Advances in Digital Light Processing (DLP) Bioprinting: A Review of Biomaterials and Its Applications, Innovations, Challenges, and Future Perspectives
Abstract
1. Introduction
| Material Type | Specific Material | AM Technique | Application Areas |
|---|---|---|---|
| Ceramic Photopolymer resin [17] | ZrO2, Al2O3, SiC, Si3N4,hydroxyapatite (HA)/Hap, TiO2, Yttria-stabilized zirconia, PEGDA poly(ethylene glycol) diacrylate, PETA, HDDA, TMPTA, HEMA, IBOA | Photopolymerization (vat polymerization) |
|
| Bioceramics [18,19] | Biphasic calcium phosphate, Hidroksiapatit, β-Trikalsiyum Fosfat, HDDA, TPO, photosensitive resin | DLP |
|
| Polymer-based materials [20,21] | Poly L-lactic acid, trimethylolpropane trimethacrylate, poly (N-vinyl carbazole), TMPTMA, NMP | DLP |
|
| Photopolymer blends [22,23] | Craftsman Beige, Plant-Based Blue, Standard Clear, TMPTA, TEGDMA, DPGDA | DLP |
|
| Bioceramics [24,25] | LithaBone TCP300), hydroxyapatite, ZrO2, PEG600DMA, ACMO, ticari slurry (LithaSol 30, Lithoz GmbH), TPO, BAPO, BDMM, ITX) | DLP |
|
| Dental SG® reçinesi/Biyouyumlu reçineler [26,27,28] | Sheraprint SG 100, Dima Print Guide, NextDent C&B MFH (Micro-Filled Hybrid) | DLP/SLA/FDM |
|
| Bioceramics [29,30,31] | Hydroxyapatite, CaTiO3, graphene oxide, HDDA, HEMA, TMPTA | DLP |
|
| PEGDA, DMSO (dimethyl sulfoxide)TPO photoinitiatorEthylene glycol and tolüene [32,33] | DLP |
| |
| Bioceramics [31,34,35] | β-TCP (100 nm), hydroxyapatite (HA, 100–200 nm), HDDA, HEMA, TMPTA, Ticari UV-reçine (Anycubic 405 nm) | DLP |
|
| Polymer-based materials [36,37] | Dimethyl sulfoxide (DMSO), 2-butoxyethyl acetate (EGBEA), and 1:1 DMSO/EGBEA), and degree of PGSA acrylation | DLP |
|
| Bioceramics [38,39,40] | β-TCP (LithaBone TCP300), BCP (HA + β-TCP), hydroxyapatite (HA, P100), HDDA, PEGDA-400, urethane acrylate (Neorad U25-20D) | DLP |
|
| PEGDA hydrogel/LAP (lithium phenyl-2,4,6-trimethylbenzoylphosphinate (photoinitiator) [41,42] | DLP |
| |
| Photopolymer blends [43,44] | BGB Ultra™, 100–150 nm, Blue Goose Biorefineries, Nano-PCC (CaCO3, 40 nm), Nanoclay (Cloisite 20A), hydroxyapatite (HA), calcium phosphate cements, Bioglass®, ZrO2, fluorcanasite glass ceramics | DLP |
|
| Resin/PLA/VeroWhitePlus™ [45,46] | DLP, FDM, PolyJet |
| |
| Smart materials and hydrogels [47,48] | GelMA, GelGMA, Silk-MA, Silk-GMA, HAMA, HAGM, PEGDA-200/400/700 | DLP |
|
| Nanocomposite hydrogels [49,50,51] | Polyurethane acrylate (PUA), ZnO nanopartiküller (0.5–2 wt. %), PEGDA, GelMA, barium titanate/hydroxyapatite, DMSO, ethylene glycol, toluene | DLP/SLA/FFF |
|
| Smart materials and hydrogels [52,53] | MPA, OPBI (aromatic heterochain polymers), PCL-based LCE with Sm-A liquid crystal moieties | DLP |
|
| ABA triblok kopolimer/Trimetilolpropan [54] | DLP |
| |
| GelMA bioink/LAP [55,56] | DLP |
| |
| GelMA bioink/LAP/oxygen-sensitive LiNc-BuO crystals [57,58] | DLP |
| |
| Bioceramics/Bioglass [59,60,61] | HA + β-TCP + 45S5 Bioglass® (20 wt%), HDDA + TPGDA/β-TCP + 58S Bioglass (20 wt%), PEGDA + 3,3-dimethacrylate + polypropylene glycol | DLP |
|
| Acrylamide-based hydrogel/PEGDA and MXene nanosheet [62,63] | DLP |
| |
| Polymer-based materials [64,65] | DEGDA + PEG + PEG-g-BN, PEGDMA, PCLMA, PU-acrylate | Photopolymerization (vat polymerization) |
|
2. Technological Fundamentals and Biomedical Applications of DLP
2.1. Case Studies on the Development of Porous Scaffolds for Bone Tissue Engineering
2.2. Case Studies on the Utilization of Natural Reinforcements and Advanced Biomaterials
2.3. Case Studies on Hydrogel Systems and Smart Materials via DLP Printing
2.4. Case Studies on Direct Bioprinting with Cell-Containing Bioinks Using DLP
| Study | Bioink | Cell Type | Cell Viability (%) | Light Source | Application |
|---|---|---|---|---|---|
| Su et al. (2021) [96] | GelMA + Si-HAp | MG63, hBMSCs | >90% | Visible light (maskless DLP) | Bone regeneration |
| Zhu et al. (2017) [97] | GelMA | Endothelial + support cells | High (qualitative) | mCOB (optical bioprinting) | Pre-vascularized tissue fabrication |
| Duong & Lin (2023) [98] | GelNB + PEG4SH + QK peptide | HUVEC | >90% | DLP (visible light) | Vascularized scaffold printing |
| Choi et al. (2023) [99] | Gel-GMA + Silk-GMA | Keratinocyte, fibroblast, endothelial | High (after 4-week culture) | DLP (405 nm LED) | Artificial skin and wound healing |
| He et al. (2024) [100] | CSMA + CoLMA | Keratinocyte, fibroblast | Good proliferation | DLP (blue light) | Biomimetic skin engineering |
| Tilton et al. (2023) [101] | GelMA + PEGDA | Human cells (unspecified) | >72% (post-injection) | Visible light (DLP) | Injectable regenerative scaffold |
| Xie et al. (2022) [102] | GelMA + CAM microtissue | Chondrocytes (from microtia) | 92–98% (over 20 days) | DLP (14 mW/cm2, 405 nm) | Auricular cartilage regeneration |
| Chang et al. (2022) [103] | GelMA + PEGDMA | Human fibroblasts | High (after 5-day culture) | DLP (25 µm layers, 5–8 s/layer) | Microchannel-based soft tissue engineering |
3. The Role of the DLP Method in the Production of Smart Materials
4. Integration of Artificial Intelligence and Machine Learning into DLP-Based Biomanufacturing Processes
5. Production of Functional Biological Structures with DLP Technology: Applications in Literature and Technical Challenges
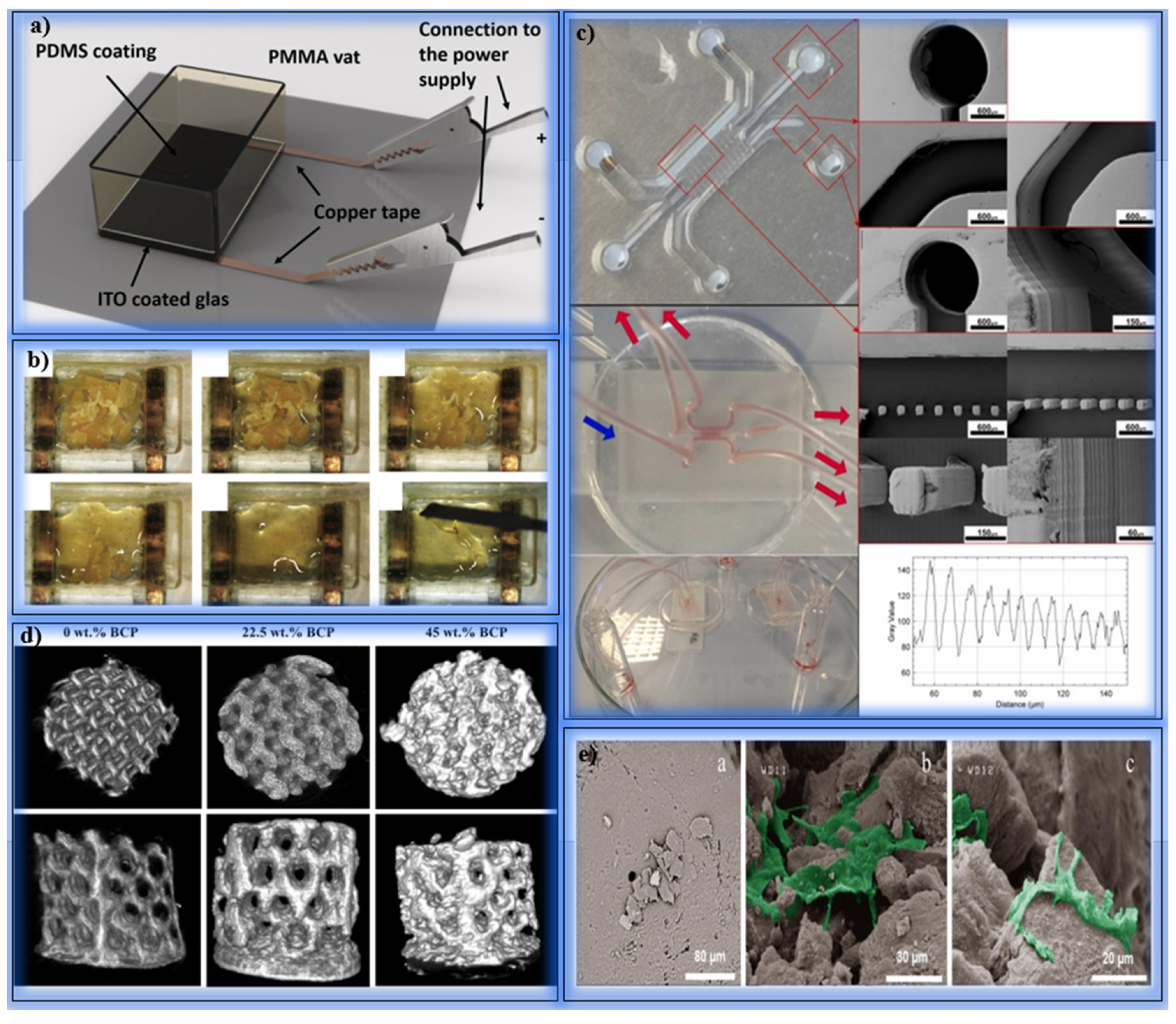
5.1. Drug Delivery and Microinjection Systems
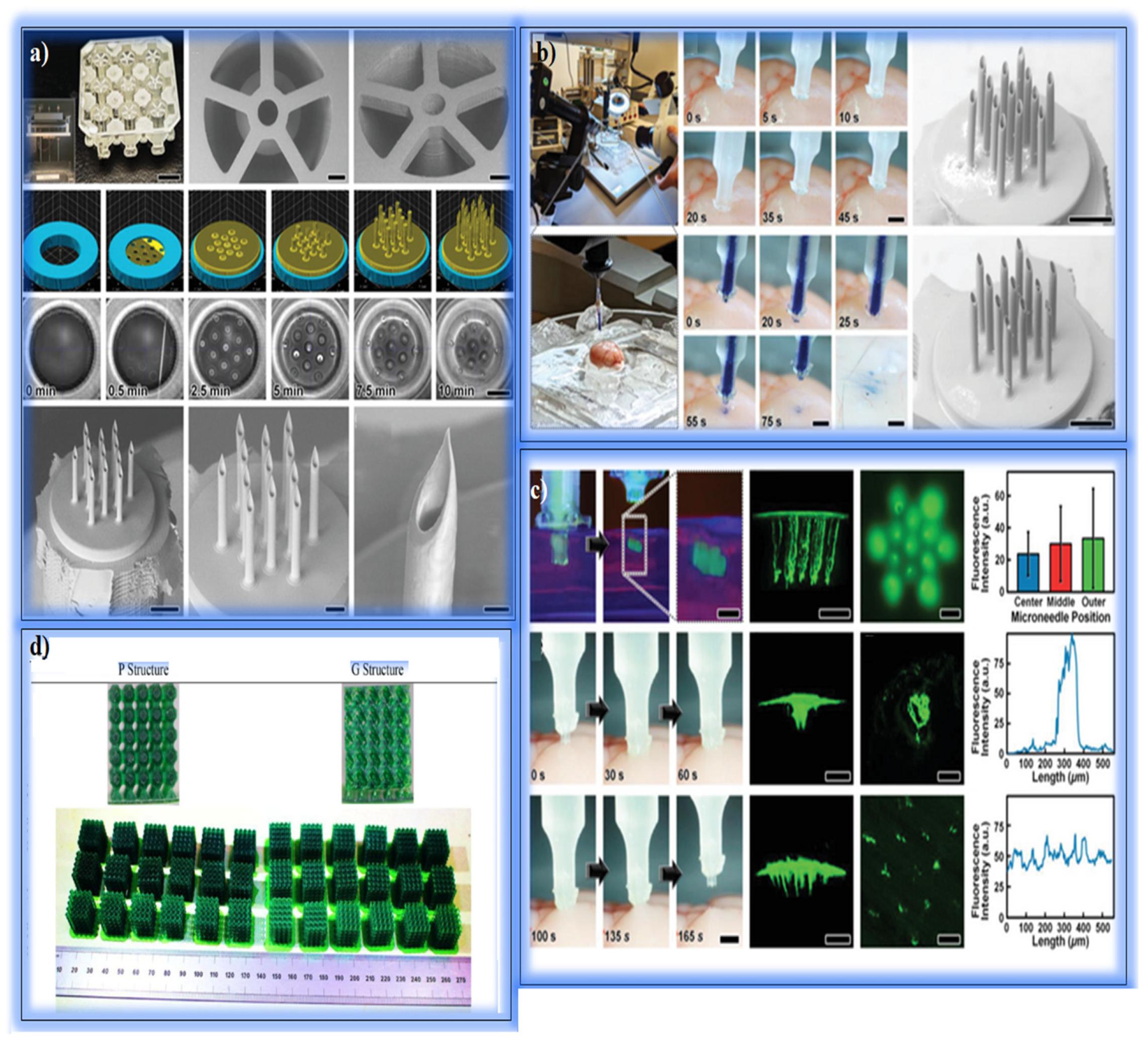
5.2. Soft Tissue and Skin Applications
5.3. Bone Tissue Engineering
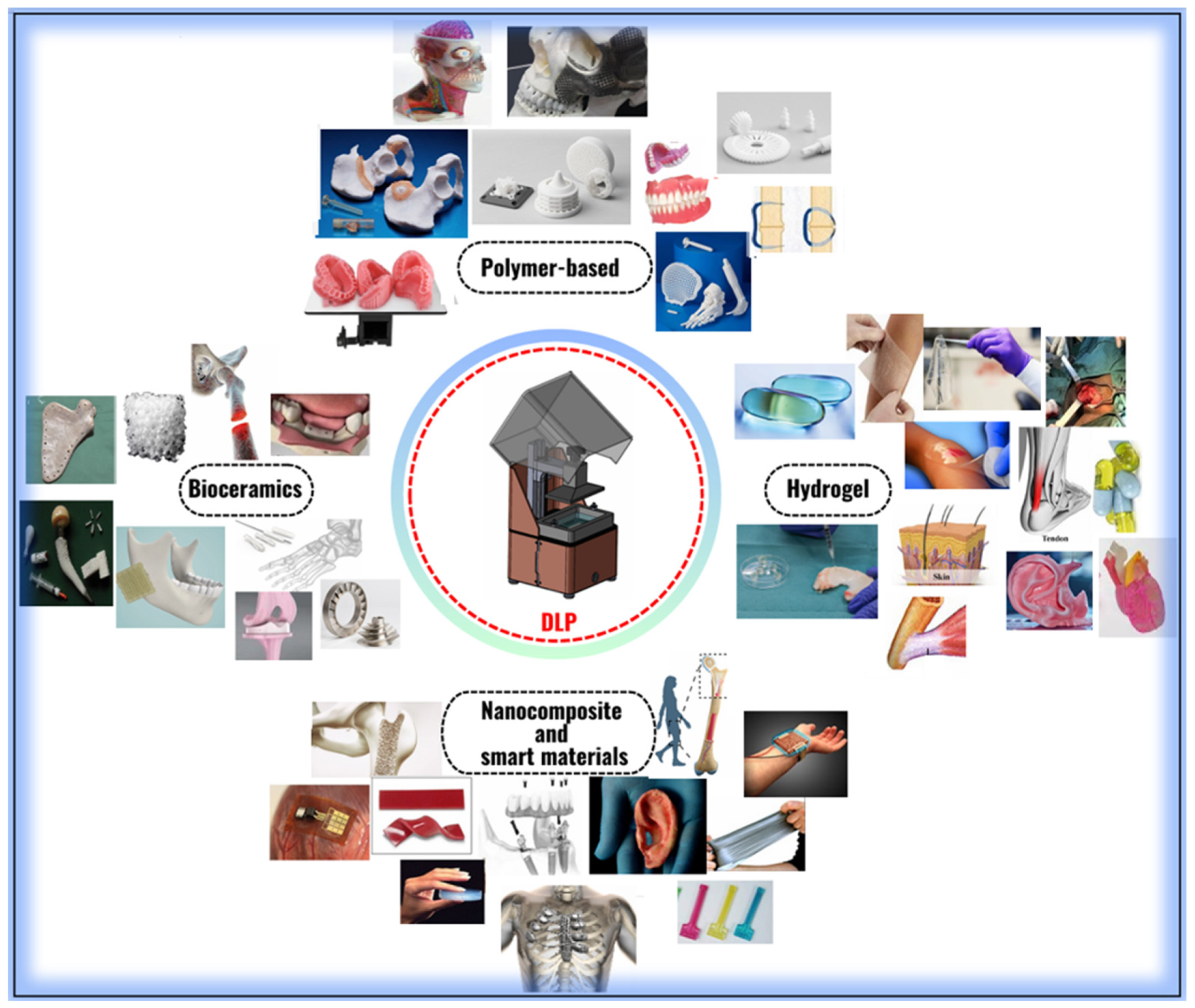
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Kardos, K.; Told, R.; Pentek, A.; Sahai, N.; Banfai, K.; Vizi, A.; Koltai, A.; Szabo, P.; Gurdan, Z.; Bovari-Biri, J.; et al. Surface Disinfection Change the Mechanical, Structural and Biological Properties of Flexible Materials Used for Additive Manufacturing of Medical Devices. Mater. Des. 2024, 237, 112616. [Google Scholar] [CrossRef]
- Soni, N.; Leo, P. Artificial Recreation of Human Organs by Additive Manufacturing. In Mechanical Engineering in Biomedical Applications: Bio-3D Printing, Biofluid Mechanics, Implant Design, Biomaterials, Computational Biomechanics, Tissue Mechanics; Wiley: Hoboken, NJ, USA, 2024; pp. 23–42. [Google Scholar]
- Ukwaththa, J.; Herath, S.; Meddage, D.P.P. A Review of Machine Learning (ML) and Explainable Artificial Intelligence (XAI) Methods in Additive Manufacturing (3D Printing). Mater. Today Commun. 2024, 41, 110294. [Google Scholar] [CrossRef]
- Bayraktar, Ş.; Alparslan, C. Comparison of the SLM, SLS, and DLMS Techniques in Additive Manufacture of AlSi10Mg Alloys. In Innovation and Sustainable Manufacturing; Woodhead Publishing: Sawston, UK, 2023; pp. 231–253. [Google Scholar]
- Şentürk, E.; Alparslan, C.; Bayraktar, Ş.; Korkmaz, M.E.; Günay, M. A Comprehensive Review on Sustainability in EDM Process of Additive Manufactured Materials. Measurement 2025, 245, 116626. [Google Scholar] [CrossRef]
- Alparslan, C.; Bayraktar, Ş.; Gupta, K. A Comparative Study on Mechanical Performance of PLA, ABS, and CF Materials Fabricated by Fused Deposition Modeling. Facta Univ. Ser. Mech. Eng. 2024, 1–18. [Google Scholar]
- Brighenti, R.; Marsavina, L.; Marghitas, M.P.; Montanari, M.; Spagnoli, A.; Tatar, F. The Effect of Process Parameters on Mechanical Characteristics of Specimens Obtained via DLP Additive Manufacturing Technology. Mater. Today Proc. 2023, 78, 331–336. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, K. Direct Slicing of Microcellular Structures for Digital Light Processing (DLP) Additive Manufacturing. Rapid Prototyp. J. 2024, 30, 633–642. [Google Scholar] [CrossRef]
- Li, H.; Dai, J.; Wang, Z.; Zheng, H.; Li, W.; Wang, M.; Cheng, F. Digital Light Processing (DLP)-Based (Bio) Printing Strategies for Tissue Modeling and Regeneration. Aggregate 2023, 4, e270. [Google Scholar] [CrossRef]
- Tao, J.; Zhu, S.; Liao, X.; Wang, Y.; Zhou, N.; Li, Z.; Wan, H.; Tang, Y.; Yang, S.; Du, T.; et al. DLP-Based Bioprinting of Void-Forming Hydrogels for Enhanced Stem-Cell-Mediated Bone Regeneration. Mater. Today Bio 2022, 17, 100487. [Google Scholar] [CrossRef]
- Nam, J.; Kim, M. Advances in Materials and Technologies for Digital Light Processing 3D Printing. Nano Converg. 2024, 11, 45. [Google Scholar]
- Cosola, A.; Sangermano, M.; Terenziani, D.; Conti, R.; Messori, M.; Grützmacher, H.; Pirri, C.F.; Chiappone, A. DLP 3D–Printing of Shape Memory Polymers Stabilized by Thermoreversible Hydrogen Bonding Interactions. Appl. Mater. Today 2021, 23, 101060. [Google Scholar] [CrossRef]
- Ji, Y.; Luan, C.; Yao, X.; Fu, J.; He, Y. Recent Progress in 3D Printing of Smart Structures: Classification, Challenges, and Trends. Adv. Intell. Syst. 2021, 3, 2000271. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Dong, R.; Gilchrist, M.D.; Zhang, N. High-Precision Digital Light Processing (DLP) Printing of Microstructures for Microfluidics Applications Based on a Machine Learning Approach. Virtual Phys. Prototyp. 2024, 19, e2318774. [Google Scholar] [CrossRef]
- Zhu, L.; Rong, Y.; Wang, Y.; Bao, Q.; An, J.; Huang, D.; Huang, X. DLP Printing of Tough Organogels for Customized Wearable Sensors. Eur. Polym. J. 2023, 187, 111886. [Google Scholar] [CrossRef]
- Trembecka-Wójciga, K.; Ortyl, J. Enhancing 3D Printed Ceramic Components: The Function of Dispersants, Adhesion Promoters, and Surface-Active Agents in Photopolymerization-Based Additive Manufacturing. Adv. Colloid Interface Sci. 2024, 332, 103251. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, X.; Peng, Y.; Liu, M.; Lu, F.; Yang, X.; Ye, J. Digital Light Processing Strength-Strong Ultra-Thin Bioceramic Scaffolds for Challengeable Orbital Bone Regeneration and Repair In Situ. Appl. Mater. Today 2021, 22, 100889. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, L.; Zhang, B.; Zhang, G.; Huo, F.; Zhou, C.; Liang, X.; Fan, Y.; Tian, W.; Tan, Y. Preparation of BMP-2/PDA-BCP Bioceramic Scaffold by DLP 3D Printing and Its Ability for Inducing Continuous Bone Formation. Front. Bioeng. Biotechnol. 2022, 10, 854693. [Google Scholar] [CrossRef]
- Saed, A.B.; Behravesh, A.H.; Hasannia, S.; Ardebili, S.A.A.; Akhoundi, B.; Pourghayoumi, M. Functionalized Poly L-Lactic Acid Synthesis and Optimization of Process Parameters for 3D Printing of Porous Scaffolds via Digital Light Processing (DLP) Method. J. Manuf. Process. 2020, 56, 550–561. [Google Scholar] [CrossRef]
- Edaugal, J.P.; Ribeiro, E.L.; Mitchell, M.K.; Cheng, X.; Buckner, E.M.; Chen, J.; Ivanov, I.N.; Ellermann, M.; Advincula, R.C. Digital Light Processing (DLP): 3D Printing of Polymer-Based Graphene Oxide Nanocomposites—Efficient Antimicrobial Material for Biomedical Devices. MRS Commun. 2023, 13, 594–602. [Google Scholar] [CrossRef]
- Kornfellner, E.; Königshofer, M.; Unger, E.; Moscato, F. Elastic and Dimensional Properties of Newly Combined 3D-Printed Multimaterials Fabricated by DLP Stereolithography. Front. Mater. 2023, 10, 1272147. [Google Scholar] [CrossRef]
- Harnany, D.; Ramadhan, M.A.; Ardianto, H.; Muflikhun, M.A. Synergizing Strength and Flexibility: Investigating Mechanical Properties of Photopolymer Resin Blends in DLP 3D Printing. Prog. Addit. Manuf. 2024, 1–16. [Google Scholar] [CrossRef]
- Butler, N.; Zhao, Y.; Lu, S.; Yin, S. Effects of Light Exposure Intensity and Time on Printing Quality and Compressive Strength of β-TCP Scaffolds Fabricated with Digital Light Processing. J. Eur. Ceram. Soc. 2024, 44, 2581–2589. [Google Scholar] [CrossRef]
- Du, Y.; Hu, T.; You, J.; Ye, Y.; Zhang, B.; Bao, B.; Li, M.; Liu, Y.; Wang, Y.; Wang, T. Study of Falling-Down-Type DLP 3D Printing Technology for High-Resolution Hydroxyapatite Scaffolds. Int. J. Appl. Ceram. Technol. 2022, 19, 268–280. [Google Scholar] [CrossRef]
- Rouzé l’Alzit, F.; Cade, R.; Naveau, A.; Babilotte, J.; Meglioli, M.; Catros, S. Accuracy of Commercial 3D Printers for the Fabrication of Surgical Guides in Dental Implantology. J. Dent. 2022, 117, 103909. [Google Scholar] [CrossRef]
- Farkas, A.Z.; Galatanu, S.V.; Nagib, R. The Influence of Printing Layer Thickness and Orientation on the Mechanical Properties of DLP 3D-Printed Dental Resin. Polymers 2023, 15, 1113. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Raju, K.; Lee, J.; Jung, J.; Jeong, S.; Kim, J.I.; Cho, J. 3D Printing of CNT- and YSZ-Added Dental Resin-Based Composites by Digital Light Processing and Their Mechanical Properties. Materials 2023, 16, 1873. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, H.; Lai, Q.; Zhao, Y.; Chen, Q.; Zou, B. Additive Manufacturing of Graphene Oxide-Hydroxyapatite Bioceramic Scaffolds with Reinforced Osteoinductivity Based on Digital Light Processing Technology. Mater. Des. 2022, 223, 111231. [Google Scholar] [CrossRef]
- Chen, J.; Gui, X.; Qiu, T.; Lv, Y.; Fan, Y.; Zhang, X.; Zhou, C.; Guo, W. DLP 3D Printing of High-Resolution Root Scaffold with Bionic Bioactivity and Biomechanics for Personalized Bio-Root Regeneration. Biomater. Adv. 2023, 151, 213475. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, D.; Yao, X.; Wang, Y.; Ma, H.; Yu, X.; Du, L.; Lin, H.; Wu, C. 3D Printing of Pink Bioceramic Scaffolds for Bone Tumor Tissue Therapy. Appl. Mater. Today 2022, 27, 101443. [Google Scholar] [CrossRef]
- Senusi, F.; Mahmood, S.; Ngadiman, N.H.A.; Saman, M.Z.M. Environmental Impact for 3D Bone Tissue Engineering Scaffolds Life Cycle: An Assessment. Biointerface Res. Appl. Chem. 2022, 12, 6504–6515. [Google Scholar]
- Zanon, M.; Baruffaldi, D.; Sangermano, M.; Pirri, C.F.; Frascella, F.; Chiappone, A. Visible Light-Induced Crosslinking of Unmodified Gelatin with PEGDA for DLP-3D Printable Hydrogels. Eur. Polym. J. 2021, 160, 110813. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Chen, T.; Zeng, Y. Fabrication of Trabecular-Like Beta-Tricalcium Phosphate Biomimetic Scaffolds for Bone Tissue Engineering. Ceram. Int. 2021, 47, 13187–13198. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, K.; He, R.; Ding, G.; Xia, M.; Jin, X.; Xie, C. Additive Manufacturing of Hydroxyapatite Bioceramic Scaffolds: Dispersion, Digital Light Processing, Sintering, Mechanical Properties, and Biocompatibility. J. Adv. Ceram. 2020, 9, 360–373. [Google Scholar] [CrossRef]
- Wu, Y.-L.; D’amato, A.R.; Yan, A.M.; Wang, R.Q.; Ding, X.; Wang, Y. Three-Dimensional Printing of Poly(Glycerol Sebacate) Acrylate Scaffolds via Digital Light Processing. ACS Appl. Bio Mater. 2020, 3, 7531–7542. [Google Scholar] [CrossRef] [PubMed]
- Linares-Alvelais, J.A.R.; Figueroa-Cavazos, J.O.; Chuck-Hernandez, C.; Siller, H.R.; Rodríguez, C.A.; Martínez-López, J.I. Hydrostatic High-Pressure Post-Processing of Specimens Fabricated by DLP, SLA, and FDM: An Alternative for the Sterilization of Polymer-Based Biomedical Devices. Materials 2018, 11, 2540. [Google Scholar] [CrossRef]
- Ryan, E.; Yin, S. Compressive Strength of β-TCP Scaffolds Fabricated via Lithography-Based Manufacturing for Bone Tissue Engineering. Ceram. Int. 2022, 48, 15516–15524. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Liang, H.; Liu, Y.; Bai, J.; Wang, M. Digital Light Processing (DLP) of Nano Biphasic Calcium Phosphate Bioceramic for Making Bone Tissue Engineering Scaffolds. Ceram. Int. 2022, 48, 27681–27692. [Google Scholar] [CrossRef]
- Zhang, B.; Xing, F.; Chen, L.; Zhou, C.; Gui, X.; Su, Z.; Fan, S.; Zhou, Z.; Jiang, Q.; Zhao, L.; et al. DLP Fabrication of Customized Porous Bioceramics with Osteoinduction Ability for Remote Isolation Bone Regeneration. Biomater. Adv. 2023, 145, 213261. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, C.; Su, Y.; Curran, J.; Henstock, J.R.; Tseng, F. A 4D Printed Self-Assembling PEGDA Microscaffold Fabricated by Digital Light Processing for Arthroscopic Articular Cartilage Tissue Engineering. Prog. Addit. Manuf. 2024, 9, 3–14. [Google Scholar] [CrossRef]
- Nguyen, M.T.H.; Kim, S.Y.; Jeong, T.H.; Kim, J.H.; Cho, H.S.; Ha, T.H.; Ahn, S.J.; Kim, Y.H. Preparation and Stability of PEGDA/GO Conductive Materials by DLP 3D Printing. Electron. Mater. Lett. 2022, 18, 275–281. [Google Scholar] [CrossRef]
- Vyas, A.; Garg, V.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S. Photopolymerizable Resin-Based 3D Printed Biomedical Composites: Factors Affecting Resin Viscosity. Mater. Today Proc. 2022, 62, 1435–1439. [Google Scholar] [CrossRef]
- Bae, S.U.; Kim, B.J. Effects of Cellulose Nanocrystal and Inorganic Nanofillers on the Morphological and Mechanical Properties of Digital Light Processing (DLP) 3D-Printed Photopolymer Composites. Appl. Sci. 2021, 11, 6835. [Google Scholar] [CrossRef]
- Benca, E.; Eckhart, B.; Stoegner, A.; Unger, E.; Moscato, F. Dimensional Accuracy and Precision and Surgeon Perception of Additively Manufactured Bone Models: Effect of Manufacturing Technology and Part Orientation. 3D Print. Med. 2024, 10, 5. [Google Scholar] [CrossRef]
- Rade, P.; Swami, S.; Pawane, V.; Hawaldar, R.; Giramkar, V.; Joseph, S.; Kale, B. Effect of Functionality of Diluents on Digital Light Processing (DLP)-Based Three-Dimensional (3D) Printing of UV-Curable Bisphenol A-Based Epoxy Acrylate Resin. Polym. Eng. Sci. 2024, 64, 2202–2213. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, W.; Liu, F.; Cui, J.; Feng, S.; Liang, C.; Guo, Y.; Wang, Z.; Mao, Z.; Zhang, B. Vat Photopolymerization-Based Digital Light Processing 3D Printing Hydrogels in Biomedical Fields: Key Parameters and Perspective. Addit. Manuf. 2024, 94, 104443. [Google Scholar] [CrossRef]
- Kröger, F.; Schulte, L.; Spiegel, C.A.; Vazquez-Martel, C.; Blasco, E. Multi-Material Single-Vat Dual-Wavelength DLP 4D Printing of Shape Memory Polymers. Smart Mater. Struct. 2024, 34, 025001. [Google Scholar] [CrossRef]
- Shashikumar, U.; Saraswat, A.; Deshmukh, K.; Hussain, C.M.; Chandra, P.; Tsai, P.-C.; Huang, P.-C.; Chen, Y.-H.; Ke, L.-Y.; Lin, Y.-C.; et al. Innovative Technologies for the Fabrication of 3D/4D Smart Hydrogels and Its Biomedical Applications: A Comprehensive Review. Adv. Colloid Interface Sci. 2024, 328, 103163. [Google Scholar] [CrossRef] [PubMed]
- Baronins, J.; Antonov, M.; Abramovskis, V.; Rautmane, A.; Lapkovskis, V.; Bockovs, I.; Goel, S.; Thakur, V.K.; Shishkin, A. The Effect of Zinc Oxide on DLP Hybrid Composite Manufacturability and Mechanical-Chemical Resistance. Polymers 2023, 15, 4679. [Google Scholar] [CrossRef]
- Xue, R.; Yuan, P.; Zhao, B.; Jing, F.; Kou, X.; Yue, W.; Wang, Y.; Wang, D.; Sewvandi, G.A.; Hu, D. DLP Printing of BT/HA Nanocomposite Ceramic Scaffolds Using Low Refractive Index BT Crystals. J. Materiomics 2024, 10, 1036–1048. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Üstünel, S.; Clements, R.J.; Hegmann, E. Physical Models from Physical Templates Using Biocompatible Liquid Crystal Elastomers as Morphologically Programmable Inks for 3D Printing. Macromol. Biosci. 2023, 23, 2200343. [Google Scholar] [CrossRef]
- Bardakova, K.N.; Kholkhoev, B.C.; Farion, I.A.; Epifanov, E.O.; Korkunova, O.S.; Efremov, Y.M.; Minaev, N.V.; Solovieva, A.B.; Timashev, P.S.; Burdukovskii, V.F. 4D Printing of Shape-Memory Semi-Interpenetrating Polymer Networks Based on Aromatic Heterochain Polymers. Adv. Mater. Technol. 2022, 7, 2100790. [Google Scholar] [CrossRef]
- Yeazel-Klein, T.R.; Davis, A.G.; Becker, M.L. Thiol-Ene-Based 3D Printing of Bioresorbable Fumarate-Based ABA Triblock Copolyester Elastomers. Adv. Mater. Technol. 2023, 8, 2201904. [Google Scholar] [CrossRef]
- Li, Y.; Mao, Q.; Xu, K.; Yang, H.; Huang, Y.; Yin, J. Vat Photopolymerization Bioprinting with a Dynamic Support Bath. Addit. Manuf. 2023, 69, 103533. [Google Scholar] [CrossRef]
- Song, P.; Gui, X.; Wu, L.; Su, X.; Zhou, W.; Luo, Z.; Zhang, B.; Feng, P.; Wei, W.; Fan, C.; et al. DLP Fabrication of Multiple Hierarchical Biomimetic GelMA/SilMA/HAp Scaffolds for Enhancing Bone Regeneration. Biomacromolecules 2024, 25, 1871–1886. [Google Scholar] [CrossRef]
- Sarvari, S.; McGee, D.; O’connell, R.; Tseytlin, O.; Bobko, A.A.; Tseytlin, M. Electron Spin Resonance Probe Incorporation into Bioinks Permits Longitudinal Oxygen Imaging of Bioprinted Constructs. Mol. Imaging Biol. 2024, 26, 511–524. [Google Scholar] [CrossRef]
- Song, P.; Li, M.; Zhang, B.; Gui, X.; Han, Y.; Wang, L.; Zhou, W.; Guo, L.; Zhang, Z.; Li, Z.; et al. DLP Fabricating of Precision GelMA/HAp Porous Composite Scaffold for Bone Tissue Engineering Application. Compos. B Eng. 2022, 244, 110163. [Google Scholar] [CrossRef]
- Tesavibul, P.; Felzmann, R.; Gruber, S.; Liska, R.; Thompson, I.; Boccaccini, A.R.; Stampfl, J. Processing of 45S5 Bioglass® by Lithography-Based Additive Manufacturing. Mater. Lett. 2012, 74, 81–84. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Shen, Y.; Xiong, Y.; Dong, L.; Zheng, J.; Zhao, S. Fabrication of Porous β-TCP/58S Bioglass Scaffolds via Top-Down DLP Printing with High Solid Loading Ceramic-Resin Slurry. Mater. Chem. Phys. 2021, 267, 124587. [Google Scholar] [CrossRef]
- Hua, S.B.; Su, J.; Deng, Z.L.; Wu, J.M.; Cheng, L.J.; Yuan, X.; Chen, F.; Zhu, H.; Qi, D.-H.; Xiao, J.; et al. Microstructures and Properties of 45S5 Bioglass® & BCP Bioceramic Scaffolds Fabricated by Digital Light Processing. Addit. Manuf. 2021, 45, 102074. [Google Scholar]
- Ma, H.; Liu, Z.; Lu, X.; Zhang, S.; Tang, C.; Cheng, Y.; Zhang, H.; Liu, G.; Sui, C.; Ding, C.; et al. 3D Printed Multi-Coupled Bioinspired Skin-Electronic Interfaces with Enhanced Adhesion for Monitoring and Treatment. Acta Biomater. 2024, 187, 183–198. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Influence of a Non-Reactive Additive on the Photocuring and 3D-VAT Printing Processes of PEGDA: Complementary Studies. Eur. Polym. J. 2022, 180, 111588. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; John, S.; Choudhury, N.R.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise, and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, J. Enhanced Thermal and Mechanical Properties of 3D-Printed PEG Containing Acrylate Composite with Surface-Treated BN via Digital Light Processing. Polym. Test. 2023, 118, 107898. [Google Scholar] [CrossRef]
- Hosseinabadi, H.G.; Nieto, D.; Yousefinejad, A.; Fattel, H.; Ionov, L.; Miri, A.K. Ink Material Selection and Optical Design Considerations in DLP 3D Printing. Appl. Mater. Today 2023, 30, 101721. [Google Scholar] [CrossRef]
- Ge, Q.; Jian, B.; Li, H. Shaping Soft Materials via Digital Light Processing-Based 3D Printing: A Review. Forces Mech. 2022, 6, 100074. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Li, M.; Zhu, S.; Chen, S.; Dong, L.; Niu, F.; Yang, R. 3D-Printed Light-Driven Microswimmer with Built-In Micromotors. Adv. Mater. Technol. 2022, 7, 2100687. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Yoo, J.J.; Lee, S.J.; Kim, M.S. 3D Digital Light Process Bioprinting: Cutting-Edge Platforms for Resolution of Organ Fabrication. Mater. Today Bio. 2024, 29, 101284. [Google Scholar] [CrossRef]
- Peng, H.; Han, B.; Tong, T.; Jin, X.; Peng, Y.; Guo, M.; Li, B.; Ding, J.; Kong, Q.; Wang, Q. 3D Printing Processes in Precise Drug Delivery for Personalized Medicine. Biofabrication 2024, 16, 032001. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J. 3D Printing Techniques in Medicine and Surgery. In 3D Printing in Medicine and Surgery; Woodhead Publishing: Sawston, UK, 2021; pp. 15–45. [Google Scholar]
- Wegmüller, L.; Halbeisen, F.; Sharma, N.; Kühl, S.; Thieringer, F.M. Consumer vs. High-End 3D Printers for Guided Implant Surgery—An In Vitro Accuracy Assessment Study of Different 3D Printing Technologies. J. Clin. Med. 2021, 10, 4894. [Google Scholar] [CrossRef]
- Tahir, N.; Abduo, J. An In Vitro Evaluation of the Effect of 3D Printing Orientation on the Accuracy of Implant Surgical Templates Fabricated by Desktop Printer. J. Prosthodont. 2022, 31, 791–798. [Google Scholar] [CrossRef]
- Hussain, M.I.; Xia, M.; Ren, X.; Ge, C.; Jamil, M.; Gupta, M.K. Digital Light Processing 3D Printing of Ceramic Materials: A Review on Basic Concept, Challenges, and Applications. Int. J. Adv. Manuf. Technol. 2024, 130, 2241–2267. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, S.; Wang, R.; Ge, Q. Digital Light Processing-Based Multimaterial 3D Printing: Challenges, Solutions and Perspectives. Int. J. Extrem. Manuf. 2024, 6, 042006. [Google Scholar] [CrossRef]
- WWang, L.; Wang, Q.; Slita, A.; Backman, O.; Gounani, Z.; Rosqvist, E.; Peltonen, J.; Willför, S.; Xu, C.; Rosenholm, J.M.; et al. Digital Light Processing (DLP) 3D-Fabricated Antimicrobial Hydrogel with a Sustainable Resin of Methacrylated Woody Polysaccharides and Hybrid Silver-Lignin Nanospheres. Green Chem. 2022, 24, 2129–2145. [Google Scholar] [CrossRef]
- LM, C.; Dimitrov, R. Rapid Prototyping Technologies—Advantages and Disadvantages. Ann. Constantin Brancusi Univ. Targu-Jiu Eng. Ser. 2021, 4, 136–142. [Google Scholar]
- Ozyilmaz, E.D.; Turan, A.; Comoglu, T. An Overview on the Advantages and Limitations of 3D Printing of Microneedles. Pharm. Dev. Technol. 2021, 26, 923–933. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Kordi, O.; Behravesh, A.H.; Hasannia, S.; Hedayati, S.K.; Pourghaumi, M.; Mazdi, M.; Ghaderi, I.; Rizvi, G. Additive Manufacture of PLLA Scaffolds Reinforced with Graphene Oxide Nanoparticles via Digital Light Processing (DLP). J. Biomater. Appl. 2023, 38, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Bahati, D.; Bricha, M.; El Mabrouk, K. Vat Photopolymerization Additive Manufacturing Technology for Bone Tissue Engineering Applications. Adv. Eng. Mater. 2023, 25, 2200859. [Google Scholar] [CrossRef]
- Chivate, A.; Zhou, C. Additive Manufacturing of Micropatterned Functional Surfaces: A Review. Int. J. Extrem. Manuf. 2024, 6, 042004. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, L.; Li, P.; Wang, E.; Pang, Y.; Wei, Y.; Li, B.; Huang, Y.; Liu, B.; Wang, S.; et al. All-Natural Ceramic Composite Bone Scaffolds of Whitlockite/Wollastonite Fibers: DLP Additive Manufacturing, Microstructure, and Performance. J. Mater. Res. Technol. 2024, 33, 7391–7405. [Google Scholar] [CrossRef]
- Elsayed, H.; Colombo, P.; Crovace, M.C.; Zanotto, E.D.; Bernardo, E. Suitability of Biosilicate® Glass-Ceramic Powder for Additive Manufacturing of Highly Porous Scaffolds. Ceram. Int. 2021, 47, 8200–8207. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2022.
- Bucciarelli, A.; Petretta, M.; Grigolo, B.; Gambari, L.; Bossi, A.M.; Grassi, F.; Maniglio, D. Methacrylated Silk Fibroin Additive Manufacturing of Shape Memory Constructs with Possible Application in Bone Regeneration. Gels 2022, 8, 833. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.W.; Zhang, Z.W.; Cui, X.Y.; Hu, R.Y.; Li, Y.; Huang, S.D.; Du, S.Q.; Dao, J.W.; Wei, D.X. ZnCur Nanoparticle-Enhanced Multifunctional Hydrogel Platform: Synergistic Antibacterial and Immunoregulatory Effects for Infected Diabetic Wound Healing. Chem. Eng. J. 2025, 503, 158387. [Google Scholar] [CrossRef]
- Ding, Y.W.; Li, Y.; Zhang, Z.W.; Dao, J.W.; Wei, D.X. Hydrogel Forming Microneedles Loaded with VEGF and Ritlecitinib/Polyhydroxyalkanoates Nanoparticles for Mini-Invasive Androgenetic Alopecia Treatment. Bioact. Mater. 2024, 38, 95–108. [Google Scholar] [CrossRef]
- Ren, Z.W.; Wang, Z.Y.; Ding, Y.W.; Dao, J.W.; Li, H.R.; Ma, X.; Yang, X.Y.; Zhou, Z.Q.; Liu, J.X.; Mi, C.H.; et al. Polyhydroxyalkanoates: The Natural Biopolyester for Future Medical Innovations. Biomater. Sci. 2023, 11, 6013–6034. [Google Scholar] [CrossRef]
- Yang, X.; Yao, L.; Sun, X.; Wang, L.; Xiao, J. Low-Temperature DLP 3D Printing of Low-Concentration Collagen Methacryloyl for the Fabrication of Durable and Bioactive Personalized Scaffolds. Chem. Eng. J. 2024, 497, 155650. [Google Scholar] [CrossRef]
- Kumar, H.; Sakthivel, K.; Mohamed, M.G.; Boras, E.; Shin, S.R.; Kim, K. Designing Gelatin Methacryloyl (GelMA)-Based Bioinks for Visible Light Stereolithographic 3D Biofabrication. Macromol. Biosci. 2021, 21, 2000317. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, Y.; Zhang, Y. Applications of Light-Based 3D Bioprinting and Photoactive Biomaterials for Tissue Engineering. Materials 2023, 16, 7461. [Google Scholar] [CrossRef]
- Lee, S.Y.; Phuc, H.D.; Um, S.H.; Mongrain, R.; Yoon, J.K.; Bhang, S.H. Photocuring 3D Printing Technology as an Advanced Tool for Promoting Angiogenesis in Hypoxia-Related Diseases. J. Tissue Eng. 2024, 15, 20417314241282476. [Google Scholar] [CrossRef]
- Fan, J.; Ding, Z.; Cai, Y.; Lai, Y.; Huang, C.; Jiang, B.; Zhou, Z.; Luo, Z. Revolutionizing Bone Regeneration: Vascularized Bone Tissue Engineering with Advanced 3D Printing Technology. Aggregate 2025, 6, e731. [Google Scholar] [CrossRef]
- Fang, W.; Yu, Z.; Gao, G.; Yang, M.; Du, X.; Wang, Y.; Fu, Q. Light-Based 3D Bioprinting Technology Applied to Repair and Regeneration of Different Tissues: A Rational Proposal for Biomedical Applications. Mater. Today Bio 2024, 27, 101135. [Google Scholar] [CrossRef] [PubMed]
- Su, J.J.M.; Lin, C.H.; Chen, H.; Lee, S.Y.; Lin, Y.M. Biofabrication of Cell-Laden Gelatin Methacryloyl Hydrogels with Incorporation of Silanized Hydroxyapatite by Visible Light Projection. Polymers 2021, 13, 2354. [Google Scholar] [CrossRef]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.E.; Gou, M.; Xu, Y.; et al. Direct 3D Bioprinting of Prevascularized Tissue Constructs with Complex Microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef]
- Duong, V.T.; Lin, C.C. Digital Light Processing 3D Bioprinting of Gelatin-Norbornene Hydrogel for Enhanced Vascularization. Macromol. Biosci. 2023, 23, 2300213. [Google Scholar] [CrossRef]
- Choi, K.Y.; Ajiteru, O.; Hong, H.; Suh, Y.J.; Sultan, T.; Lee, H.; Lee, J.S.; Lee, Y.J.; Lee, O.J.; Kim, S.H.; et al. A Digital Light Processing 3D-Printed Artificial Skin Model and Full-Thickness Wound Models Using Silk Fibroin Bioink. Acta Biomater. 2023, 164, 159–174. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, H.; Chi, B.; Zhang, X.; Wang, Y.; Wu, J.; Huang, Q. Construction of a Dual-Component Hydrogel Matrix for 3D Biomimetic Skin Based on Photo-Crosslinked Chondroitin Sulfate/Collagen. Int. J. Biol. Macromol. 2024, 254, 127940. [Google Scholar] [CrossRef]
- Tilton, M.; Camilleri, E.T.; Potes, M.D.A.; Gaihre, B.; Liu, X.; Lucien, F.; Elder, B.D.; Lu, L. Visible Light-Induced 3D Bioprinted Injectable Scaffold for Minimally Invasive Tissue Regeneration. Biomater. Adv. 2023, 153, 213539. [Google Scholar] [CrossRef]
- Xie, X.; Wu, S.; Mou, S.; Guo, N.; Wang, Z.; Sun, J. Microtissue-Based Bioink as a Chondrocyte Microshelter for DLP Bioprinting. Adv. Healthc. Mater. 2022, 11, 2201877. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Ching, T.; Hashimoto, M. Bioprinting Using PEGDMA-Based Hydrogel on DLP Printer. Mater. Today Proc. 2022, 70, 179–183. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Tariq, A.; Hossain, M.; Khan, K.A.; Umer, R. 3D Printing of Magneto-Active Smart Materials for Advanced Actuators and Soft Robotics Applications. Eur. Polym. J. 2024, 205, 112718. [Google Scholar] [CrossRef]
- Sajjad, R.; Chauhdary, S.T.; Anwar, M.T.; Zahid, A.; Khosa, A.A.; Imran, M.; Sajjad, M.H. A Review of 4D Printing–Technologies, Shape Shifting, Smart Polymer-Based Materials, and Biomedical Applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 20–36. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, J.; Luo, Z.; Li, Z. Recent Research Developments of 4D Printing Technology for Magnetically Controlled Smart Materials: A Review. Magnetochemistry 2023, 9, 204. [Google Scholar] [CrossRef]
- Cortés, A.; Cosola, A.; Sangermano, M.; Campo, M.; Prolongo, S.G.; Pirri, C.F.; Jiménez-Suárez, A.; Chiappone, A. DLP 4D-Printing of Remotely, Modularly, and Selectively Controllable Shape Memory Polymer Nanocomposites Embedding Carbon Nanotubes. Adv. Funct. Mater. 2021, 31, 2106774. [Google Scholar] [CrossRef]
- Wu, H.; Chen, P.; Yan, C.; Cai, C.; Shi, Y. Four-Dimensional Printing of a Novel Acrylate-Based Shape Memory Polymer Using Digital Light Processing. Mater. Des. 2019, 171, 107704. [Google Scholar] [CrossRef]
- Mahjoubnia, A.; Cai, D.; Wu, Y.; King, S.D.; Torkian, P.; Chen, A.C.; Talaie, R.; Chen, S.-Y.; Lin, J. Digital Light 4D Printing of Bioresorbable Shape Memory Elastomers for Personalized Biomedical Implantation. Acta Biomater. 2024, 177, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Liu, Z.; Sun, L.; Cao, Y.; Shen, Z.; Li, M.; An, Y.; Zhang, H.; Sang, S. A Multicrosslinked Network Composite Hydrogel Scaffold Based on DLP Photocuring Printing for Nasal Cartilage Repair. Biotechnol. Bioeng. 2024, 121, 2752–2766. [Google Scholar] [CrossRef]
- Mo, X.; Ouyang, L.; Xiong, Z.; Zhang, T. Advances in Digital Light Processing of Hydrogels. Biomed. Mater. 2022, 17, 042002. [Google Scholar] [CrossRef]
- De Grave, L.; Di Meo, C.; Gréant, C.; Van Durme, B.; Gérard, M.; La Gatta, A.; Schiraldi, C.; Thorrez, L.; Bernaerts, K.V.; Van Vlierberghe, S. Photo-Crosslinkable Poly(Aspartic Acid) for Light-Based Additive Manufacturing: Chain-Growth versus Step-Growth Crosslinking. Eur. Polym. J. 2023, 190, 112017. [Google Scholar] [CrossRef]
- Han, K.; Cheng, Y.; Han, Q.; Chen, J. Extraction of Type I Collagen and Development of Collagen Methacryloyl (ColMA)/PEGDA Ink for Digital Light Processing Printing. Int. J. Biol. Macromol. 2024, 282, 137253. [Google Scholar] [CrossRef]
- Zanon, M.; Cue-López, R.; Martínez-Campos, E.; Bosch, P.; Versace, D.-L.; Hayek, H.; Garino, N.; Pirri, C.F.; Sangermano, M.; Chiappone, A. Bioderived Dyes-Mediated Vat Photopolymerization 3D Printing of Chitosan Hydrogels for Tissue Engineering. Addit. Manuf. 2023, 69, 103553. [Google Scholar] [CrossRef]
- Lu, G.; Tang, R.; Nie, J.; Zhu, X. Photocuring 3D Printing of Hydrogels: Techniques, Materials, and Applications in Tissue Engineering and Flexible Devices. Macromol. Rapid Commun. 2024, 45, 2300661. [Google Scholar] [CrossRef] [PubMed]
- Soullard, L.; Schlepp, A.; Buret, R.; Lancelon-Pin, C.; Nonglaton, G.; Texier, I.; Jean, B.; Rolere, S. Towards the 3D Printing of Innovative Hydrogel Scaffolds through Vat Polymerization Techniques Using Methacrylated Carboxymethylcellulose Aqueous Formulations. Prog. Addit. Manuf. 2024, 10, 744. [Google Scholar] [CrossRef]
- Sachdeva, I.; Ramesh, S.; Chadha, U.; Punugoti, H.; Selvaraj, S.K. Computational AI Models in VAT Photopolymerization: A Review, Current Trends, Open Issues, and Future Opportunities. Neural Comput. Appl. 2022, 34, 17207–17229. [Google Scholar] [CrossRef]
- Ma, L.; Yu, S.; Xu, X.; Amadi, S.M.; Zhang, J.; Wang, Z. Application of Artificial Intelligence in 3D Printing Physical Organ Models. Mater. Today Bio 2023, 23, 100792. [Google Scholar] [CrossRef]
- Bax, M.; Thorpe, J.; Romanov, V. The Future of Personalized Cardiovascular Medicine Demands 3D and 4D Printing, Stem Cells, and Artificial Intelligence. Front. Sens. 2023, 4, 1294721. [Google Scholar] [CrossRef]
- Goh, G.D.; Hamzah, N.M.B.; Yeong, W.Y. Anomaly Detection in Fused Filament Fabrication Using Machine Learning. 3D Print. Addit. Manuf. 2023, 10, 428–437. [Google Scholar] [CrossRef]
- Fouly, A.; Albahkali, T.; Abdo, H.S.; Salah, O. Investigating the Mechanical Properties of Annealed 3D-Printed PLA–Date Pits Composite. Polymers 2023, 15, 3395. [Google Scholar] [CrossRef]
- Rezapour Sarabi, M.; Alseed, M.M.; Karagoz, A.A.; Tasoglu, S. Machine Learning-Enabled Prediction of 3D-Printed Microneedle Features. Biosensors 2022, 12, 491. [Google Scholar] [CrossRef]
- Chen, J.; Liu, A.; Shi, Y.; Luo, Y.; Li, J.; Ye, M.; Guo, W. Skin-Inspired Bimodal Receptors for Object Recognition and Temperature Sensing Simulation. Adv. Funct. Mater. 2024, 34, 2403528. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, K.; Demoly, F.; Zhao, R.R.; Qi, H.J. Perspective: Machine Learning in Design for 3D/4D Printing. J. Appl. Mech. 2024, 91, 030801. [Google Scholar] [CrossRef]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Harnessing Artificial Intelligence for the Next Generation of 3D Printed Medicines. Adv. Drug Deliv. Rev. 2021, 175, 113805. [Google Scholar] [CrossRef]
- Gong, J.; Qian, Y.; Lu, K.; Zhu, Z.; Siow, L.; Zhang, C.; Yang, H. Digital Light Processing (DLP) in Tissue Engineering: From Promise to Reality, and Perspectives. Biomed. Mater. 2022, 17, 062004. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Hsieh, C.Y. Studying the Influence of the Photopolymer Material Properties to the Creation of MLAs with Using Digital Light Processing (DLP) Stereolithography Printing (SLA). Sens. Actuators A Phys. 2023, 360, 114546. [Google Scholar] [CrossRef]
- Hong, H.; Seo, Y.B.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Park, C.H. Digital Light Processing 3D Printed Silk Fibroin Hydrogel for Cartilage Tissue Engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Kollipara, P.S.; Zheng, Y. Digital Manufacturing of Advanced Materials: Challenges and Perspective. Mater. Today 2019, 28, 49–62. [Google Scholar] [CrossRef]
- Sarker, S.; Colton, A.; Wen, Z.; Xu, X.; Erdi, M.; Jones, A.; Kofinas, P.; Tubaldi, E.; Walczak, P.; Janowski, M.; et al. 3D-Printed Microinjection Needle Arrays via a Hybrid DLP-Direct Laser Writing Strategy. Adv. Mater. Technol. 2023, 8, 2201641. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Paolelli, X.; De Vitis, E.; Selicato, N.; Gervaso, F.; Gigli, G.; Moroni, L.; Polini, A. VAT Photopolymerization 3D Printing Optimization of High Aspect Ratio Structures for Additive Manufacturing of Chips towards Biomedical Applications. Addit. Manuf. 2022, 60, 103200. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital Light Processing-Based 3D Printing for Medical Applications. Int. J. Bioprint. 2020, 6, 242. [Google Scholar]
- Keshavarzan, M.; Kadkhodaei, M.; Badrossamay, M.; Ravarib, M.R.K. Investigation on the Failure Mechanism of Triply Periodic Minimal Surface Cellular Structures Fabricated by Vat Photopolymerization Additive Manufacturing under Compressive Loadings. Mech. Mater. 2020, 140, 103150. [Google Scholar] [CrossRef]
- Wang, R.; Damanik, F.; Kuhnt, T.; Jaminon, A.; Hafeez, S.; Liu, H.; Ippel, H.; Dijkstra, P.J.; Bouvy, N.; Schurgers, L.; et al. Biodegradable Poly(Ester) Urethane Acrylate Resins for Digital Light Processing: From Polymer Synthesis to 3D Printed Tissue Engineering Constructs. Adv. Healthc. Mater. 2023, 12, 2202648. [Google Scholar] [CrossRef]
- Kuhnt, T.; Morgan, F.L.C.; Baker, M.B.; Moroni, L. An Efficient and Easily Adjustable Heating Stage for Digital Light Processing Set-Ups. Addit. Manuf. 2021, 46, 102102. [Google Scholar] [CrossRef]
- Guo, W.; Li, P.; Wei, Y.; Zhao, L.; Pang, Y.; Huang, Y.; Ye, X.; Wang, S.; Liu, B.; You, H.; et al. Ionic Substitution through Bredigite Doping for Microstructure and Performance Adjustment in DLP 3D-Printed TPMS Porous HA Bone Scaffolds. Virtual Phys. Prototyp. 2024, 19, e2423840. [Google Scholar] [CrossRef]
- Martinez, J.S.; Peterson, S.; Hoel, C.A.; Erno, D.J.; Murray, T.; Boyd, L.; Her, J.-H.; Mclean, N.; Davis, R.; Ginty, F.; et al. High Resolution DLP Stereolithography to Fabricate Biocompatible Hydroxyapatite Structures That Support Osteogenesis. PLoS ONE 2022, 17, e0272283. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Saed, A.; Behravesh, A.H.; Hasannia, S.; Akhoundi, B.; Hedayati, S.K.; Gashtasbi, F. An In Vitro Study on the Key Features of Poly L-Lactic Acid/Biphasic Calcium Phosphate Scaffolds Fabricated via DLP 3D Printing for Bone Grafting. Eur. Polym. J. 2020, 141, 110057. [Google Scholar] [CrossRef]
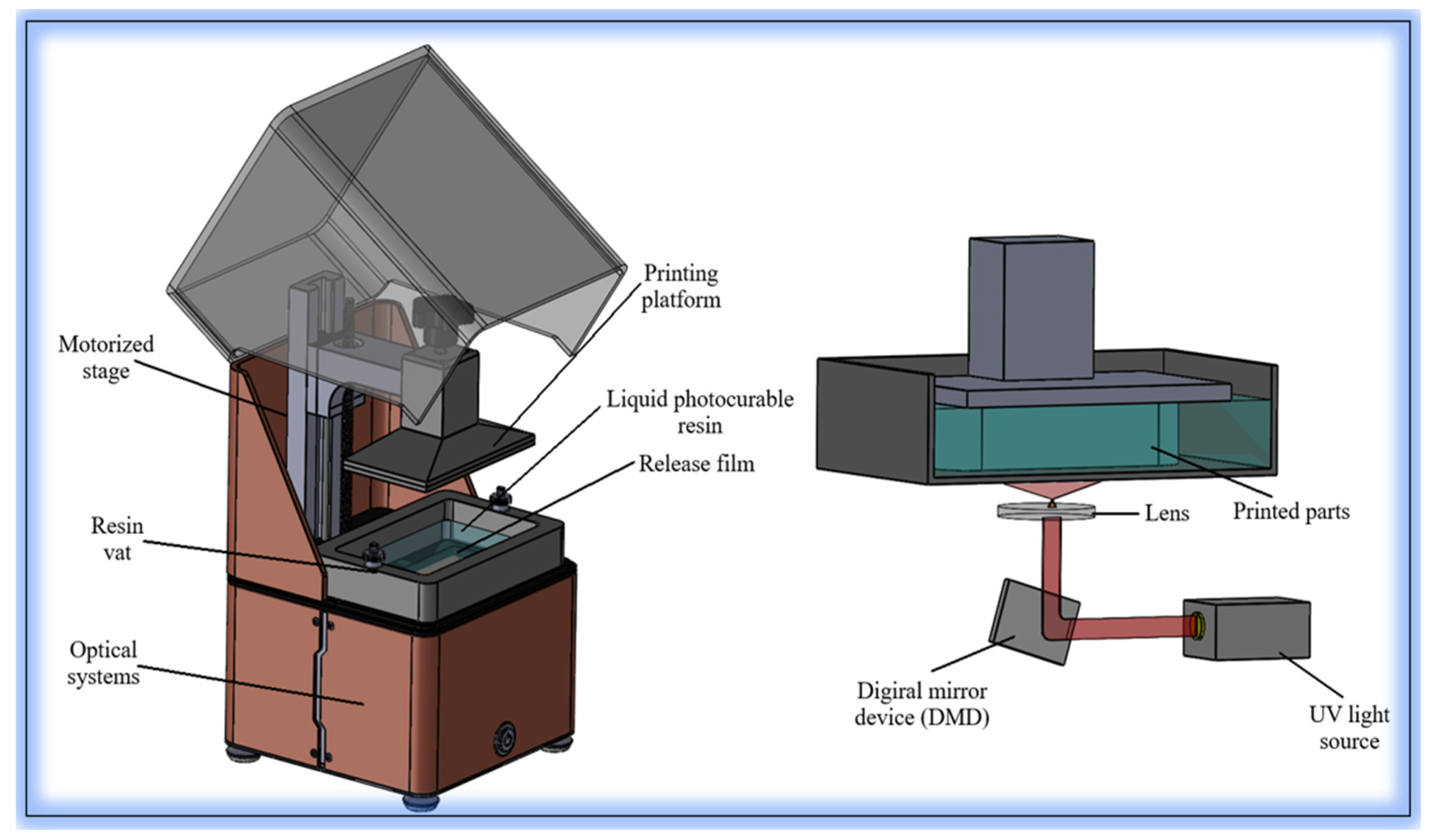
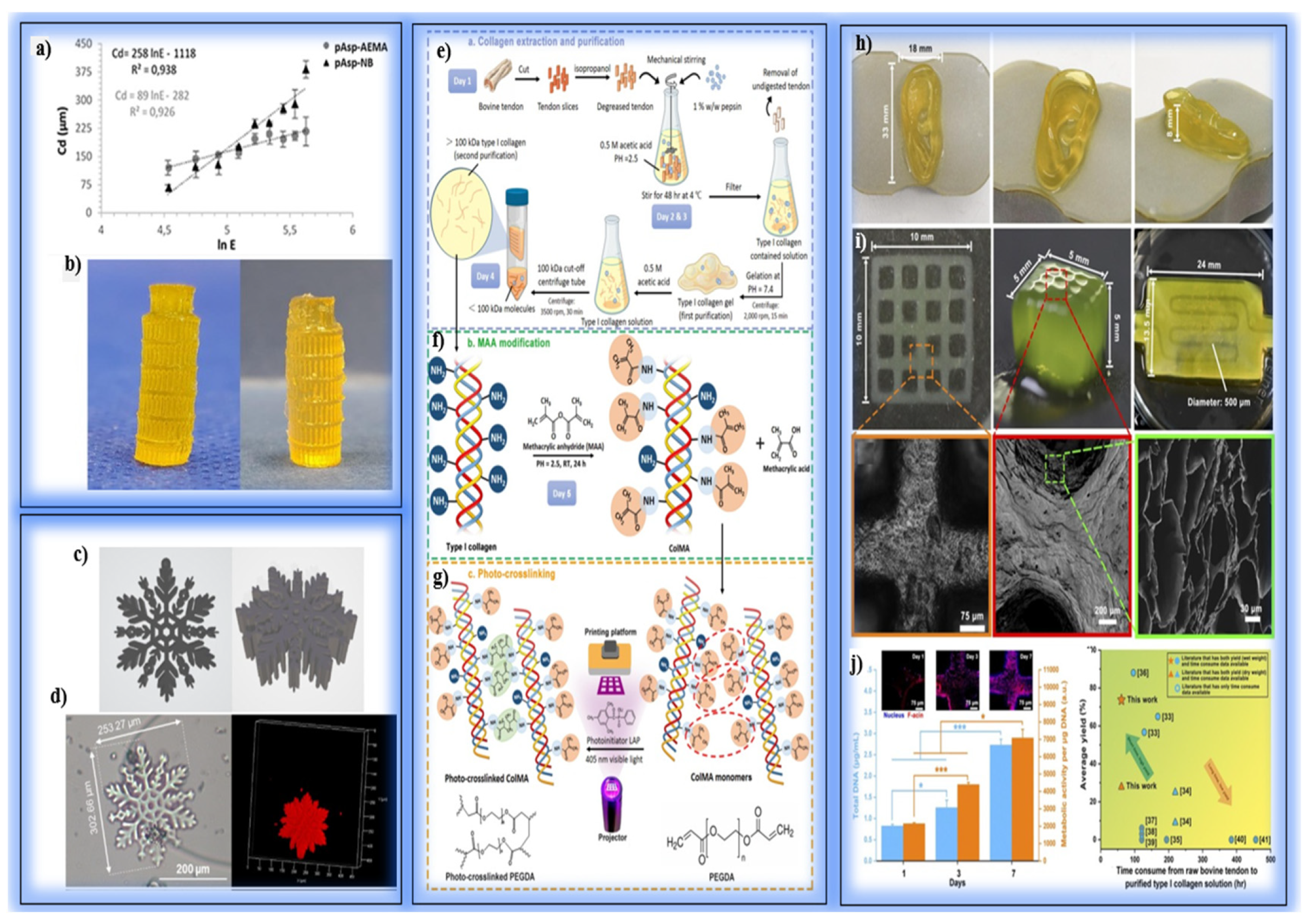
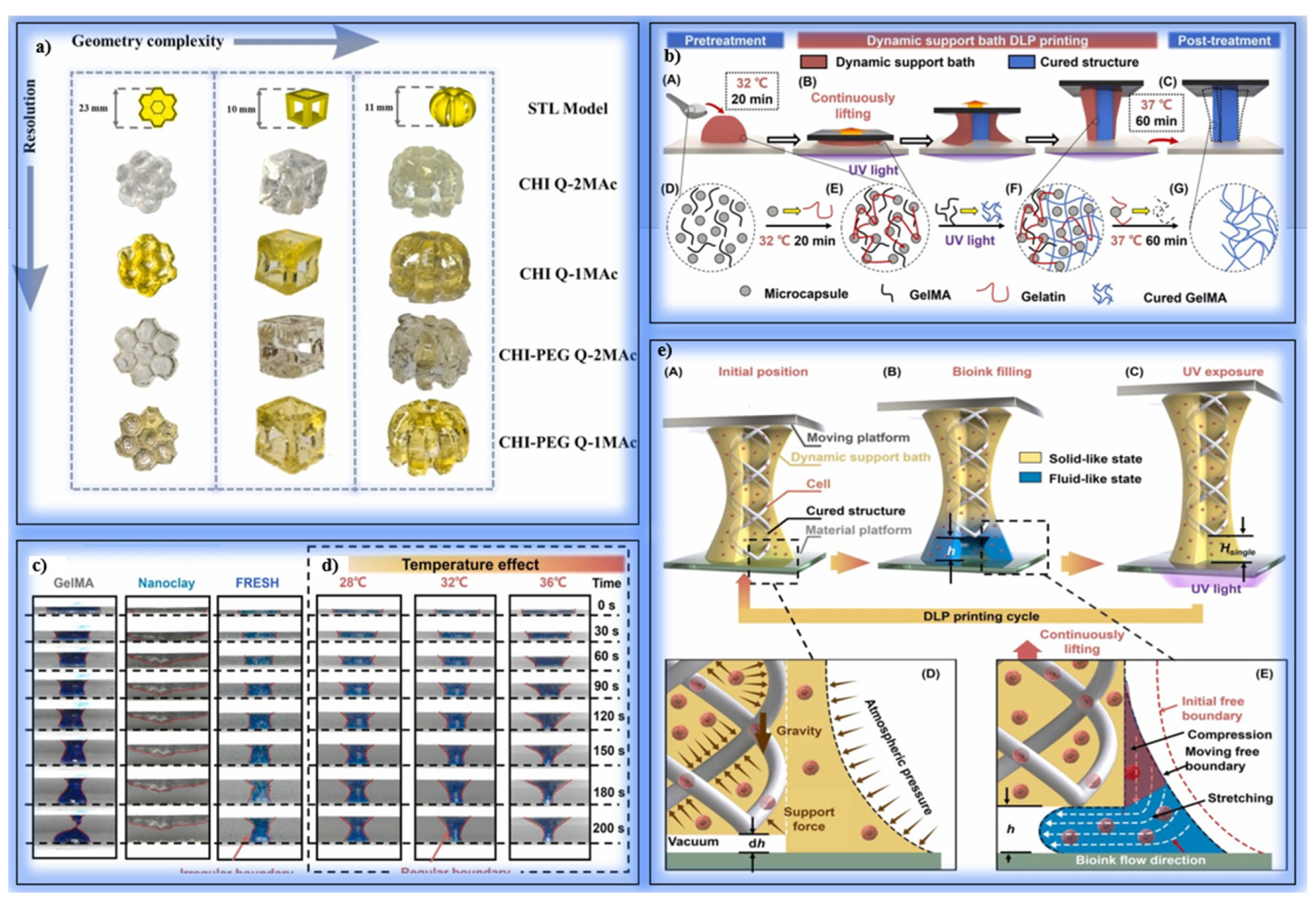
| Printing Technology | Resolution | Printing Speed | Cell Viability | Bioink Compatibility | Structural Complexity | Application Area |
|---|---|---|---|---|---|---|
| DLP | High (micron-level) | High (layer-wide projection) | Medium–high (in light-exposed environments) | Photopolymer-based bioinks | High (complex 3D structures possible) | Soft tissues, microstructures, vascular constructs |
| Extrusion-based | Low–Medium | Low–Medium | High (low mechanical stress) | Wide variety of bioinks | Limited (collapse risk) | Cartilage, bone, skin tissue |
| Inkjet-based | Medium–High | High | Medium (thermal/mechanical stress may occur) | Low-viscosity inks only | Limited (due to low viscosity) | Skin, neural tissue |
| Laser-assisted | High | Medium | High | Photoreactive bioinks | High | Vascular, skin, neural tissues |
| Type | Examples | Advantages | Disadvantages | Applications |
|---|---|---|---|---|
| Photopolymer Resins |
|
|
|
|
| Smart Photopolymers |
|
|
|
|
| Hydrogels |
|
|
|
|
| Ceramic Modified Resins |
|
|
|
|
| Nanocomposite Resins |
|
|
|
|
| Advantages | Details |
|---|---|
| Optimization of the Production Process | Production parameters (light exposure time, layer thickness, curing time) can be optimized with AI algorithms. |
| Error Detection and Correction | ML models can instantly detect errors (layer shifting, deformation) that may occur during production and suggest corrective steps. |
| Material Selection and Adaptation | With data analysis, appropriate options can be offered according to the mechanical strength, biocompatibility, and biodegradation behavior of different smart materials. |
| Predictive Performance Models | Before the production process, the mechanical and biological performances of the structures can be predicted by simulations. |
| Personalized Building Design | AI-powered design tools help design patient-based bio-constructs faster and more efficiently. |
| Data-Based Continuous Improvement | Data obtained from each production process are analyzed using ML algorithms, ensuring continuous improvement of processes. |
| Increased Speed and Efficiency | Thanks to optimum parameters, production time is shortened and energy/material waste is reduced. |
| Model Compatibility and Scalability | It can be easily adapted to production scale and new materials, increasing process flexibility. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alparslan, C.; Bayraktar, Ş. Advances in Digital Light Processing (DLP) Bioprinting: A Review of Biomaterials and Its Applications, Innovations, Challenges, and Future Perspectives. Polymers 2025, 17, 1287. https://doi.org/10.3390/polym17091287
Alparslan C, Bayraktar Ş. Advances in Digital Light Processing (DLP) Bioprinting: A Review of Biomaterials and Its Applications, Innovations, Challenges, and Future Perspectives. Polymers. 2025; 17(9):1287. https://doi.org/10.3390/polym17091287
Chicago/Turabian StyleAlparslan, Cem, and Şenol Bayraktar. 2025. "Advances in Digital Light Processing (DLP) Bioprinting: A Review of Biomaterials and Its Applications, Innovations, Challenges, and Future Perspectives" Polymers 17, no. 9: 1287. https://doi.org/10.3390/polym17091287
APA StyleAlparslan, C., & Bayraktar, Ş. (2025). Advances in Digital Light Processing (DLP) Bioprinting: A Review of Biomaterials and Its Applications, Innovations, Challenges, and Future Perspectives. Polymers, 17(9), 1287. https://doi.org/10.3390/polym17091287





