Photocurable Crosslinker from Bio-Based Non-Isocyanate Poly(hydroxyurethane) for Biocompatible Hydrogels
Abstract
1. Introduction: Green Synthesis from Bio-Based Source and Functionalization of Non-Isocyanate Poly(hydroxyurethane)
- The development and investigation of bio-based polyhydroxyurethanes (BPHUs) as a specialized form of non-isocyanate polyurethanes (NIPUs), derived from NIPU chemistry. We focus on their synthesis routes, starting from bio-based cyclic carbonates and amines, exploring the effects of aromatic versus aliphatic precursors on the resulting materials.
- The suitability of the synthesized BPHU compounds as alternative crosslinkers in hydrogels, specifically compared to the commonly used GelMa. These photocurable hydrogels are evaluated as HEAA photoinks for medical additive manufacturing applications, such as biocompatible implant structures.
2. Materials and Methods
2.1. Compounds
2.2. Analytics
- Chromatography: The reactions were monitored by TLC (thin-layer chromatography) performed by using the POLYGRAM® SIL G/UV254 from Macherey-Nagel, Düren, Germany. Column chromatography was performed by using Merck silica gel (particle size: 0.040–0.063 mm), Darmstadt, Germany.
- Gel Permeation Chromatography (GPC): The average molecular weights and molecular weight distributions (polydispersity index, PDI) of the produced polymers were determined by gel permeation chromatography (GPC). All of the measurements were performed on a Waters GPC system with a column of polysulfone styrenes. The GPC was equipped with a differential refractive index and UV detectors. Polymer solutions (2 mg/mL) were prepared in DMSO and filtered (1 μm PTFE), and then 100 μL of the solution was added to the column. The measurements were carried out at 80 °C with a flow rate of 1 mL/min. The samples were read by a UV detector at a wavelength of 280 nm. Pullulan standards in DMSO with 0.1 M LiBr were used for calibration.
- FTIR Spectroscopy: The Fourier transform infrared (FTIR) spectra were collected using an ATR-equipped Nicolet iS5 FTIR spectrometer (Thermo Fisher Scientific, USA). Samples of the NIPU intermediates and methacrylated derivatives (BPHU-MA) were recorded in the range of 4000–600 cm−1 with a resolution of 4 cm−1. Characteristic vibrations were monitored to confirm urethane formation (ν(N–H), ~1514 cm−1), the ring opening of cyclic carbonates (ν(C=O), 1809/1044 cm−1), and methacrylate functionalities (ν(C=C), 1640 cm−1).
- NMR spectra were measured on a Unity INOVA 500 NB spectrometer (Varian, Palo Alto, CA, USA) at 298 K. The 1H-NMR spectra and the 13C signals were recorded for the 13C-NMR spectra. Coupling constants (J) are reported in Hertz. Abbreviations to denote the multiplicity of a particular signal include s (singlet), d (doublet), t (triplet), dd (double doublet), q (quartet), and m (multiplet). The polymer composition was determined by 1H-NMR. The aromatic protons were set in relation to the hydroxy group of the HEAA component.
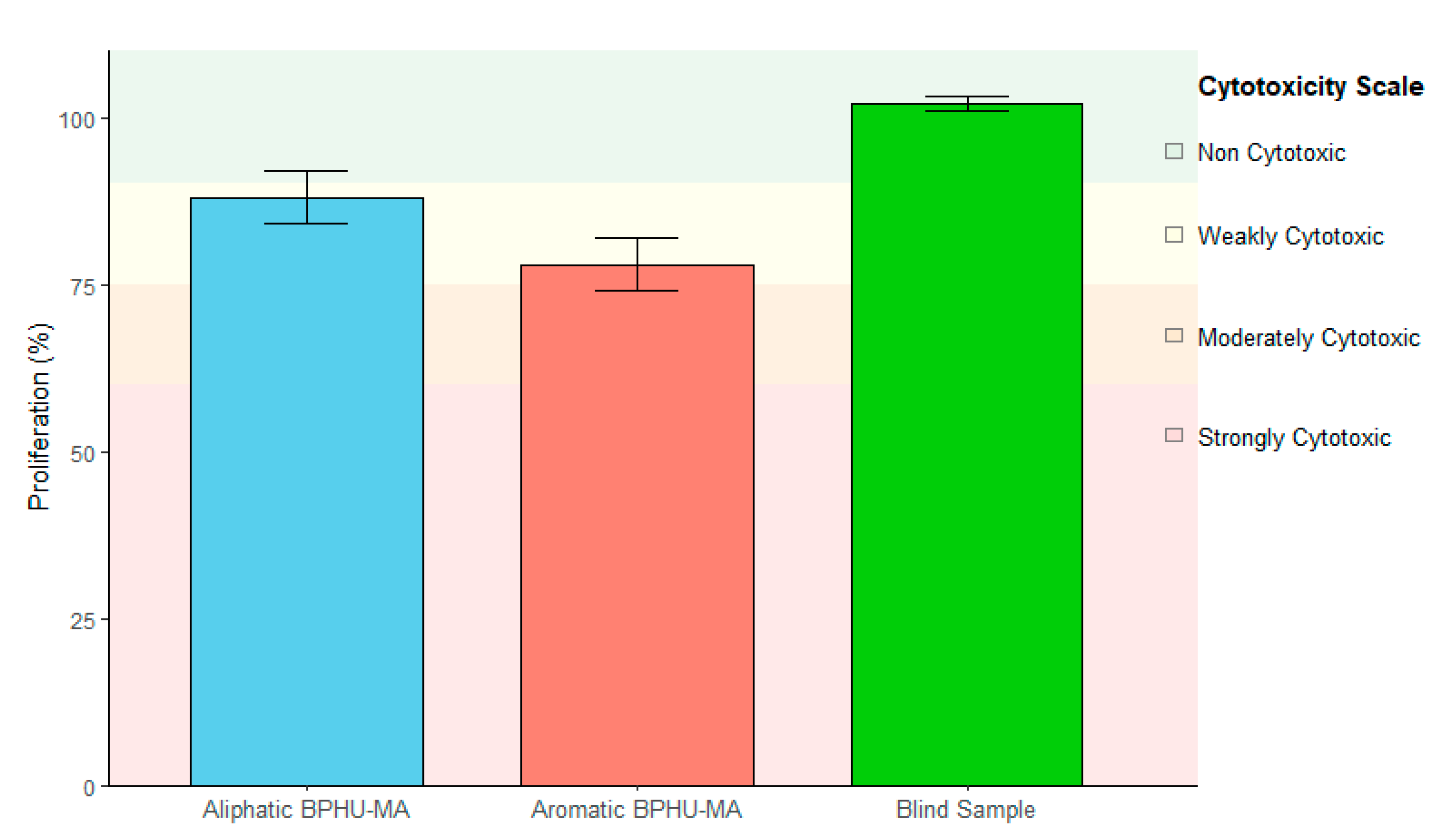
- Biocompatibility: The biocompatibility of methacrylated polyhydroxyurethanes (BPHU 3 and BPHU 4) was evaluated following standardized cytotoxicity protocols. The materials were formulated into a hydrogel matrix and UV-cured for 1 min. Cytotoxicity testing was conducted by the accredited laboratory at Fraunhofer Stuttgart in accordance with DIN EN ISO 10993-5:2009 [28], which assesses the in vitro cytotoxic potential of medical devices. Extraction of the test material was performed following DIN EN ISO 10993-12:2012 [29] ensuring reproducible conditions for the preparation of the extracts. Human HaCaT keratinocyte cells were incubated with these extracts for 24 ± 2 h. Negative (fresh cell culture medium), positive (known cytotoxic substance), and blind controls (extraction medium subjected to the same extraction conditions as the material samples) were included in parallel to validate the test setup. After the incubation period, the cell viability was assessed to determine any cytotoxic effects. The results classified both materials as non-cytotoxic based on the criteria defined in the standard. This standardized approach ensured robust, reproducible, and internationally comparable results for assessing the cytocompatibility of hydrogel-based materials.
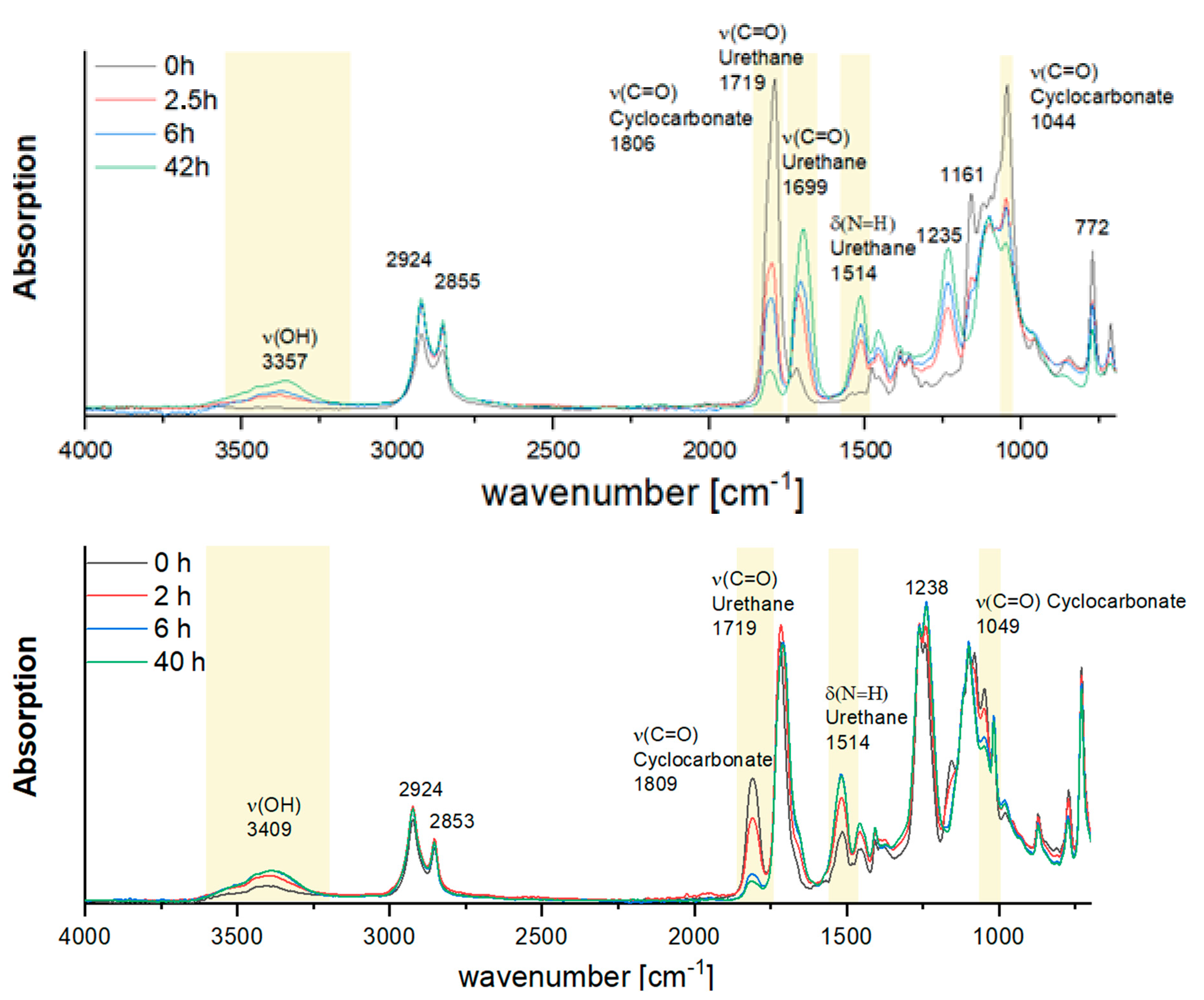
- Photostability: To assess the structural stability of the crosslinked hydrogels under prolonged irradiation, samples of aliphatic and aromatic BPHU-MA were exposed to continuous UV-A light (365 nm and 10 mW/cm2) for up to 42 h. At predefined time points (0 h, 2.5 h, 6 h, and 42 h), the samples were analyzed via ATR-FTIR spectroscopy using a Nicolet iS5 spectrometer (Thermo Fisher Scientific, USA) equipped with an iD7 ATR accessory. The IR spectra were collected in the range of 4000–600 cm−1 at a resolution of 4 cm−1 with 32 scans per measurement. Changes in the characteristic absorption bands, including ν(N–H), ν(OH), and ν(C=O), were evaluated to monitor potential degradation or structural changes in the polymer network.
- Determination of Swelling Degree: The swelling degree (Q) of the crosslinked polymer networks was determined to assess their ability to absorb aqueous fluids, an important parameter for biomedical applications. After determining the dry mass (mdry) of the polymer samples, they were immersed in distilled water at room temperature for a defined period (typically 24 h). Following incubation, the swollen samples were carefully removed, blotted to remove excess surface water, and immediately weighed (mswollen). The swelling degree was then calculated according to the following formula:where
- Determination of Gel Content: To evaluate the efficiency of the crosslinking reaction in the photopolymerized networks, the gel content (G) was determined gravimetrically. Polymer films were first weighed in their dry state (initial mass: mpolymer), and then subjected to swelling in an appropriate solvent to remove all soluble, non-crosslinked fractions. After a defined swelling time, the remaining insoluble polymer was thoroughly dried and reweighed (mgew). The gel content was calculated using the following equation:where
2.3. Synthesis
3. Results
3.1. FTIR Analysis of BPHU Formation with Aromatic and Aliphatic Dicyclic Carbonates
3.2. GPC Analysis of Methacrylated BPHUs
3.3. Biocompatibility Testing
3.4. Gel Content and Swelling Behavior
3.5. UV Stability
3.6. 1H- and 13C-NMR Analysis and Degree of Methacrylation (DoM) of Endproducts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirchberg, A.; Khabazian Esfahani, M.; Röpert, M.-C.; Wilhelm, M.; Meier, M.A.R. Sustainable Synthesis of Non-Isocyanate Polyurethanes Based on Renewable 2,3-Butanediol. Macromol. Chem. Phys. 2022, 223, 2200010. [Google Scholar] [CrossRef]
- Figovsky, O.; Shapovalov, L.; Leykin, A.; Birukova, O.; Potashnikova, R. Recent Advances in the Development of Non-Isocyanate Polyurethanes Based on Cyclic Carbonates. PU Mag. 2013, 10, 256–263. [Google Scholar]
- Jin, L.; Zhao, W.; Feng, Z.; Zhao, Y.; Zhang, Y.; Chen, N.; Ni, Y. Environmentally Friendly Synthesis of Poly(Hydroxyurethane) Vitrimer Microspheres and Their Potential Application in Moisture-Enabled Electric Generation. ACS Appl. Polym. Mater. 2024, 6, 2294–2304. [Google Scholar] [CrossRef]
- Kuang, G.; Bakhshi, H.; Meyer, W. Urethane-Acrylate-Based Photo-Inks for Digital Light Processing of Flexible Materials. J. Polym. Res. 2023, 30, 141. [Google Scholar] [CrossRef]
- Bakhshi, H.; Kuang, G.; Wieland, F.; Meyer, W. Photo-Curing Kinetics of 3D-Printing Photo-Inks Based on Urethane-Acrylates. Polymers 2022, 14, 2974. [Google Scholar] [CrossRef]
- Kuang, G.; Bakhshi, H.; Meyer, W. The Role of Photo-Initiators in the Inks for Medical 3D Printing. In Proceedings of the ProMatLeben—Midtermkonferenz 2021, Online, 18–19 May 2021. [Google Scholar]
- Herwig, G.; Pérez-Madrigal, M.M.; Dove, A.P. Customized Fading Scaffolds: Strong Polyorthoester Networks via Thiol–Ene Cross-Linking for Cytocompatible Surface-Eroding Materials in 3D Printing. Biomacromolecules 2021, 22, 1472–1483. [Google Scholar] [CrossRef]
- Farkhondehnia, M.; Maric, M. Design of Crosslinked Networks with Hydroxyurethane Linkages via Bio-based Alkyl Methacrylates and Diamines. J. Appl. Polym. Sci. 2023, 140, e54039. [Google Scholar] [CrossRef]
- Visser, D.; Bakhshi, H.; Rogg, K.; Fuhrmann, E.; Wieland, F.; Schenke-Layland, K.; Meyer, W.; Hartmann, H. Green Chemistry for Biomimetic Materials: Synthesis and Electrospinning of High-Molecular-Weight Polycarbonate-Based Nonisocyanate Polyurethanes. ACS Omega 2022, 7, 39772–39781. [Google Scholar] [CrossRef]
- Comin, E.; Aquino, A.S.; Favero, C.; Mignoni, M.L.; De Souza, R.F.; De Souza, M.O.; Pergher, S.B.C.; Campos, C.X.D.S.; Ber-nardo-Gusmão, K. Cyclic Carbonate Synthesis via Cycloaddition of CO2 and Epoxides Catalysed by Beta Zeolites Containing Alkyl Imidazolium Ionic Liquids Used as Structure-Directing Agents. Mol. Catal. 2022, 530, 112624. [Google Scholar] [CrossRef]
- Usman, M.; Rehman, A.; Saleem, F.; Abbas, A.; Eze, V.C.; Harvey, A. Synthesis of Cyclic Carbonates from CO2 Cycloaddition to Bio-Based Epoxides and Glycerol: An Overview of Recent Development. RSC Adv. 2023, 13, 22717–22743. [Google Scholar] [CrossRef]
- Hebda, E.; Ozimek, J.; Szołdrowska, K.; Pielichowski, K. Synthesis of Bis(Cyclic Carbonates) from Epoxy Resin under Microwave Irradiation: The Structural Analysis and Evaluation of Thermal Properties. Molecules 2024, 29, 250. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, Y.; Zhu, X.; Yang, G.; Wu, G. Simultaneous Activation of Carbon Dioxide and Epoxides to Produce Cyclic Carbonates by Cross-linked Epoxy Resin Organocatalysts. ChemCatChem 2023, 15, e202300360. [Google Scholar] [CrossRef]
- Chen, C.; Gnanou, Y.; Feng, X. Ultra-Productive Upcycling CO2 into Polycarbonate Polyols via Borinane-Based Bifunctional Organocatalysts. Macromolecules 2023, 56, 892–898. [Google Scholar] [CrossRef]
- Lv, Z.; Tang, Y.; Dong, S.; Zhou, Q.; Cui, G. Polyurethane-Based Polymer Electrolytes for Lithium Batteries: Advances and Perspectives. Chem. Eng. J. 2022, 430, 132659. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Mindt, M.; Pérez-García, F. Biotechnological Production of Mono- and Diamines Using Bacteria: Recent Progress, Applications, and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 3583–3594. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, L.; Wang, F.; Deng, Y.; Jiang, Z.; Li, A. Transforming Inert Cycloalkanes into α,ω-Diamines by Designed Enzymatic Cascade Catalysis. Angew. Chem. 2023, 135, e202215935. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef]
- Mhd Haniffa, M.A.C.; Munawar, K.; Ching, Y.C.; Illias, H.A.; Chuah, C.H. Bio-based Poly (hydroxy urethane) s: Synthesis and Pre/Post-Functionalization. Chem. Asian J. 2021, 16, 1281–1297. Available online: https://aces.onlinelibrary.wiley.com/doi/full/10.1002/asia.202100226 (accessed on 2 December 2024). [CrossRef]
- Bibolet, E.R.; Fernando, G.E.; Shah, S.M. Renewable 1,4-Butanediol; University of Pennsylvania: Philadelphia, PA, USA, 2011. [Google Scholar]
- Cheng, J.; Li, J.; Zheng, L. Achievements and Perspectives in 1,4-Butanediol Production from Engineered Microorganisms. J. Agric. Food Chem. 2021, 69, 10480–10485. [Google Scholar] [CrossRef]
- Kumar, P.; Park, H.; Yuk, Y.; Kim, H.; Jang, J.; Pagolu, R.; Park, S.; Yeo, C.; Choi, K.-Y. Developed and Emerging 1,4-Butanediol Commercial Production Strategies: Forecasting the Current Status and Future Possibility. Crit. Rev. Biotechnol. 2024, 44, 530–546. [Google Scholar] [CrossRef]
- Huo, G.; Foulquié-Moreno, M.R.; Thevelein, J.M. Development of an Industrial Yeast Strain for Efficient Production of 2,3-Butanediol. Microb. Cell Factories 2022, 21, 199. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, F.; Mohammadi, A. Assessing the environmental footprints and material flow of 2,3-butanediol production in a wood-based biorefinery. Bioresour. Technol. 2023, 387, 129642. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.H. Biobased Terephthalic Acid. In Industrial Arene Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 2037–2058. ISBN 978-3-527-82799-2. [Google Scholar]
- He, Y.; Luo, Y.; Yang, M.; Zhang, Y.; Zhu, L.; Fan, M.; Li, Q. Selective Catalytic Synthesis of Bio-Based Terephthalic Acid from Lignocellulose Biomass. Appl. Catal. A Gen. 2022, 630, 118440. [Google Scholar] [CrossRef]
- Ogunjobi, J.K.; Farmer, T.J.; McElroy, C.R.; Breeden, S.W.; Macquarrie, D.J.; Thornthwaite, D.; Clark, J.H. Synthesis of Biobased Diethyl Terephthalate via Diels–Alder Addition of Ethylene to 2,5-Furandicarboxylic Acid Diethyl Ester: An Alternative Route to 100% Biobased Poly(Ethylene Terephthalate). ACS Sustain. Chem. Eng. 2019, 7, 8183–8194. [Google Scholar] [CrossRef]
- DIN EN ISO 10993-5:2009-10; Biologische Beurteilung von Medizinprodukten_- Teil_5: Prüfungen Auf In-Vitro-Zytotoxizität (ISO_10993-5:2009); Deutsche Fassung EN_ISO_10993-5:2009. DIN Media Kommentar: Berlin, Germany, 2009. [CrossRef]
- BS EN ISO 10993-12:2021; Biological Evaluation of Medical Devices Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021. Available online: https://www.en-standard.eu/bs-en-iso-10993-12-2021-biological-evaluation-of-medical-devices-sample-preparation-and-reference-materials/ (accessed on 26 March 2025).

| Aromatic BPHU-MA 4 | Aliphatic BPHU-MA 4′ | |
|---|---|---|
| Mw [g/mol] | 42.974 | 2867 |
| Mn [g/mol] | 2631 | 1905 |
| PDI | 17.4 | 1.5 |
| Yield [%] | 62 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennig, K.; Vacun, G.; Thude, S.; Meyer, W. Photocurable Crosslinker from Bio-Based Non-Isocyanate Poly(hydroxyurethane) for Biocompatible Hydrogels. Polymers 2025, 17, 1285. https://doi.org/10.3390/polym17091285
Hennig K, Vacun G, Thude S, Meyer W. Photocurable Crosslinker from Bio-Based Non-Isocyanate Poly(hydroxyurethane) for Biocompatible Hydrogels. Polymers. 2025; 17(9):1285. https://doi.org/10.3390/polym17091285
Chicago/Turabian StyleHennig, Kathleen, Gabriele Vacun, Sibylle Thude, and Wolfdietrich Meyer. 2025. "Photocurable Crosslinker from Bio-Based Non-Isocyanate Poly(hydroxyurethane) for Biocompatible Hydrogels" Polymers 17, no. 9: 1285. https://doi.org/10.3390/polym17091285
APA StyleHennig, K., Vacun, G., Thude, S., & Meyer, W. (2025). Photocurable Crosslinker from Bio-Based Non-Isocyanate Poly(hydroxyurethane) for Biocompatible Hydrogels. Polymers, 17(9), 1285. https://doi.org/10.3390/polym17091285








