New Explosive-Circulation Technology of Tire Recycling for the Production of Crumb Rubber with Modified Surface
Abstract
1. Introduction
- Barodestruction of tires, in which tire rubber is brought to a fluid state in an industrial press;
- Pyrolysis. This tire recycling method produces pyrolysis oil, carbon residue, and metal cord. Pyrolysis oil can be further purified and used as fuel. Carbon residue can be used to make new rubber or dyes. Metal cord is remelted [13];
- Foam concentrates in electric arc furnaces [14];
2. Materials and Methods
2.1. Materials
- Radial solid-steel cord: 385/65R22.5; 315/60R22.5; 295/80R22.5; 245/70R; 215/75 R (Michelin, Bridgestone, Continental, as well as those made in Russia, a total of about 38,000 kg);
- Radial solid-steel cord: 385/65R22.5; 315/60R22.5; 295/80R22.5; 215/75 R (People’s Republic of China, a total of about 6000 kg);
- Diagonal tires: 3270 kg;
- Tires with the maximum possible visible defects on the surface associated with long-term storage (about 1000 kg);
- Aircraft tires with textile cord (about 1000 kg).
2.2. Methods
2.2.1. Wettability Test
2.2.2. X-Ray Diffraction Study
2.2.3. Atomic Force Microscopy Study
2.2.4. Gel Permeation Chromatography Study
2.2.5. Gas Chromatography Study
2.2.6. Thermodesorption Mass Spectrometry Study
2.2.7. X-Ray Photoelectron Spectroscopy Study
3. Results and Discussion
3.1. Characteristics of Explosive-Circulation Technology
3.1.1. Description of the Design of the Explosion Circulator and Characteristics of Its Operation
- -
- Increase the efficiency of tire shredding by explosion;
- -
- Ensure localization of the explosion and guaranteed protection of personnel and equipment from all types of damaging effects of a charge explosion (shock wave, gaseous products, spread of tire fragments).
- -
- The strength (to explosion) of the device is calculated in accordance with the industry calculation method OST-92, which guarantees the operability of the device with a 20-fold safety margin for the destruction conditions;
- -
- The device has passed preliminary strength tests by the action of an explosion of an explosive charge (EC) and has shown a 20-fold safety margin for the destruction conditions.
- -
- Commissioning of the device is carried out in accordance with the permission of Federal Environmental, Industrial and Nuclear Supervision Service of Russia upon fulfillment of a number of requirements that ensure the safe operation of the device (Operating Manual, Blasting Operations Project, Expertise on Exploding Operations Project, etc.).
3.1.2. Description of the Technological Process
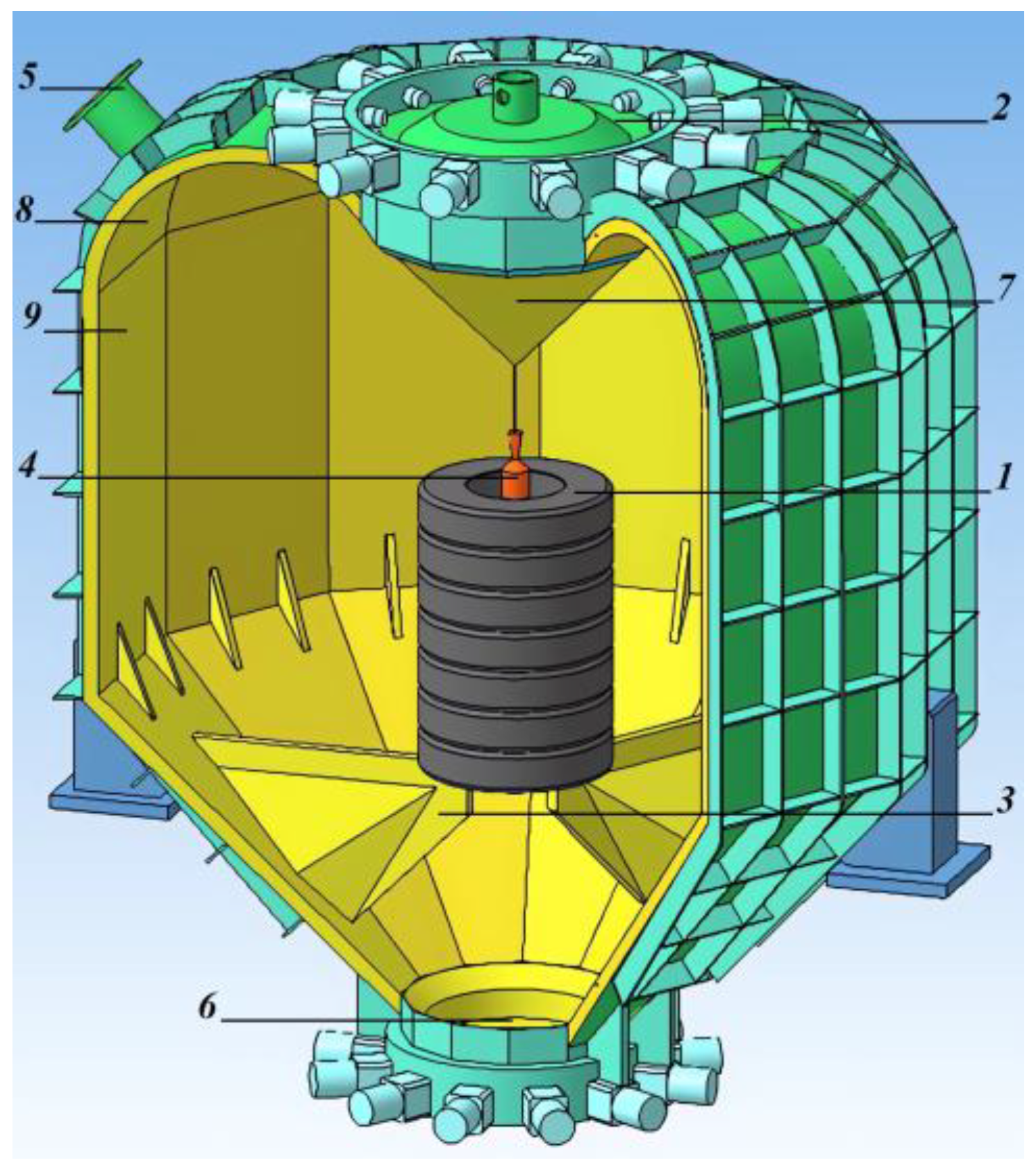
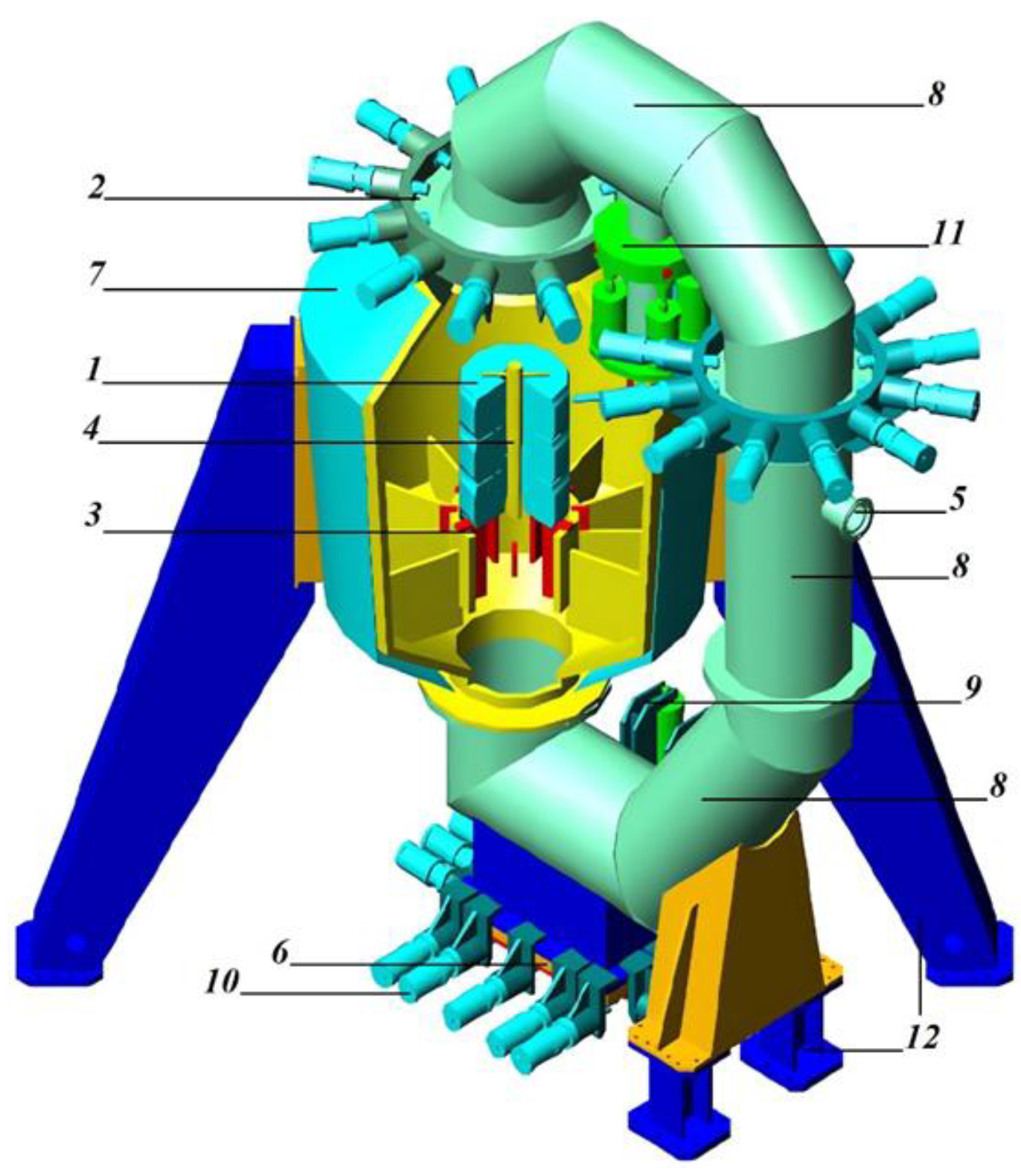
- The formation of tire packages with a height of about 2.4 m and a mass of up to 1000 kg (1 in Figure 1). Each package consists of seven tire briquettes stacked on top of each other. Each of the briquettes can contain one truck tire into which three–four car tires or one truck tire is inserted.
- This package is cooled by air turbo-cooling machines to a temperature of minus 70–80 °C. Then, through the hatch (2 in Figure 1), the package is placed into the explosion circulator and installed on internal supports (3 in Figure 1). A coaxial cylindrical explosive charge is installed inside the tire package (4 in Figure 1). Around the coaxial cylindrical explosive charge there is a cylindrical shell filled with an aqueous solution of technological additives. These additives can significantly reduce the amount of toxic gases generated during an explosion and then escaping through the gaseous products removal pipe (5 in Figure 1).
- The explosion circulator is sealed. The explosive charge is initiated. Under the influence of gaseous explosion products, the tire package expands with an increase in the diameter of the package by three–four times. This leads to the division of tires into separate factions. The fractions are further crushed upon impact with the surface of the explosion circulator. The explosion circulator is designed in such a way that the shape of its surface causes the movement of shock waves along a trajectory close to a tangent to its surface. After the completion of the circulation of shock waves and explosion products, static pressure of a gaseous medium of up to 1 atm is formed inside the explosion circulator.
- Gaseous products of the explosion are removed through the pipe branch (5 in Figure 1) into the gas purification system. After the excess pressure of the medium in the explosion circulator becomes equal to zero, the tire-shredding products are removed from the explosion circulator through the hatch (6 in Figure 1).
- The technology allows for the processing of 10 packages of tires per hour, which equals up to 30 thousand tons of tires per year.
- Rubber crumbs with particle sizes of 0–10 mm—up to 65% of the total mass of crushed tire packages.
- Metal cord—19–23% in the form of needles and wire tangles, cleared of rubber (with rubber residues up to 1–2%).
- Textile cord—8–12%, in the form of short threads and fluff.
- -
- Particles with a size of 0–1 mm (CR 0–1 mm) make up to 30% of the rubber mass;
- -
- Particles with a size 1–3 (CR 1–3 mm), up to 25%;
- -
- Particles with a size 3–5 mm (CR 3–5 mm), up to 20%;
- -
- Particles with a size of 5–10 mm (CR 5–10 mm), up to 20%;
- -
- Particles larger than 10 mm, approximately 10%.
3.1.3. Description of the Explosive and Strength Characteristics of the Explosion Circulator
3.1.4. Advantages of Explosion-Circulation Technology
- -
- Production areas for explosion-circulation technology are three–four times smaller than for mechanical technology;
- -
- The price of equipment for explosion-circulation technology is approximately 30% less than the price of equipment for mechanical technology;
- -
- The energy consumption for crushing one ton of tires using explosion-circulation technology is about 250 kWh/t, or approximately two times less than when using mechanical technology;
- -
- The high wear of equipment used in mechanical method: repair of shredders and sharpening of knives once a week; replacement of knives on average every 2.5 months after nine sharpenings; shredders wear out after 5–10 years. This requires frequent shutdown of the process, which reduces its economic efficiency;
- -
- The detected presence of polar groups on the surface of the crumb produced using explosion-circulation technology leads to a significant improvement in the adhesion of the crumb;
- -
- Rubber crumb produced using explosion-circulation technology has improved adhesion in composites due to the presence of polar groups on the crumb surface, which in turn eliminates additional costs for modifying the crumb surface.
3.1.5. Application of Crumb Rubber in Composites
- -
- Needle penetration at 25 °C according to [56],
- -
- Softening point of ring and ball according to [57],
- -
- Deformation behavior in the dynamic shear rheometer (DSR) according to [58] Tests were carried out in the temperature range of 30–90 °C,
- -
- Behavior at low temperatures—bending beam rheometer (BBR) according to [58],
- -
- Elastic recovery according to [59].
- -
- Needle penetration decreased from 50.3 to 39.3 1/10 mm;
- -
- Hardness at −16 °C decreased from 217 to 161 MPa;
- -
- Complex shear modulus in the rheometer increased from 416,100 to 580,300 and from 15,461 to 39,700 Pa at 30 °C and 50 °C, respectively.
3.2. Structure and Physicochemical Properties of the Resulting Crumb Rubber
3.2.1. Wettability of Regenerated Crumb Rubber
3.2.2. X-Ray Diffraction Study
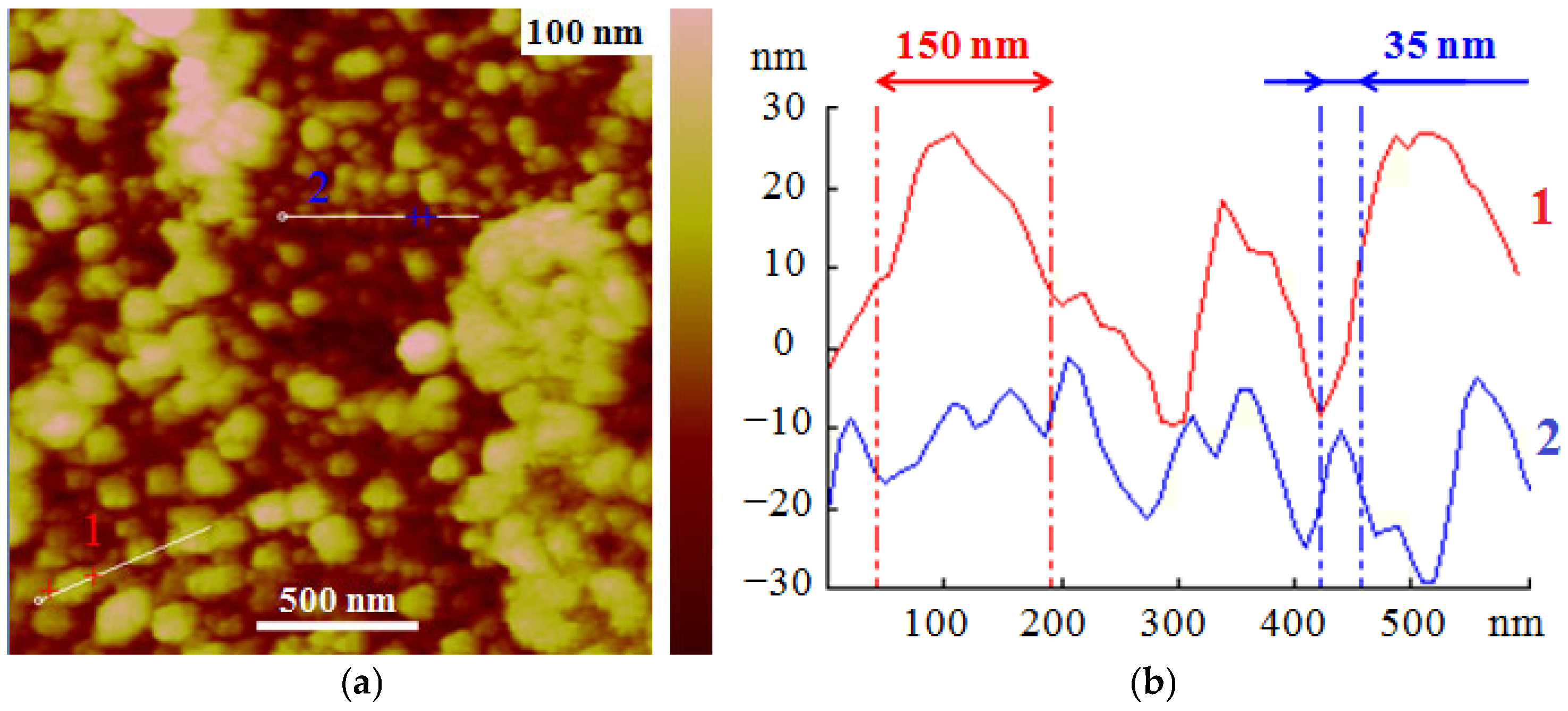
3.2.3. Surface Topography
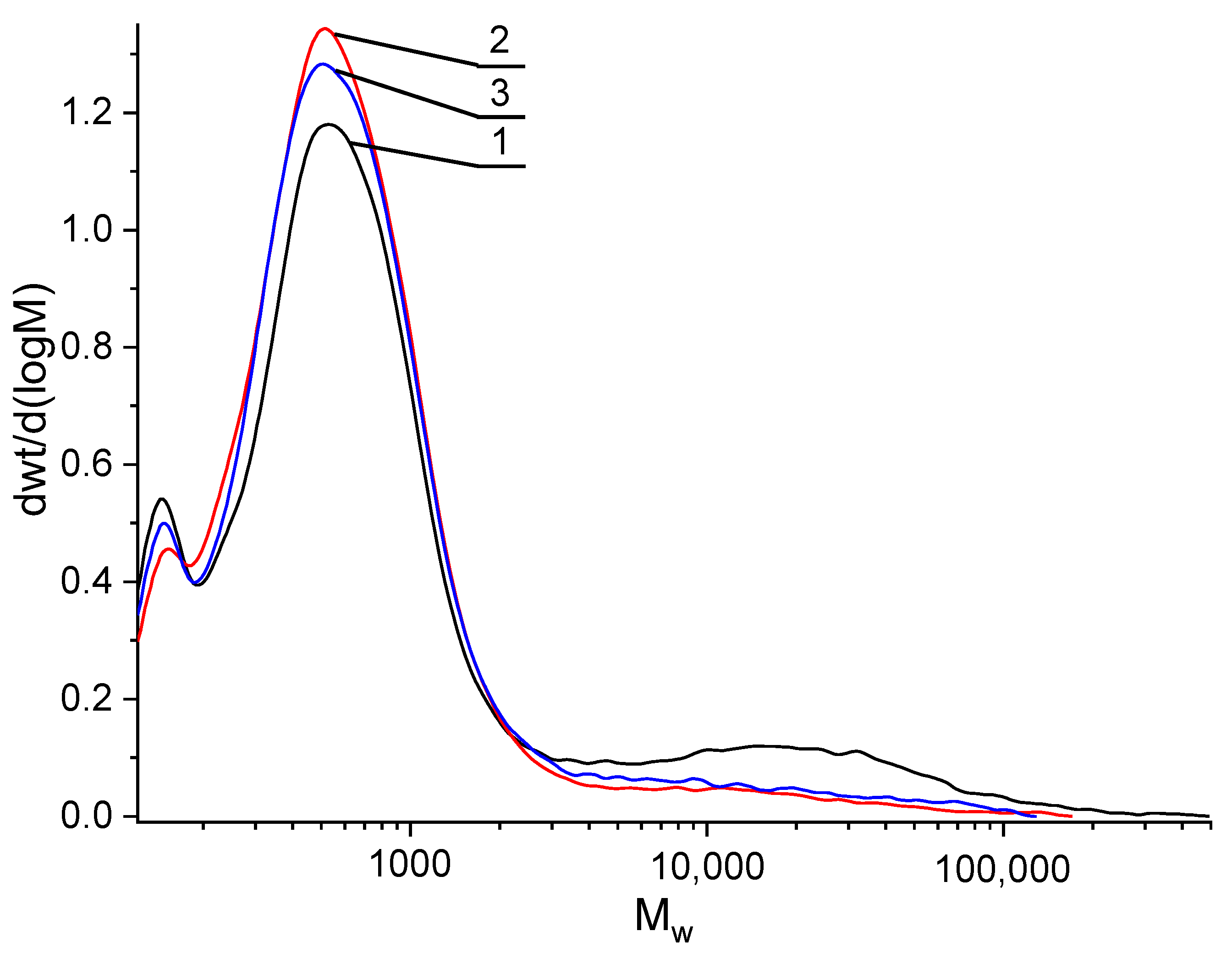
3.2.4. Gel Permeation Chromatography
3.2.5. Gas Phase Analysis by Means of Gas Chromatography
3.2.6. Mass Spectrometry
3.2.7. X-Ray Photoelectron Spectroscopy
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CR | Crumb rubber |
| GPC | Gel permeation chromatography |
| AFM | Atomic force microscopy |
| XPS | X-ray photoelectron spectroscopy |
| SPME | Solid-phase microextraction |
| EC | Explosive charge |
References
- Ghabchi, R.; Arshadi, A.; Zaman, M.; March, F. Technical challenges of utilizing ground tire rubber in asphalt pavements in the United States. Materials 2021, 14, 4482. [Google Scholar] [CrossRef] [PubMed]
- Luhar, I.; Luhar, S. Rubberized Geopolymer Composites: Value-Added Applications. J. Compos. Sci. 2021, 5, 312. [Google Scholar] [CrossRef]
- Ćetković, J.; Lakić, S.; Žarković, M.; Vujadinović, R.; Knežević, M.; Živković, A.; Cvijović, J. Environmental Benefits of Air Emission Reduction in the Waste Tire Management Practice. Processes 2022, 10, 787. [Google Scholar] [CrossRef]
- Saurabh, V.S. Allied Market Research. Tire Recycling Market by Process (Pyrolysis, Shredding), by Product (Crumbed Rubber, Tire Derived Fuel, Others), by Application (Manufacturing, Construction, Rubber Products, Others): Global Opportunity Analysis and Industry Forecast 2021, 2021–2031. Available online: https://www.alliedmarketresearch.com/tire-recycling-market-A17016 (accessed on 26 September 2023).
- Aoudia, K.; Azem, S.; Hocine, N.A.; Gratton, M.; Pettarin, V.; Seghar, S. Recycling of waste tire rubber: Microwave devulcanization and incorporation in a thermoset resin. Waste Manag. 2017, 60, 471–481. [Google Scholar] [CrossRef]
- Hussain, T.A. Using the scrap tires to produce a flexible coupler. Kufa J. Eng. 2018, 9, 106–117. [Google Scholar] [CrossRef]
- Shen, M.; Liu, J.; Xin, Z. Mechanical Properties of Rubber Sheets Produced by Direct Molding of Ground Rubber Tire Powder. J. Macromol. Sci. Part B Phys. 2018, 58, 16–27. [Google Scholar] [CrossRef]
- Forrest, M.J. Recycling and Re-Use of Waste Rubber, 2nd ed.; De Gruyter STEM: Berlin, Germany; Boston, MA, USA,, 2019. [Google Scholar]
- Lapkovskis, V.; Mironovs, V.; Kasperovich, A.; Myadelets, V.; Goljandin, D. Crumb Rubber as a Secondary Raw Material from Waste Rubber: A Short Review of End-Of-Life Mechanical Processing Methods. Recycling 2020, 5, 32. [Google Scholar] [CrossRef]
- Kiss, L.; Simon, D.A.; Petrény, R.; Kocsis, D.; Bárány, T.; Mészáros, L. Ground tire rubber filled low-density polyethylene: The effect of particle size. Adv. Ind. Eng. Polym. Res. 2022, 5, 12–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, G.; Leng, Z.; Kong, P.; Wang, H.; Yang, J.; Qiu, W.; Xu, Z.; Chen, X. Investigation on storage stability and rheological properties of environment-friendly devulcanized rubber modified asphalt. Phys. Chem. Earth Parts A/B/C 2023, 129, 103332. [Google Scholar] [CrossRef]
- Singh, P.; Singh, D.N.; Debbarma, S. Macro- and micro- mechanisms associated with valorization of waste rubber in cement-based concrete and thermoplastic polymer composites: A critical review. Constr. Build. Mater. 2023, 371, 130807. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Testa, D.; Lorenzi, R.; Vanacore, G.M.; Poli, F.; Soavi, F.; Specchia, S.; Giurlani, W.; Innocenti, M.; Rosi, L.; et al. Iron-based electrocatalysts derived from scrap tires for oxygen reduction reaction: Evolution of synthesis-structure-performance relationship in acidic, neutral and alkaline media. Electrochim. Acta 2022, 433, 141254. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Zaharia, M.; Rahman, M.; Khanna, R.; Saha-Chaudhury, N.; O’Kane, P.; Dicker, J.; Skidmore, C.; Knights, D. Recycling Rubber Tyres and Waste Plastics in EAF Steelmaking. Steel Res. Int. 2011, 82, 566–572. [Google Scholar] [CrossRef]
- Nakomcic-Smaragdakis, B.; Cepic, Z.; Senk, N.; Doric, J.; Radovanovic, L. Use of scrap tires in cement production and their impact on nitrogen and sulfur oxides emissions. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 485–493. [Google Scholar] [CrossRef]
- Vasiliu, L.; Gencel, O.; Damian, I.; Harja, M. Capitalization of tires waste as derived fuel for sustainable cement production. Sustain. Energy Technol. Assess. 2023, 56, 103104. [Google Scholar] [CrossRef]
- Mungyeko Bisulandu, B.-J.R.; Marias, F. Numerical Modeling of Thermochemical Conversion of Biomass and Tires as Fuels for Cement Clinker Production. Recycling 2023, 8, 41. [Google Scholar] [CrossRef]
- Fraile-Garcia, E.; Ferreiro-Cabello, J.; Defez, B.; Peris-Fajanes, G. Acoustic Behavior of Hollow Blocks and Bricks Made of Concrete Doped with Waste-Tire Rubber. Materials 2016, 9, 962. [Google Scholar] [CrossRef]
- Gupta, T.; Siddique, S.; Sharma, R.K.; Chaudhary, S. Effect of elevated temperature and cooling regimes on mechanical and durability properties of concrete containing waste rubber fiber. Constr. Build. Mater. 2017, 137, 35–45. [Google Scholar] [CrossRef]
- Zhang, B.; Poon, C.S. Sound insulation properties of rubberized lightmass aggregate concrete. J. Clean. Prod. 2018, 172, 3176–3185. [Google Scholar] [CrossRef]
- Rahimi, S.R.; Nikbin, I.M.; Allahyari, H.; Habibi, S.T. Sustainable approach for recycling waste tire rubber and polyethylene terephthalate (PET) to produce green concrete with resistance against sulfuric acid attack. J. Clean. Prod. 2016, 126, 166–177. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Guo, S.; Huang, Y.; Xu, W. Experimental study on fatigue properties of normal and rubberized self-compacting concrete under bending. Constr. Build. Mater. 2019, 205, 10–20. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Markovski, S.; Rodwell, G.; Rahman, M.T.; Kurmus, H.; Mirzababaei, M.; Arulrajah, A.; Horpibulsuk, S.; et al. Recycling waste rubber tyres in construction materials and associated environmental considerations: A review. Resour. Conserv. Recycl. 2020, 155, 104679. [Google Scholar] [CrossRef]
- Elshazly, F.A.; Mustafa, S.A.; Fawzy, H.M. Rubberized concrete properties and its structural engineering applications–an overview. Egypt. Int. J. Eng. Sci. Technol. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Mhaya, A.M.; Huseien, G.F.; Faridmehr, I.; Razin Zainal Abidin, A.; Alyousef, R.; Ismail, M. Evaluating mechanical properties and impact resistance of modified concrete containing ground Blast Furnace slag and discarded rubber tire crumbs. Constr. Build. Mater. 2021, 295, 123603. [Google Scholar] [CrossRef]
- Bu, C.; Zhu, D.; Liu, L.; Lu, X.; Sun, Y.; Yan, Z.; Yu, L.; Wei, Q. A Study on the Mechanical Properties and Microcosmic Mechanism of Basalt Fiber Modified Rubber Ceramsite Concrete. Buildings 2022, 12, 103. [Google Scholar] [CrossRef]
- Chen, A.; Han, X.; Wang, Z.; Zhang, Q.; Xia, X.; Ji, Y.; Li, K. Analytical evaluation of compressive strength for concrete with rubber fine aggregates and the predictive model. Constr. Build. Mater. 2022, 345, 128359. [Google Scholar] [CrossRef]
- Bu, C.; Zhu, D.; Lu, X.; Liu, L.; Sun, Y.; Yu, L.; Zhang, W.; Xiao, T. Optimization of the water–cement ratio of rubberized ceramsite concrete. J. Rubber Res. 2023, 26, 27–36. [Google Scholar] [CrossRef]
- Momeen Ul Islam, M.; Li, J.; Roychand, R.; Saberian, M. Investigation of durability properties for structural lightmass concrete with discarded vehicle tire rubbers: A study for the complete replacement of conventional coarse aggregates. Constr. Build. Mater. 2023, 369, 130634. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zhang, Z.X.; Pal, K.; Xin, Z.X.; Suh, J.; Kim, J.K. Prediction of mechanical properties of waste polypropylene/waste ground rubber tire powder blends using artificial neural networks. Mater. Des. 2010, 31, 3624–3629. [Google Scholar] [CrossRef]
- Hadiwardoyo, S.P.; Senawibowo, O.; Sumabrata, R.J.; Iskandar, D. Laboratory investigation on skid resistance of hot mix asphalt pavement with nano crumb rubber contribution. Civ. Eng. Archit. 2020, 8, 662–668. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, H.; Chen, Y.; Ji, J.; You, Z.; Zhang, Y. A review on compatibility between crumb rubber and asphalt binder. Constr. Build. Mater. 2021, 297, 123820. [Google Scholar] [CrossRef]
- Bueno, M.; Haag, R.; Heeb, N.; Mikhailenko, P.; Boesiger, L.; Poulikakos, L.D. Functional and environmental performance of plant-produced crumb rubber asphalt mixtures using the dry process. Mater. Struct. 2021, 54, 194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Wang, Z.; Jing, H.; Yang, B. Investigations on adhesion characteristics between high-content rubberized asphalt and aggregates. Polymers 2022, 14, 5474. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Ji, J.; Wang, D.; Li, R. Assessing the Potential for Sustainable Cold-Mix Asphalt Mixtures Based on Crumb-Rubberized Asphalt Binder. J. Transp. Eng. Part B Pavements 2022, 148, 04022044. [Google Scholar] [CrossRef]
- Vishnu, T.B.; Singh, K.L. A performance study on asphalt concrete mixes with different waste materials as modifiers in pavement application. J. Mater. Cycles Waste Manag. 2023, 25, 1519–1533. [Google Scholar] [CrossRef]
- White, G.; Kidd, A.; Shadforth, T. Effect of low dosage crumbed rubber on the mechanical properties of a dense graded asphalt mixture. Road Mater. Pavement Des. 2023, 24, 2464–2482. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.; He, W.; Zhang, Y.; Xin, Y. Improving anti-aging performance of terminal blend rubberized bitumen by using graft activated crumb rubber. J. Polym. Eng. 2023, 43, 855–864. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.; Zhang, Y.; Yu, F.; Luo, H.; Chen, X.; He, W. Preparation of terminal blend/grafting activated crumb rubber composite modified asphalt based on response surface methodology. Front. Mater. 2023, 10, 1193225. [Google Scholar] [CrossRef]
- Barbuta, M.; Harja, M.; Ciobanu, G. Mechanical properties of polymer concrete containing tire waste power. J. Food Agric. Environ. 2014, 12, 1185–1190. [Google Scholar]
- Jafari, K.; Toufigh, V. Experimental and analytical evaluation of rubberized polymer concrete. Constr. Build. Mater. 2017, 155, 495–510. [Google Scholar] [CrossRef]
- Dębska, B.; Lichołai, L.; Miąsik, P. Assessment of the Applicability of Sustainable Epoxy Composites Containing Waste Rubber Aggregates in Buildings. Buildings 2019, 9, 31. [Google Scholar] [CrossRef]
- Wang, J.; Dai, Q.; Guo, S.; Si, R. Mechanical and durability performance evaluation of crumb rubber-modified epoxy polymer concrete overlays. Constr. Build. Mater. 2019, 203, 469–480. [Google Scholar] [CrossRef]
- Niaki, M.H.; Ahangari, M.G. Polymer Concretes: Advanced Construction Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, Y.; Ma, L.; Zhou, L.; He, H. Tailoring the properties of ground tire rubber/high-density polyethylene blends by combining surface devulcanization and in-situ grafting technology. Mater. Chem. Phys. 2018, 220, 161–170. [Google Scholar] [CrossRef]
- Moghaddamzadeh, S.; Rodrigue, D. The effect of polyester recycled tire fibers mixed with ground tire rubber on polyethylene composites. Part I: Morphological analysis. Prog. Rubber Plast. Recycl. Technol. 2018, 34, 200–220. [Google Scholar] [CrossRef]
- Khan, R.M.; Mushtaq, A.; Ali, Z.U. Effect of Ground Tire Rubber on Mechanical Properties of Low Density Polyethylene. Int. J. Membr. Sci. Technol. 2021, 8, 85–92. [Google Scholar] [CrossRef]
- Kenawy, S.H.; Khalil, A.M. Reclaiming waste rubber for a green environment. Biointerface Res. Appl. Chem. 2021, 11, 8413–8423. [Google Scholar] [CrossRef]
- Bhatti, I.A.; Ahmad, N.; Iqbal, N.; Zahid, M.; Iqbal, M. Chromium adsorption using waste tire and conditions optimization by response surface methodology. J. Environ. Chem. Eng. 2017, 5, 2740–2751. [Google Scholar] [CrossRef]
- Shirajuddin, S.S.M.; Ratnam, C.T.; Hussin, K.; Shukri, N.A.; Ishak, N.S. Quantification of Tripropylene Glycol Diacrylate grafted onto Waste Tire Dust from Fourier Transform Infrared Spectroscopy. Mater. Today Proc. 2020, 29, 63–67. [Google Scholar] [CrossRef]
- Zedler, L.; Wang, S.; Formela, K. Ground tire rubber functionalization as a promising approach for the production of sustainable adsorbents of environmental pollutants. Sci. Total Environ. 2022, 836, 155636. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wan, C.; Yang, B.; Tang, Z.; Li, W. Performance evaluation of asphalt binder and mixture modified by pre-treated crumb rubber. Constr. Build. Mater. 2023, 362, 129777. [Google Scholar] [CrossRef]
- Nabok, A.A. Method and device for destroying worn out tyres. RF Patent 2057014, 27 March 1996. [Google Scholar]
- Nabok, A.A.; Zakharov, A.S. Armored chamber for grinding worn tire casings. Patent WO 2012/053923 A1, 26 April 2012. [Google Scholar]
- Krivandin, A.V.; Solov’eva, A.B.; Glagolev, N.N.; Shatalova, O.V.; Kotova, S.L. Structure alterations of perfluorinated sulfocationic membranes under the action of ethylene glycol (SAXS and WAXS studies). Polymer 2003, 44, 5789–5796. [Google Scholar] [CrossRef]
- DIN EN 1426; Bitumen and bituminous binders – Determination of needle penetration. German version EN 1426:2015; DIN Media: Berlin, Germany, 2015.
- DIN EN 1427; Bitumen and bitumenious benders – Detemination of the softening point – Ring and Ball method. German version EN 1427:2007; DIN Media: Berlin, Germany, 2007.
- TL Bitumen-StB 07; Technische Lieferbedingungen für Baustoffe und Baustoffgemische für Tragschichten mit hydraulischen Bindemitteln und Fahrbahndecken aus Beton, Ausgabe 2007, Änderung/Ergänzung 2014, TL Beton-StB 07. FGSV: Köln, Germany, 2007.
- DIN EN 13398; Bitumen and bituminous binders - Determination of the elastic recovery of modified bitumen DIN EN 13398:2018-02 (E). DIN Media: Berlin, Germany, 2018.
- Zarkhin, L.S.; Sheberstov, S.V.; Panfilovich, N.V.; Manevitch, L.I. Mechanodegradation of polymers. The method of molecular dynamics. Russ. Chem. Rev. 1989, 58, 381–393. [Google Scholar] [CrossRef]
- Martin, J.M.; Smith, W.K.; Bhatia, S.C. Handbook of Rubber Technology: Natural, Synthetic Rubber and Technology of Vulcanisation; CBS Pub & Dist: New Delhi, India, 2007. [Google Scholar]
- Mark, J.E.; Erman, B.; Roland, C.M. The Science and Technology of Rubber, 4th ed.; Academic Press: Cambridge, MA, USA, 2013; 816p, ISBN 978-0-12-394584-6. [Google Scholar]







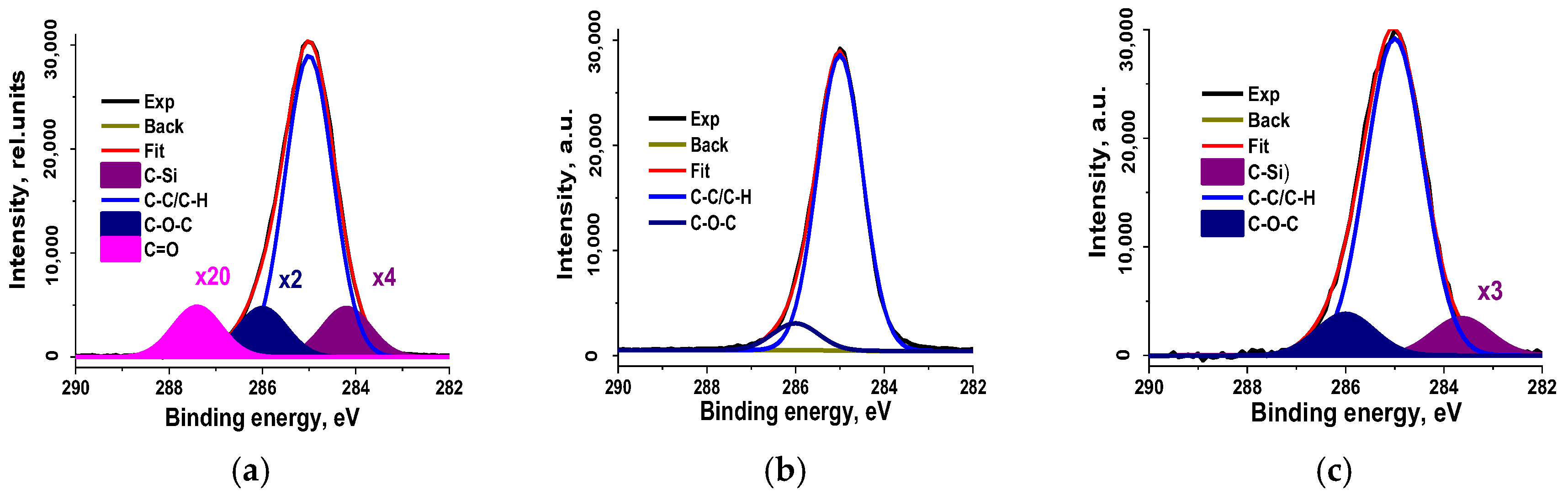
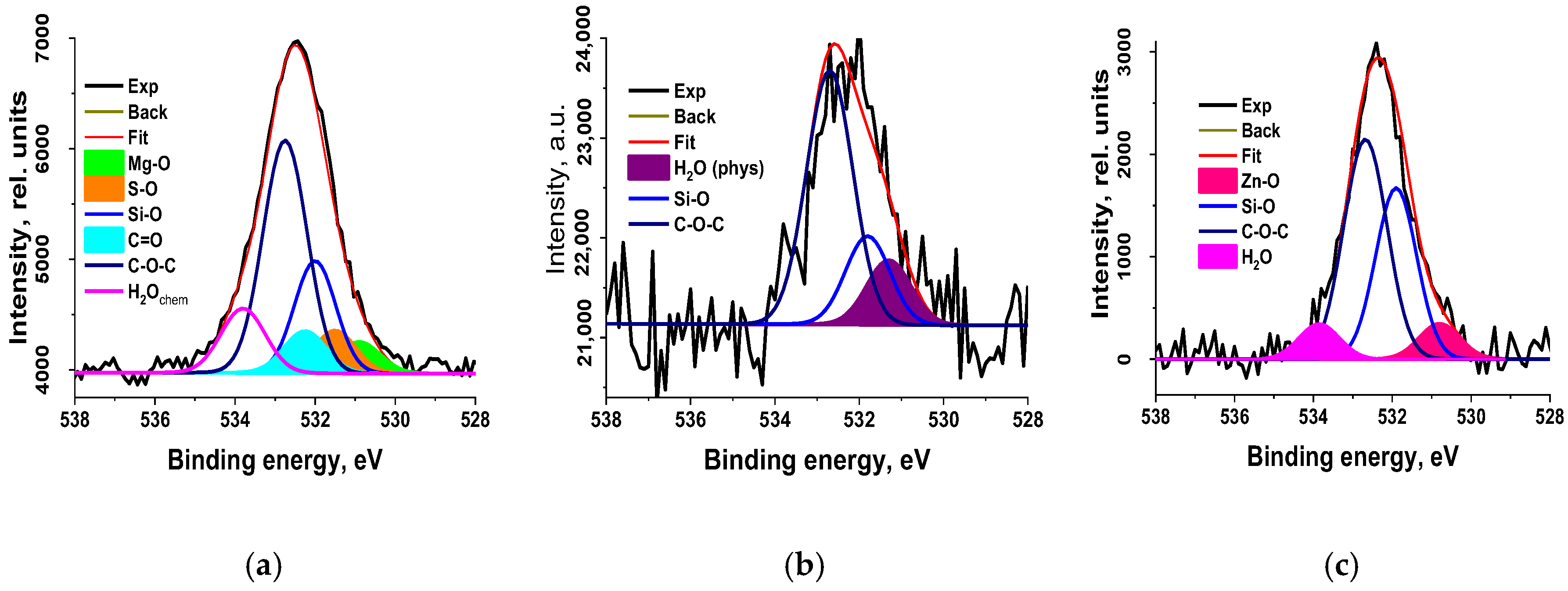

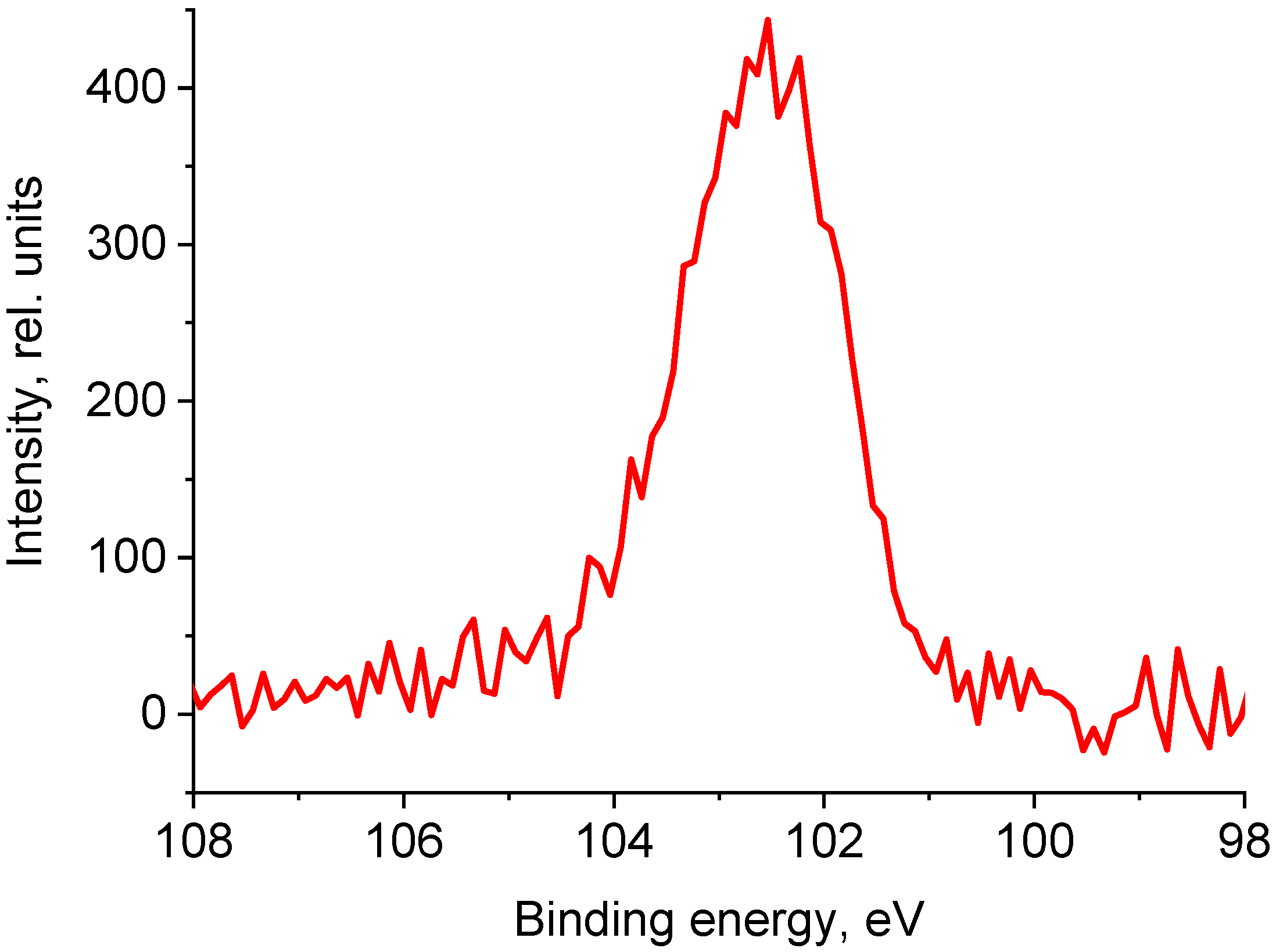
| Sample | C | O | Mg | Si | Zn | S |
|---|---|---|---|---|---|---|
| CR 0–1 | 91.5 | 5.2 | 0.3 | 1.1 | 0.5 | 1.4 |
| CR 1–3 | 99.0 | 0.8 | 0.2 | |||
| CR 3–5 | 94.4 | 3.8 | 1.5 | 0.3 |
| Sample | C-Si | C-C/C-H | C-O-C | C=O; | |
|---|---|---|---|---|---|
| CR 0–1 | Eb | 284.2 | 285.0 | 286.0 | 287.4 |
| W | 1.03 | 1.03 | 1.03 | 1.03 | |
| Irel | 0.07 | 0.80 | 0.13 | 0.01 | |
| CR 1–3 | Eb | 285.0 | 286.0 | ||
| W | 1.02 | 1.03 | |||
| Irel | 0.92 | 0.08 | |||
| CR 3–5 | Eb | 284.2 | 285.0 | 286.0 | |
| W | 1.17 | 1.17 | 1.17 | ||
| Irel | 0.05 | 0.84 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misin, V.M.; Nabok, A.A.; Zakharov, A.A.; Krivandin, A.V.; Krikunova, N.I.; Volkov, V.A.; Voronkov, M.V.; Pozin, S.I.; Buryak, A.K.; Tarasov, A.E.; et al. New Explosive-Circulation Technology of Tire Recycling for the Production of Crumb Rubber with Modified Surface. Polymers 2025, 17, 1260. https://doi.org/10.3390/polym17091260
Misin VM, Nabok AA, Zakharov AA, Krivandin AV, Krikunova NI, Volkov VA, Voronkov MV, Pozin SI, Buryak AK, Tarasov AE, et al. New Explosive-Circulation Technology of Tire Recycling for the Production of Crumb Rubber with Modified Surface. Polymers. 2025; 17(9):1260. https://doi.org/10.3390/polym17091260
Chicago/Turabian StyleMisin, Vyacheslav M., Alexander A. Nabok, Alexander A. Zakharov, Alexey V. Krivandin, Natalia I. Krikunova, Vladimir A. Volkov, Mikhail V. Voronkov, Sergey I. Pozin, Alexey K. Buryak, Alexander E. Tarasov, and et al. 2025. "New Explosive-Circulation Technology of Tire Recycling for the Production of Crumb Rubber with Modified Surface" Polymers 17, no. 9: 1260. https://doi.org/10.3390/polym17091260
APA StyleMisin, V. M., Nabok, A. A., Zakharov, A. A., Krivandin, A. V., Krikunova, N. I., Volkov, V. A., Voronkov, M. V., Pozin, S. I., Buryak, A. K., Tarasov, A. E., Naumkin, A. V., & Nikulin, S. S. (2025). New Explosive-Circulation Technology of Tire Recycling for the Production of Crumb Rubber with Modified Surface. Polymers, 17(9), 1260. https://doi.org/10.3390/polym17091260







