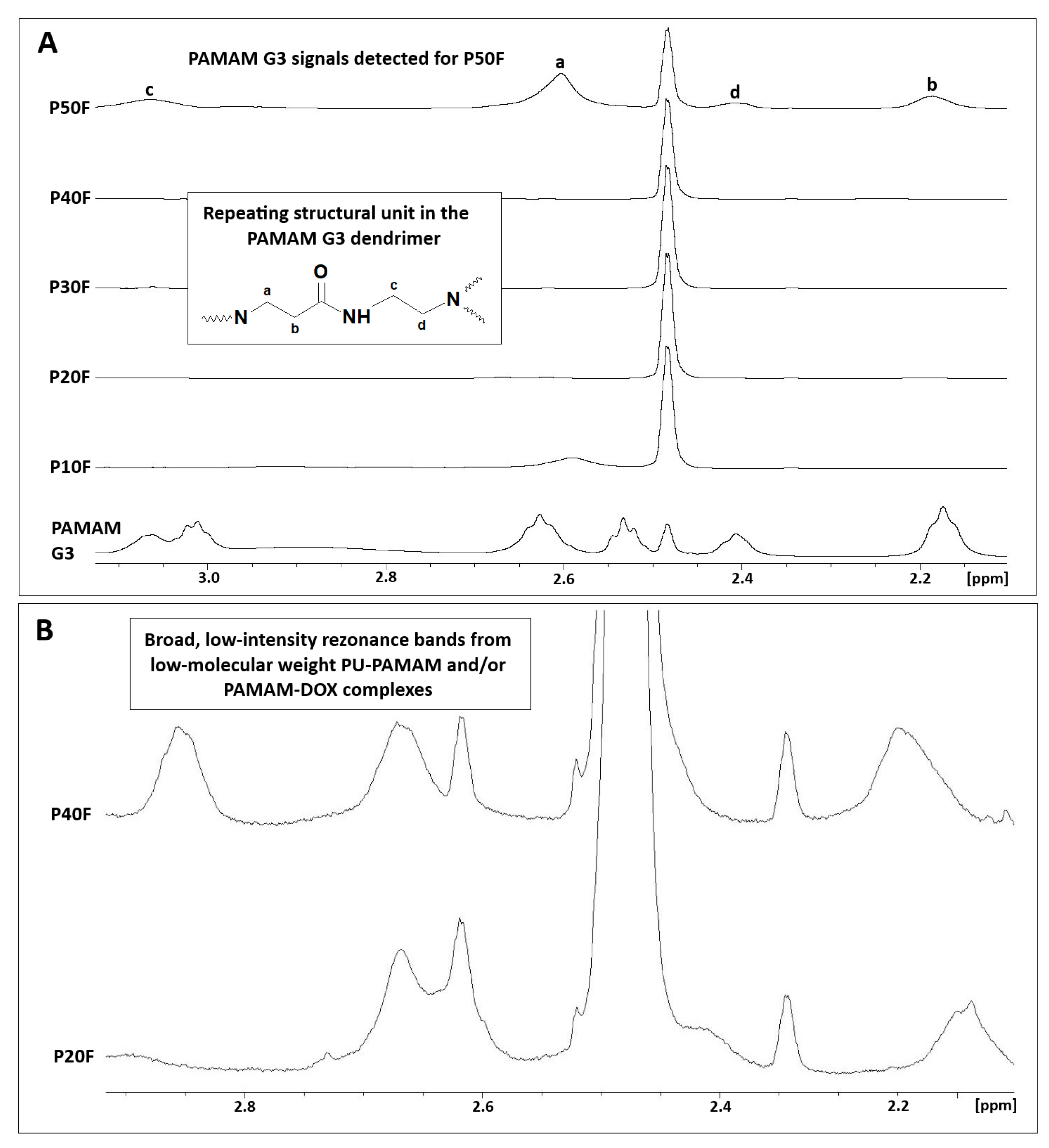
2.7. NMR Spectroscopy
2.8. Immobilization and Release of Doxorubicin from Matrices
2.8.1. DOX Encapsulation
2.8.2. DOX Release from Polymer Matrices
2.8.3. Quantitative Studies of DOX Encapsulation and Release Using HPLC with UV–Vis Detection
2.9. Cell Culture
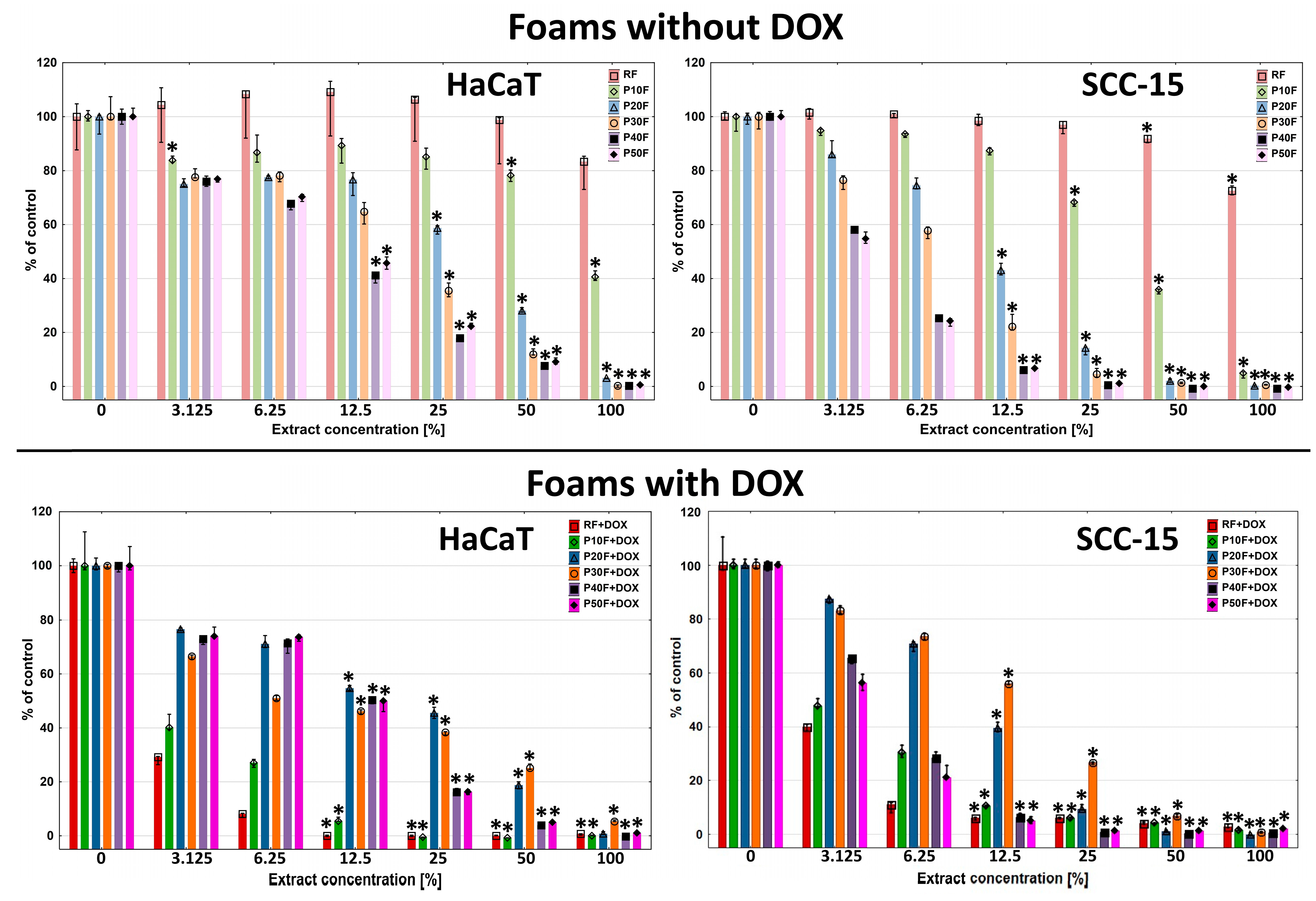
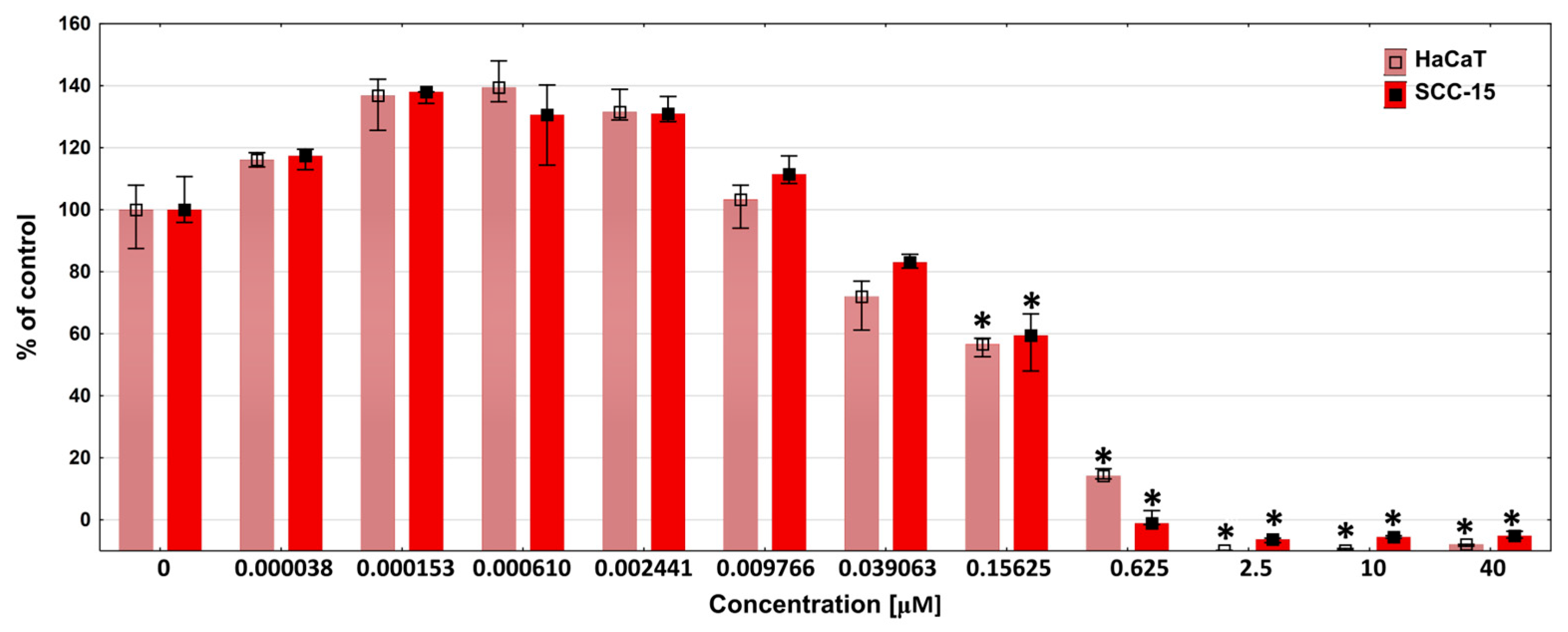
2.10. Extract and DOX Cytotoxicity Assay with Neutral Red (NR)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preparation of PU–PAMAM Matrices Containing 10, 20, 30, 40, or 50 Mass % of PAMAM G3 Dendrimer Nanoparticles
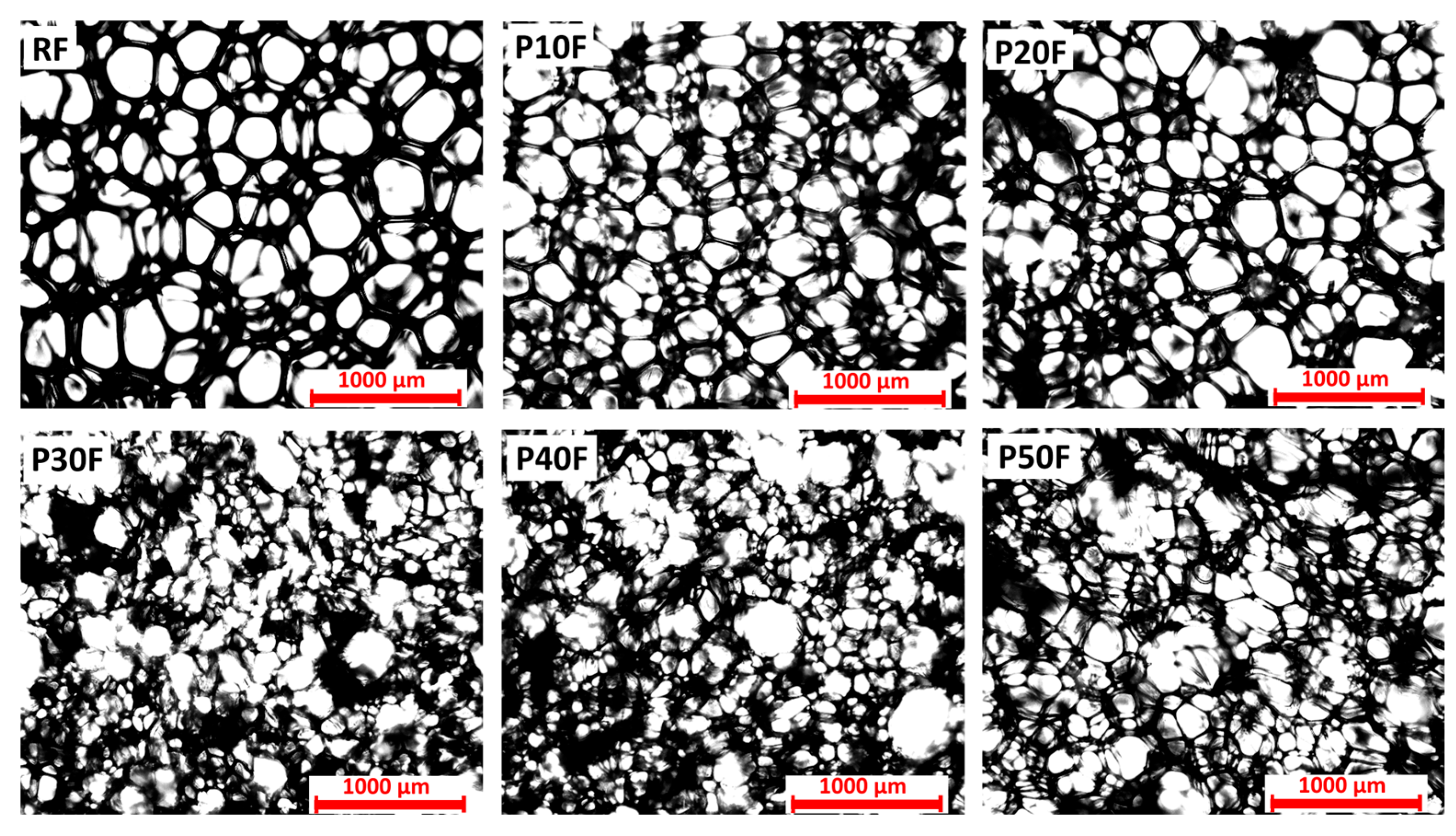
3.2. Structure of Obtained Matrices
3.3. Porosity of Foams
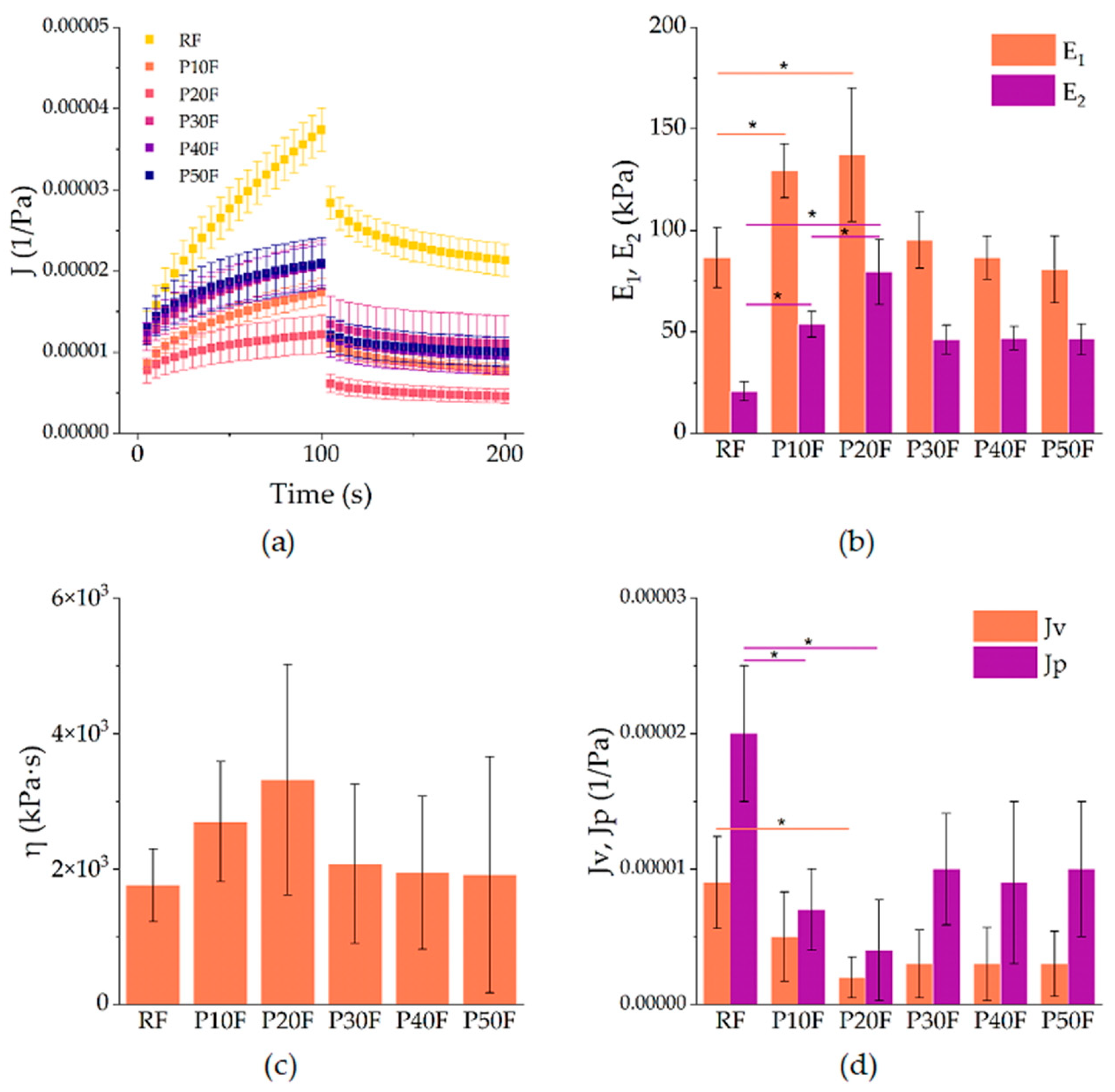
3.4. Creep–Recovery Behavior of PU–PAMAM Foams
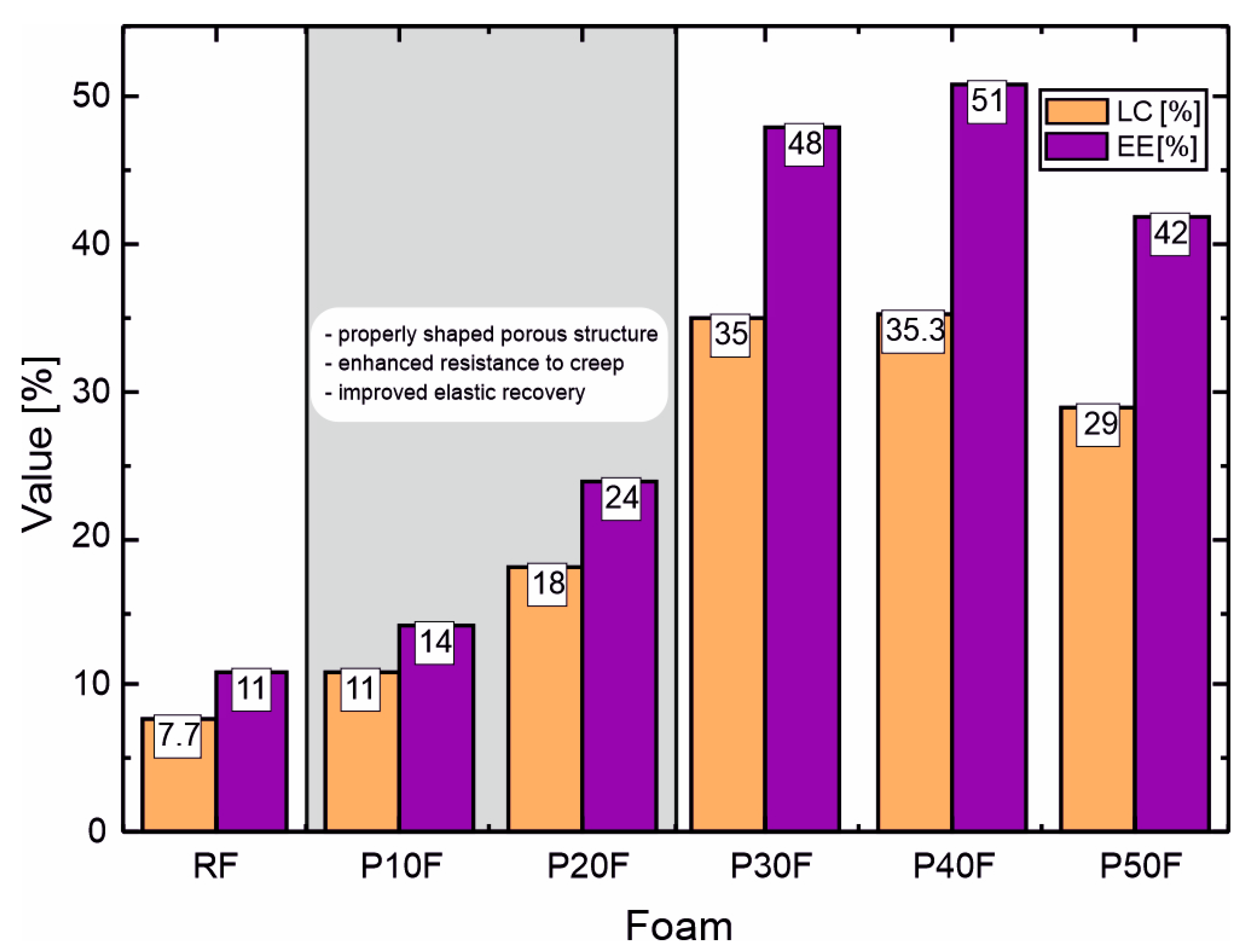
3.5. Calculation of DOX Loading Capacity and Encapsulation Efficiency
3.6. Correlation Between Structure, Rheological Behavior and Drug Loading Efficiency of PU–PAMAM Foams
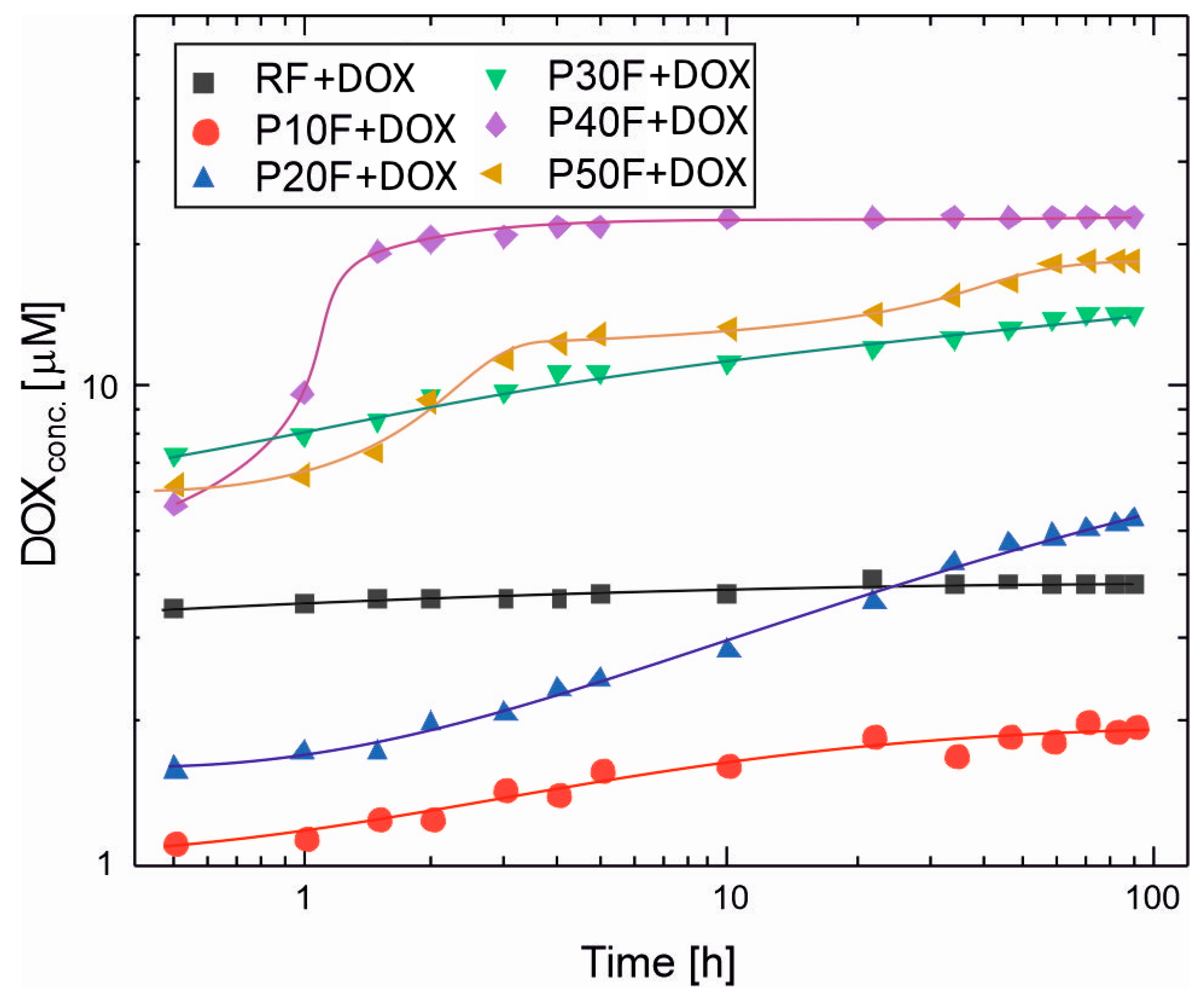
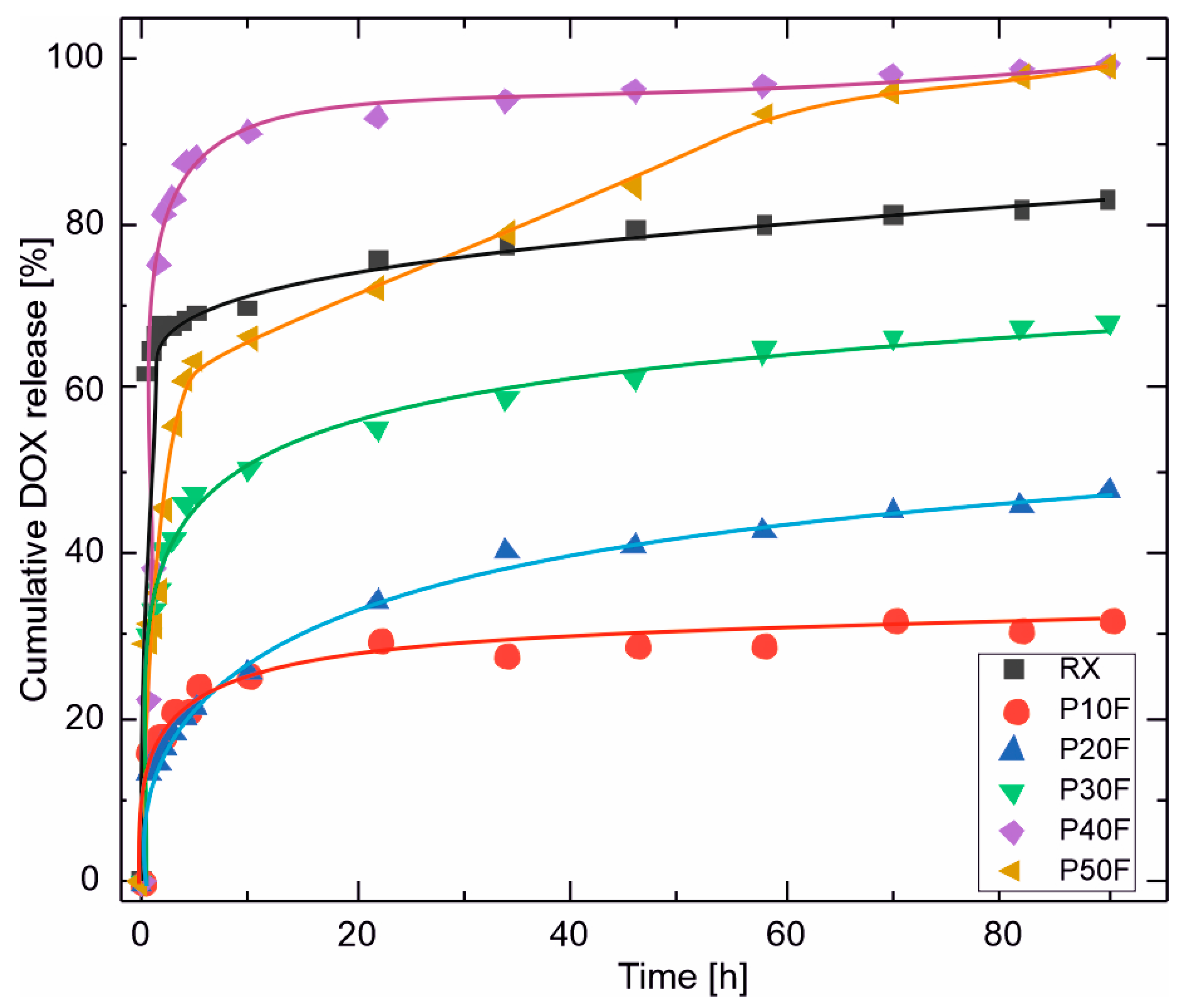
3.7. DOX Release Profiles
3.8. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dewangan, P.; Mourya, A.; Singh, P.K.; Chaudhary, M.; Sharma, R.; Bajwa, N.; Baldi, A.; Singh, K.K.; Singh, S.B.; Madan, J.; et al. Drug delivery: The conceptual perspectives and therapeutic applications. In Transdermal Drug Delivery: Concepts and Advances, 1st ed.; Saha, R.N., Vuddanda, P.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–28. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Gupta, L.; Sarita, R.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Prasada, P.; Upadhyayb, A.; Jayroniac, S.; Pathaka, S.; Tiwaria, A.; Mishrab, S. Advancements in minimally invasive transdermal drug delivery systems. Lett. Drug Des. Discov. 2025, 22, 100045. [Google Scholar] [CrossRef]
- Ghaferi, M.; Alavi, S.E.; Phan, K.; Maibach, H.; Mohammed, Y. Transdermal Drug Delivery Systems (TDDS): Recent Advances and Failure Modes. Mol. Pharm. 2024, 21, 5373–5391. [Google Scholar] [CrossRef]
- Chiarappa, G.; Abrami, M.; Farra, R.; Dapas, B.; Grassi, G.; Grassi, M. Chapter 11—Drug delivery from polymeric matrices. Comput. Aided Chem. Eng. 2018, 42, 325–356. [Google Scholar] [CrossRef]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Akanksha Kumari, A.; Mishra, N. Polymers in Drug Delivery. J. Biosci. Med. 2015, 4, 69–84. [Google Scholar] [CrossRef]
- Kandavilli, S.; Nair, V.; Panchagnula, R. Polymers in Transdermal Drug Delivery Systems. Pharm. Technol. 2002, 5, 62–80. [Google Scholar]
- Das, S.; Sarkar, P.; Majee, S.B. Polymers in Matrix Type Transdermal Patch. Int. J. Pharm. Sci. Rev. Res. 2022, 73, 77–86. [Google Scholar] [CrossRef]
- Kesarwani, A.; Yadav, A.K.; Singh, S.; Gautam, H.; Singh, H.N.; Sharma, A.; Yadav, C. Theoretical aspects of transdermal drug delivery system. Bull. Pharm. Res. 2013, 3, 78–89. [Google Scholar]
- Priyanka; Khan, R.; Sharma, B. A Review on Transdermal Patch. Int. J. Pharm. Res. Appl. 2025, 10, 518–529. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef]
- Tong, X.; Yang, F. Recent Progress in Developing Injectable Matrices for Enhancing Cell Delivery and Tissue Regeneration. Adv. Healthc. Mater. 2017, 7, 1701065. [Google Scholar] [CrossRef] [PubMed]
- Wienen, D.; Gries, T.; Cooper, S.L.; Heath, D.E. An overview of polyurethane biomaterials and their use in drug delivery. J. Control. Release 2023, 363, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Paswan, K.K.; Kumar, A.; Gupta, V.; Sonker, M.; Khan, M.A.; Kumar, A.; Shreyash, N. Recent Advancements in Polyurethane-based Tissue Engineering. ACS Appl. Bio Mater. 2023, 6, 327–348. [Google Scholar] [CrossRef]
- Bin Kamal, U.; Bakhtawar, M.; Haris, M.; Shehzadi, I.; Majeed, T.; Khan, M.A.; Noeid, S.; Ali, A. Polyurethane nanoparticles and injectable hydrogels: A synergistic approach to enhanced drug delivery systems. Eur. Chem. Bull. 2022, 11, 1185–1205. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Moustafa, A.-E.A.; Benter, I.F.; Akhtar, S. Emerging innate biological properties of nano-drug delivery systems: A focus on PAMAM dendrimers and their clinical potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Dehkordi, A.A.; Mollazadeh, S.; Talaie, A.; Yazdimamaghani, M. Engineering PAMAM dendrimers for optimized drug delivery. Nano Trends 2025, 9, 100094. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Huang, B.; Swanson, D.R.; Brothers II, H.M.; Klimash, J.W. Structure control within poly(amidoamine) dendrimers: Size, shape and regiochemical mimicry of globular proteins. Tetrahedron 2003, 59, 3799–3813. [Google Scholar] [CrossRef]
- Zhong, T.; Ai, P.; Zhou, J. Structures and properties of PAMAM dendrimer: A multi-scale simulation study. Fluid Ph. Equilib. 2011, 302, 43–47. [Google Scholar] [CrossRef]
- Kharwade, R.; More, S.; Warokar, A.; Agrawal, P.; Mahajan, N. Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications. Arab. J. Chem. 2020, 13, 6009–6039. [Google Scholar] [CrossRef]
- Sadhu, P.; Kumari, M.; Rathod, F.; Shah, N.; Patel, S. A Review on Poly(amidoamine) Dendrimers: Properties, Synthesis, and Characterization Prospects. Arch. Pharm. Pract. 2022, 13, 1–6. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, M.; Xu, T. Potential of poly(amidoamine) dendrimers as drug carriers of camptothecin based on encapsulation studies. Eur. J. Med. Chem. 2008, 43, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ta, W.; Hua, R.; Song, J.; Lu, W. A Review on Increasing the Targeting of PAMAM as Carriers in Glioma Therapy. Biomedicines 2022, 10, 2455. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Araújo, R.V.D.; Santos, S.D.S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef]
- Zaręba, M.; Chmiel-Szukiewicz, E.; Szpiłyk, M.; Uram, Ł. Sposób otrzymywania materiału porowatego oraz zastosowanie tego materiału porowatego. Poland patent PL247079B1, 2025. [Google Scholar]
- Arima, H.; Motoyama, K. Recent Findings Concerning PAMAM Dendrimer Conjugates with Cyclodextrins as Carriers of DNA and RNA. Sensors 2009, 9, 6346–6361. [Google Scholar] [CrossRef]
- Shao, N.; Su, Y.; Hu, J.; Zhang, J.; Zhang, H.; Cheng, Y. Comparison of generation 3 polyamidoamine dendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, release behavior, and cytotoxicity. Int. J. Nanomed. 2011, 6, 3361–3372. [Google Scholar] [CrossRef]
- Ke, W.; Zhao, Y.; Huang, R.; Jiang, C.h.; Pei, Y. Enhanced Oral Bioavailability of Doxorubicin in a Dendrimer Drug Delivery System. J. Pharm. Sci. 2008, 97, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Micallef, I.; Baron, B. Doxorubicin: An Overview of the Anti-Cancer and Chemoresistance Mechanisms. Ann. Clin. Toxicol. 2020, 3, 1031. [Google Scholar]
- D’Angelo, N.A.; Noronha, M.A.; Câmara, M.C.C.; Kurnik, I.S.; Feng, C.; Araujo, V.H.S.; Santos, J.H.P.M.; Feitosa, V.; Molino, J.V.D.; Rangel-Yagui, C.O.; et al. Doxorubicin nanoformulations on therapy against cancer: An overview from the last 10 years. Biomater. Adv. 2022, 133, 112623. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Babunagappan, K.V.; Seetharaman, A.; Ariraman, S.; Santhosh, P.B.; Genova, J.; Ulrih, N.P.; Sudhakar, S. Doxorubicin loaded thermostable nanoarchaeosomes: A next-generation drug carrier for breast cancer therapeutics. Nanoscale Adv. 2024, 6, 2026–2037. [Google Scholar] [CrossRef]
- Agudelo, D.; Bérubé, G.; Tajmir-Riahi, H.A. An overview on the delivery of antitumor drug doxorubicin by carrier proteins. Int. J. Biol. Macromol. 2016, 88, 354–360. [Google Scholar] [CrossRef]
- Zaręba, M.; Chmiel-Szukiewicz, E.; Uram, Ł.; Noga, J.; Rzepna, M.; Wołowiec, S. A Novel PAMAM G3 Dendrimer-Based Foam with PolyetherPolyol and Castor Oil Components as Drug Delivery System intoCancer and Normal Cells. Materials 2024, 17, 3905. [Google Scholar] [CrossRef]
- Uram, Ł.; Szuster, M.; Gargasz, K.; Filipowicz, A.; Wałajtys-Rode, E.; Wołowiec, S. In vitro cytotoxicity of the ternary PAMAM G3–pyridoxal–biotin bioconjugate. Int. J. Nanomed. 2013, 8, 4707–4720. [Google Scholar] [CrossRef]
- UNE EN ISO 845:2010; Cellular Plastics and Rubbers-Determination of Apparent Density (ISO 845:2006). International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.en-standard.eu/une-en-iso-845-2010-cellular-plastics-and-rubbers-determination-of-apparent-density-iso-845-2006 (accessed on 8 March 2025).
- Rampf, M.; Speck, O.; Speck, T.; Luchsinger, R.H. Structural and mechanical properties of flexible polyurethane foams cured under pressure. J. Cell. Plast. 2011, 48, 53–69. [Google Scholar] [CrossRef]
- ASTM International. ASTM 51631-20e1 Standard Practice for Use of Calorimetric Dosimetry Systems for Dose Measurements and Routine Dosimetry System Calibration in Electron Beams; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- European Medicines Agency. Guideline on the Sterilisation of the Medicinal Product, Active Substance, Excipient and Primary Container; EMA/CHMP/CVMP/QWP/850374/2015; European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Briggs, S.T.; Monroe, M.B.B.; Wierzbicki, M.A.; Hasan, S.M.; Maitland, D.J. Influence of Aging, Sterilization, and Composition on the Degradation of Polyurethane Foams. Recent Prog. Mater. 2021, 3, 025. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Giraldo-Gomez, D.M.; Figueroa-Gonzalez, G.; Reyes-Hernandez, O.D.; González-Del Carmen, M.; González-Torres, M.; Cortés, H.; Leyva-Gómez, G. Insights into Terminal Sterilization Processes of Nanoparticles for Biomedical Applications. Molecules 2021, 26, 2068. [Google Scholar] [CrossRef]
- Magill, E.; Demartis, S.; Gavini, E.; Permana, A.D.; Thakur, R.R.S.; Adrianto, M.F.; Waite, D.; Glover, K.; Picco, C.J.; Korelidou, A.; et al. Solid implantable devices for sustained drug delivery. Adv. Drug Deliv. Rev. 2023, 199, 114950. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Hajare, A.A.; Powar, T.A.; Bhatia, N.M.; More, H.N. Development and Validation of RP HPLC Method for Determination of Doxorubicin Hydrochloride from Vacuum Foam Dried Formulation. Res. J. Pharm. Technol. 2017, 9, 1352–1356. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- Garms, B.C.; Poli, H.; Baggley, D.; Han, F.Y.; Whittaker, A.K.; Anitha, A.; Grøndahl, L. Evaluating the effect of synthesis, isolation, and characterisation variables on reported particle size and dispersity of drug loaded PLGA nanoparticles. Mater Adv. 2021, 2, 5657–5671. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; McEnery, M.; Guelcher, S.A. Polyurethanes for Bone Tissue Engineering. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 481–501. [Google Scholar] [CrossRef]
- Shah, M.; Bourner, L.; Ali, S.; Al-Enazy, S.; Youssef, M.M.; Fisler, M.; Rytting, E. HPLC Method Development for Quantification of Doxorubicin in Cell Culture and Placental Perfusion Media. Separations 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, C.; Wu, Y.; Cheng, C.-Y.; Xiac, W.; Zhang, Z. Combination delivery of Adjudin and Doxorubicin via integrating drug conjugation and nanocarrier approaches for the treatment of drug-resistant cancer cells. J. Mater. Chem. B 2015, 3, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y. Dimerization of Doxorubicin Causes Its Precipitation. ACS Omega 2020, 51, 33235–33241. [Google Scholar] [CrossRef]
- Szota, M.; Jachimska, B. Effect of Alkaline Conditions on Forming an Effective G4.0 PAMAM Complex with Doxorubicin. Pharmaceutics 2023, 15, 875. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Lu, W.; Jia, X. A poly(amidoamine) dendrimer-based drug carrier for delivering DOX to gliomas cells. RSC Adv. 2017, 7, 15475–15481. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- Chittasupho, C.; Angklomklew, J.; Thongnopkoon, T.; Senavongse, W.; Jantrawut, W.; Ruksiriwanich, W. Biopolymer Hydrogel Scaffolds Containing Doxorubicin as ALocalized Drug Delivery System for Inhibiting Lung Cancer Cell Proliferation. Polymers 2021, 13, 3580. [Google Scholar] [CrossRef]
- Lee, J.H.; Tsubota, H.; Tachibana, T. Controllable Drug Release Ratio and Rate of Doxorubicin Loaded Natural Composite Films Based on Polysaccharides: Evaluation of Transdermal Permeability Potential. ACS Omega 2023, 9, 1936–1944. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Xu, J.; Lai, Y.; Xu, R.; Li, J.; Shen, J.-V.; Chen, J.X. pH sensitive loading and release of doxorubicin by chitosan-graphene quantum dots hybridized material. Int. J. Biol. Macromol. 2025, 319, 145375. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Lo, T.-S.; Chien, Y.H.; Kuo, Y.-H.; Liu, S.H. In Vitro and In Vivo Drug Release from a Nano-Hydroxyapatite Reinforced Resorbable Nanofibrous Scaffold for Treating Female Pelvic Organ Prolapse. Polymers 2024, 16, 1667. [Google Scholar] [CrossRef] [PubMed]
- Eltahir, S.; Al homsi, R.; Jagal, J.; Ahmed, I.S.; Haider, M. Graphene Oxide/Chitosan Injectable Composite Hydrogel for Controlled Release of Doxorubicin: An Approach for Enhanced Intratumoral Delivery. Nanomaterials 2022, 12, 4261. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Wychowaniec, J.K.; Castillo Díaz, L.A.; Smith, A.M.; Miller, A.F.; Saiani, A. Controlling Doxorubicin Release from a Peptide Hydrogel through Fine-Tuning of Drug-Peptide Fiber Interactions. Biomolecules 2022, 13, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Weiyuan, J.K. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between Direct Contact and Extract Exposure Methods for PFO Cytotoxicity Evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef]
- Safety and Toxicity Issues of Dendrimers. In Dendrimer-Based Nanotherapeutics; Academic Press: Amsterdam, The Netherlands, 2021; pp. 143–162.
- Szota, M.; Szwedowicz, U.; Rembialkowska, N.; Janicka-Klos, A.; Doveiko, D.; Chen, Y.; Kulbacka, J.; Jachimska, B. Dendrimer Platforms for Targeted Doxorubicin Delivery—Physicochemical Properties in Context of Biological Responses. Int. J. Mol. Sci. 2024, 25, 7201. [Google Scholar] [CrossRef]
| Substrate Ratio [wt%] | Sample | Substrates | Foaming Process | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G3 [g] | G441 [g] | CO [g] | pMDI [g] | H2O [g] | Silicone [g] | TEA [g] | Cream Time [s] | Time of Expanding [s] | ||
| G3:G441:CO 50%:35%:15% | P50F | 2.0 | 1.4 | 0.6 | 3.57 | 0.16 | 0.16 | 0.068 | 15 | 125 |
| G3:G441:CO 40%:45%:15% | P40F | 1.6 | 1.8 | 0.6 | 3.64 | 0.16 | 0.16 | 0.068 | 14 | 120 |
| G3:G441:CO 30%:55%:15% | P30F | 1.2 | 2.2 | 0.6 | 3.71 | 0.16 | 0.16 | 0.051 | 13 | 100 |
| G3:G441:CO 20%:65%:15% | P20F | 0.8 | 2.6 | 0.6 | 3.77 | 0.16 | 0.16 | 0.034 | 12 | 50 |
| G3:G441:CO 10%:75%:15% | P10F | 0.4 | 3.0 | 0.6 | 3.84 | 0.16 | 0.16 | 0.034 | 11 | 47 |
| G441:CO 85%:15% | RF | - | 3.4 | 0.6 | 3.91 | 0.16 | 0.16 | 0.034 | 10 | 40 |
| Foam | RF | P10F | P20F | P30F | P40F | P50F |
|---|---|---|---|---|---|---|
| Apparent density [kg/m3] | 54.5 | 33.2 | 33.8 | 34.7 | 35.3 | 35.7 |
| Porosity [%] | 95.45 | 97.23 | 97.18 | 97.10 | 97.05 | 97.02 |
| Foam | RF | P10F | P20F | P30F | P40F | P50F |
|---|---|---|---|---|---|---|
| LC [%] | 7.7 | 11.0 | 17.9 | 35.0 | 35.3 | 29.1 |
| EE [%] | 11 | 14 | 24 | 48 | 51 | 42 |
| Foam | RF | P10F | P20F | P30F | P40F | P50F |
|---|---|---|---|---|---|---|
| n [-] | 0.05 | 0.14 | 0.27 | 0.15 | 0.19 | 0.21 |
| R2 [-] | 0.998 | 0.989 | 0.997 | 0.997 | 0.861 | 0.984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zaręba, M.; Twardowska, M.Z.; Błoniarz, P.; Lechowicz, J.B.; Czechowicz, J.; Łysik, D.; Rzepna, M.; Uram, Ł.S. The Optimal Amount of PAMAM G3 Dendrimer in Polyurethane Matrices Makes Them a Promising Tool for Controlled Drug Release. Polymers 2026, 18, 135. https://doi.org/10.3390/polym18010135
Zaręba M, Twardowska MZ, Błoniarz P, Lechowicz JB, Czechowicz J, Łysik D, Rzepna M, Uram ŁS. The Optimal Amount of PAMAM G3 Dendrimer in Polyurethane Matrices Makes Them a Promising Tool for Controlled Drug Release. Polymers. 2026; 18(1):135. https://doi.org/10.3390/polym18010135
Chicago/Turabian StyleZaręba, Magdalena, Magdalena Zuzanna Twardowska, Paweł Błoniarz, Jaromir B. Lechowicz, Jakub Czechowicz, Dawid Łysik, Magdalena Rzepna, and Łukasz Stanisław Uram. 2026. "The Optimal Amount of PAMAM G3 Dendrimer in Polyurethane Matrices Makes Them a Promising Tool for Controlled Drug Release" Polymers 18, no. 1: 135. https://doi.org/10.3390/polym18010135
APA StyleZaręba, M., Twardowska, M. Z., Błoniarz, P., Lechowicz, J. B., Czechowicz, J., Łysik, D., Rzepna, M., & Uram, Ł. S. (2026). The Optimal Amount of PAMAM G3 Dendrimer in Polyurethane Matrices Makes Them a Promising Tool for Controlled Drug Release. Polymers, 18(1), 135. https://doi.org/10.3390/polym18010135







