Effect of Drying Methods on the Morphological and Functional Properties of Cellulose Ester Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cellulose Transesterification
2.1.2. Solvent Casting of Cellulose Films
2.2. Characterisation
3. Results
3.1. X-Ray Diffraction Analysis (XRD)
3.2. Thermogravimetric Analysis (TGA)
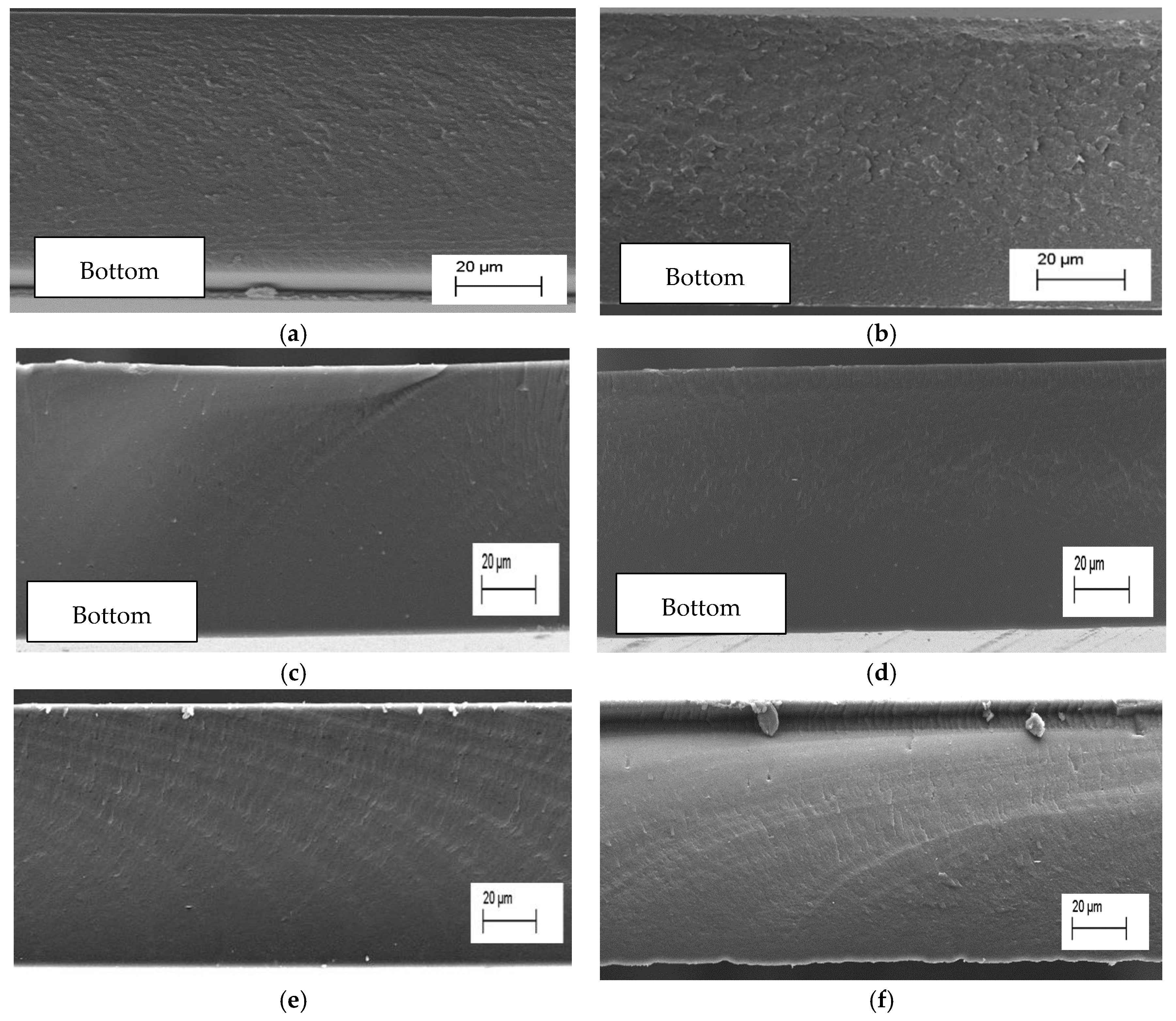
3.3. Scanning Electron Microscopy (SEM)
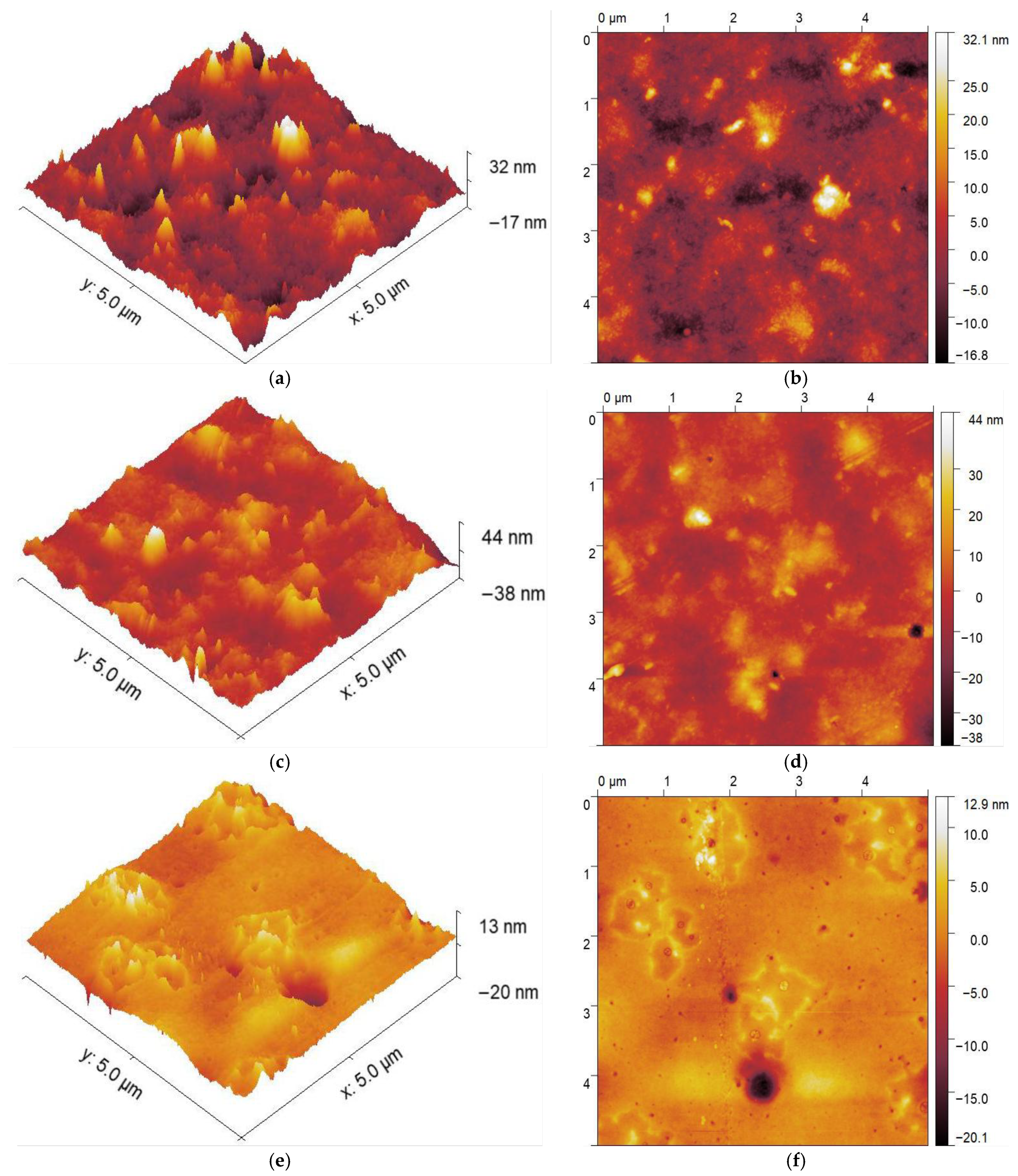
3.4. Atomic Force Microscopy (AFM)
3.5. Contact Angle Measurement
3.6. Correlation of Cellulose Ester Films’ Surface Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Sacripante, G.G.; Lawton, D.J.W.; Marway, H.S.; Thompson, M.R. Solvent-Free Modification of Lignocellulosic Wood Pulp into a Melt-Flowable Thermoplastic. Cellulose 2021, 28, 1055–1069. [Google Scholar] [CrossRef]
- Willberg-Keyriläinen, P.; Ropponen, J. Evaluation of Esterification Routes for Long Chain Cellulose Esters. Heliyon 2019, 5, e2898. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Voicu, S.I. Cellulose Acetate-Based Membranes for the Removal of Heavy Metals from Water in the Context of Circular Economy. Ind. Crops Prod. 2023, 206, 117716. [Google Scholar] [CrossRef]
- Kontturi, E.; Tammelin, T.; Österberg, M. Cellulose—Model Films and the Fundamental Approach. Chem. Soc. Rev. 2006, 35, 1287–1304. [Google Scholar] [CrossRef]
- Müller, M.; Czihak, C.; Schober, H.; Nishiyama, Y.; Vogl, G. All Disordered Regions of Native Cellulose Show Common Low-Frequency Dynamics. Macromolecules 2000, 33, 1834–1840. [Google Scholar] [CrossRef]
- Hummel, A. 3.2 Industrial Processes. Macromol. Symp. 2004, 208, 61–80. [Google Scholar] [CrossRef]
- Wu, S.; Qin, X.; Li, M. The Structure and Properties of Cellulose Acetate Materials: A Comparative Study on Electrospun Membranes and Casted Films. J. Ind. Text. 2014, 44, 85–98. [Google Scholar] [CrossRef]
- Lin, J.; Cai, Y.; Wang, X.; Ding, B.; Yu, J.; Wang, M. Fabrication of Biomimetic Superhydrophobic Surfaces Inspired by Lotus Leaf and Silver Ragwort Leaf. Nanoscale 2011, 3, 1258. [Google Scholar] [CrossRef]
- Simovich, T.; Wu, A.H.; Lamb, R.N. Hierarchically Rough, Mechanically Durable and Superhydrophobic Epoxy Coatings through Rapid Evaporation Spray Method. Thin Solid Film. 2015, 589, 472–478. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Han, L.; Tam, K.C. Superhydrophobic Surfaces from Sustainable Colloidal Systems. Curr. Opin. Colloid. Interface Sci. 2022, 57, 101534. [Google Scholar] [CrossRef]
- Li, A.; Wang, G.; Zhang, Y.; Zhang, J.; He, W.; Ren, S.; Xu, Z.; Wang, J.; Ma, Y. Preparation Methods and Research Progress of Superhydrophobic Paper. Coord. Chem. Rev. 2021, 449, 214207. [Google Scholar] [CrossRef]
- Lyytikäinen, J.; Morits, M.; Österberg, M.; Heiskanen, I.; Backfolk, K. Skin and Bubble Formation in Films Made of Methyl Nanocellulose, Hydrophobically Modified Ethyl (Hydroxyethyl) Cellulose and Microfibrillated Cellulose. Cellulose 2021, 28, 787–797. [Google Scholar] [CrossRef]
- Harini, K.; Sukumar, M. Development of Cellulose-Based Migratory and Nonmigratory Active Packaging Films. Carbohydr. Polym. 2019, 204, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Kallakas, H.; Kattamanchi, T.; Kilumets, C.; Tarasova, E.; Krasnou, I.; Savest, N.; Ahmadian, I.; Kers, J.; Krumme, A. Tensile and Surface Wettability Properties of the Solvent Cast Cellulose Fatty Acid Ester Films. Polymer 2023, 15, 2677. [Google Scholar] [CrossRef]
- ASTM D1795-13; Standard Test Method for Intrinsic Viscosity of Cellulose. ASTM International: West Conshohocken, PA, USA, 2013.
- Tarasova, E.; Savale, N.; Krasnou, I.; Kudrjašova, M.; Rjabovs, V.; Reile, I.; Vares, L.; Kallakas, H.; Kers, J.; Krumme, A. Preparation of Thermoplastic Cellulose Esters in [MTBNH][OAC] Ionic Liquid by Transesterification Reaction. Polymer 2023, 15, 3979. [Google Scholar] [CrossRef]
- Habibi, K.; Golshan Ebrahimi, N.; Jafari Nodoushan, E. Fast Solvent Evaporation by Experimental Optimization Using Central Composite Design for One-Step Fabrication of Superhydrophobic Polypropylene Surface. Iran. Polym. J. 2023, 32, 81–92. [Google Scholar] [CrossRef]
- Huang, K.; Xia, J.; Li, M.; Lian, J.; Yang, X.; Lin, G. Homogeneous Synthesis of Cellulose Stearates with Different Degrees of Substitution in Ionic Liquid 1-Butyl-3-Methylimidazolium Chloride. Carbohydr. Polym. 2011, 83, 1631–1635. [Google Scholar] [CrossRef]
- Tarasova, E.; Krasnou, I.; Enkhsaikhan, G.; Abousharabia, I.; Nunes, C.C.Z.; Karthegesu, D.; Savale, N.; Kontturi, E.; Krumme, A. Reactive Extrusion of Cellulose Esters in Ionic Liquid: Exploring Properties and Performance across Different Cellulose Types and Degrees of Polymerization. Cellulose 2024, 31, 10223–10240. [Google Scholar] [CrossRef]
- Kramar, A.; Rodríguez Ortega, I.; González-Gaitano, G.; González-Benito, J. Solution Casting of Cellulose Acetate Films: Influence of Surface Substrate and Humidity on Wettability, Morphology and Optical Properties. Cellulose 2023, 30, 2037–2052. [Google Scholar] [CrossRef]
- Li, Q.; Renneckar, S. Supramolecular Structure Characterization of Molecularly Thin Cellulose I Nanoparticles. Biomacromolecules 2011, 12, 650–659. [Google Scholar] [CrossRef]
- Fan, G.; Wang, M.; Liao, C.; Fang, T.; Li, J.; Zhou, R. Isolation of Cellulose from Rice Straw and Its Conversion into Cellulose Acetate Catalyzed by Phosphotungstic Acid. Carbohydr. Polym. 2013, 94, 71–76. [Google Scholar] [CrossRef]
- Duchatel-Crépy, L.; Joly, N.; Martin, P.; Marin, A.; Tahon, J.-F.; Lefebvre, J.-M.; Gaucher, V. Substitution Degree and Fatty Chain Length Influence on Structure and Properties of Fatty Acid Cellulose Esters. Carbohydr. Polym. 2020, 234, 115912. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose Polymorphy, Crystallite Size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional Methods in Cellulose Functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Sassi, J.-F.; Chanzy, H. Ultrastructural Aspects of the Acetylation of Cellulose. Cellulose 1995, 2, 111–127. [Google Scholar] [CrossRef]
- Jandura, P.; Riedl, B.; Kokta, B.V. Thermal Degradation Behavior of Cellulose Fibers Partially Esterified with Some Long Chain Organic Acids. Polym. Degrad. Stab. 2000, 70, 387–394. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Belgacem, M.N.; Gandini, A. Controlled Heterogeneous Modification of Cellulose Fibers with Fatty Acids: Effect of Reaction Conditions on the Extent of Esterification and Fiber Properties. J. Appl. Polym. Sci. 2006, 100, 1093–1102. [Google Scholar] [CrossRef]
- Uschanov, P.; Johansson, L.-S.; Maunu, S.L.; Laine, J. Heterogeneous Modification of Various Celluloses with Fatty Acids. Cellulose 2011, 18, 393–404. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannia, J.; Pirsa, S.; Zandi, M. Heterogeneous Modification of Softwoods Cellulose Nanofibers with Oleic Acid: Effect of Reaction Time and Oleic Acid Concentration. Fibers Polym. 2015, 16, 1715–1722. [Google Scholar] [CrossRef]
- Sonia, A.; Priya Dasan, K. Chemical, Morphology and Thermal Evaluation of Cellulose Microfibers Obtained from Hibiscus Sabdariffa. Carbohydr. Polym. 2013, 92, 668–674. [Google Scholar] [CrossRef]
- Jebrane, M.; Terziev, N.; Heinmaa, I. Biobased and Sustainable Alternative Route to Long-Chain Cellulose Esters. Biomacromolecules 2017, 18, 498–504. [Google Scholar] [CrossRef]
- Kawano, T.; Iikubo, S.; Andou, Y. The Relationship between Crystal Structure and Mechanical Performance for Fabrication of Regenerated Cellulose Film through Coagulation Conditions. Polymer 2021, 13, 4450. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, H.; Hu, X. Atomic Force Microscopy of Cellulose Membranes Prepared from the N-methylmorpholine-N -oxide/Water Solvent System. J. Appl. Polym. Sci. 2002, 86, 3389–3395. [Google Scholar] [CrossRef]
- De Souza, N.C.; Silva, J.R.; Pereira-Da-Silva, M.A.; Raposo, M.; Faria, R.M.; Giacometti, J.A.; Oliveira, O.N. Dynamic Scale Theory for Characterizing Surface Morphology of Layer-by-Layer Films of Poly(o-Methoxyaniline). J. Nanosci. Nanotechnol. 2004, 4, 548–552. [Google Scholar] [CrossRef]
- Kohout, M.; Collier, A.P.; Stepanek, F. Vacuum Contact Drying Kinetics: An Experimental Parametric Study. Dry. Technol. 2005, 23, 1825–1839. [Google Scholar] [CrossRef]
- McLoughlin, C.M.; McMinn, W.A.M.; Magee, T.R.A. Microwave-Vacuum Drying of Pharmaceutical Powders. Dry. Technol. 2003, 21, 1719–1733. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.; Qi, H.J. Solvent Assisted Pressure-Free Surface Welding and Reprocessing of Malleable Epoxy Polymers. Macromolecules 2016, 49, 5527–5537. [Google Scholar] [CrossRef]
- Bando, H.; Mcginity, J. Physicochemical Properties of Enteric Films Prepared from Aqueous Dispersions and Organic Solutions. Int. J. Pharm. 2006, 313, 43–48. [Google Scholar] [CrossRef]
- Oliveira de Moraes, J.; Scheibe, A.S.; Augusto, B.; Carciofi, M.; Laurindo, J.B. Conductive Drying of Starch-Fiber Films Prepared by Tape Casting: Drying Rates and Film Properties. LWT—Food Sci. Technol. 2015, 64, 356–366. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Dias, C.R.; Norberta de Pinho, M. Atomic Force Microscopy of Dense and Asymmetric Cellulose-Based Membranes. J. Membr. Sci. 1999, 160, 235–242. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T.; Noh, S.H. Effect of Thickness of the PPO Membranes on the Surface Morphology. J. Membr. Sci. 1998, 145, 243–251. [Google Scholar] [CrossRef]
- Granstrom, J.; Roy, A.; Rowell, G.; Moon, J.S.; Jerkunica, E.; Heeger, A.J. Improvements in Barrier Performance of Perfluorinated Polymer Films through Suppression of Instability during Film Formation. Thin Solid Films 2010, 518, 3767–3771. [Google Scholar] [CrossRef]
- Kosaka, P.M.; Kawano, Y.; Salvadori, M.C.; Petri, D.F.S. Characterization of Ultrathin Films of Cellulose Esters. Cellulose 2005, 12, 351–359. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Das, D. Cellulose: A Fascinating Biopolymer for Hydrogel Synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef] [PubMed]
- Crépy, L.; Chaveriat, L.; Banoub, J.; Martin, P.; Joly, N. Synthesis of Cellulose Fatty Esters as Plastics—Influence of the Degree of Substitution and the Fatty Chain Length on Mechanical Properties. ChemSusChem 2009, 2, 165–170. [Google Scholar] [CrossRef] [PubMed]


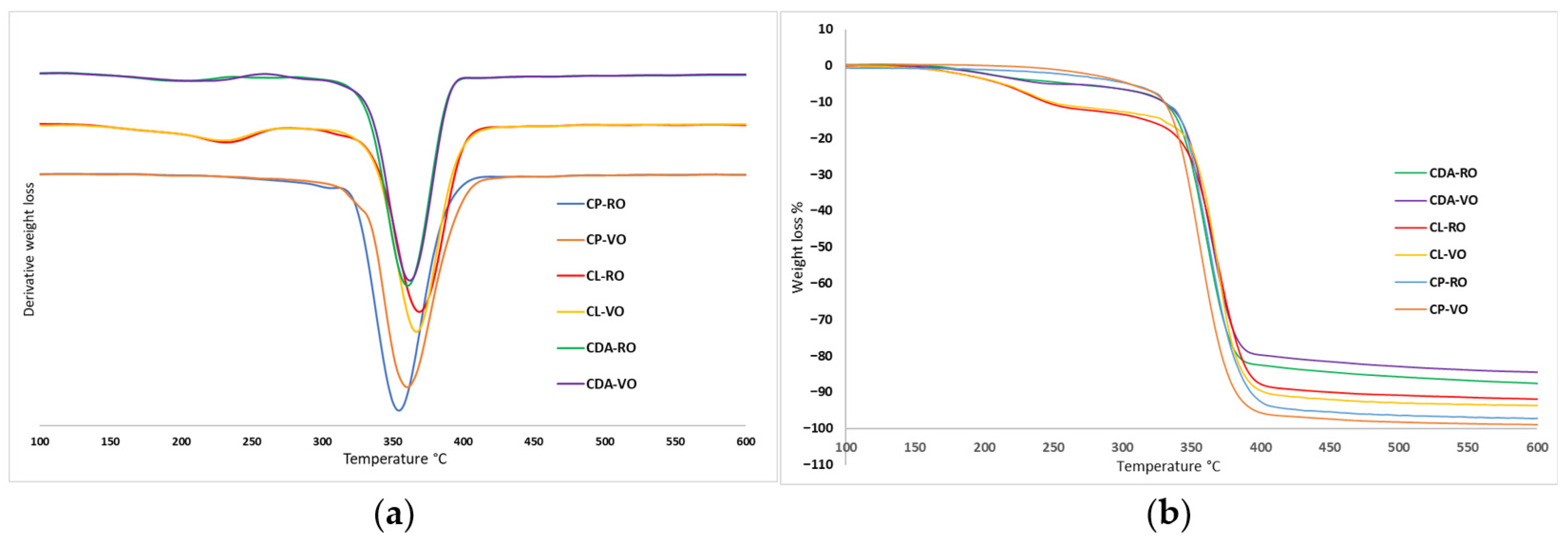


| Sample | T5% °C | Td1 °C | T50% °C | Td °C | Residue % | |
|---|---|---|---|---|---|---|
| CDA | RO | 263.2 | 201.1 | 362.4 | 360.8 | 12.2 |
| VO | 245.0 | 210.6 | 363.6 | 362.0 | 15.3 | |
| CL | RO | 212.5 | 232.0 | 366.7 | 369.2 | 7.9 |
| VO | 212.7 | 230.8 | 365.7 | 367.3 | 6.3 | |
| CP | RO | 303.4 | - | 362.2 | 360.6 | 2.6 |
| VO | 305.0 | - | 354.4 | 354.4 | 0.9 | |
| Sample | RMS Roughness (Sq) (nm) | Mean Roughness (Sa) (nm) | ||
|---|---|---|---|---|
| Drying Method | RO | VO | RO | VO |
| CDA | 8.12 (±1.9) | 6.55 (±0.3) | 5.83 (±1.3) | 4.71 (±0.1) |
| CL | 3.42 (±0.5) | 2.06 (±0.6) | 2.32 (±0.3) | 1.27 (±0.3) |
| CP | 3.09 (±0.4) | 2.14 (±0.3) | 2.30 (±0.3) | 1.66 (±0.2) |
| Ester | RO (°) | VO (°) |
|---|---|---|
| CDA | 80 (±5) | 85 (±8) |
| CL | 106 (±2) | 113 (±3) |
| CP | 121 (±2) | 124 (±3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kattamanchi, T.; Kallakas, H.; Tarasova, E.; Alao, P.F.; Kaljuvee, T.; Mere, A.; Katerski, A.; Lõhmus, R.; Krumme, A.; Kers, J. Effect of Drying Methods on the Morphological and Functional Properties of Cellulose Ester Films. Polymers 2025, 17, 3026. https://doi.org/10.3390/polym17223026
Kattamanchi T, Kallakas H, Tarasova E, Alao PF, Kaljuvee T, Mere A, Katerski A, Lõhmus R, Krumme A, Kers J. Effect of Drying Methods on the Morphological and Functional Properties of Cellulose Ester Films. Polymers. 2025; 17(22):3026. https://doi.org/10.3390/polym17223026
Chicago/Turabian StyleKattamanchi, Tanuj, Heikko Kallakas, Elvira Tarasova, Percy Festus Alao, Tiit Kaljuvee, Arvo Mere, Atanas Katerski, Rünno Lõhmus, Andres Krumme, and Jaan Kers. 2025. "Effect of Drying Methods on the Morphological and Functional Properties of Cellulose Ester Films" Polymers 17, no. 22: 3026. https://doi.org/10.3390/polym17223026
APA StyleKattamanchi, T., Kallakas, H., Tarasova, E., Alao, P. F., Kaljuvee, T., Mere, A., Katerski, A., Lõhmus, R., Krumme, A., & Kers, J. (2025). Effect of Drying Methods on the Morphological and Functional Properties of Cellulose Ester Films. Polymers, 17(22), 3026. https://doi.org/10.3390/polym17223026









