Effects of Organophosphorus Flame Retardants on the Dissipation Factor of Flame-Retardant Polymers
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Flame Retardants
2.3. Preparation of Flame-Retardant Polymers
2.4. Characterizations
3. Results and Discussion
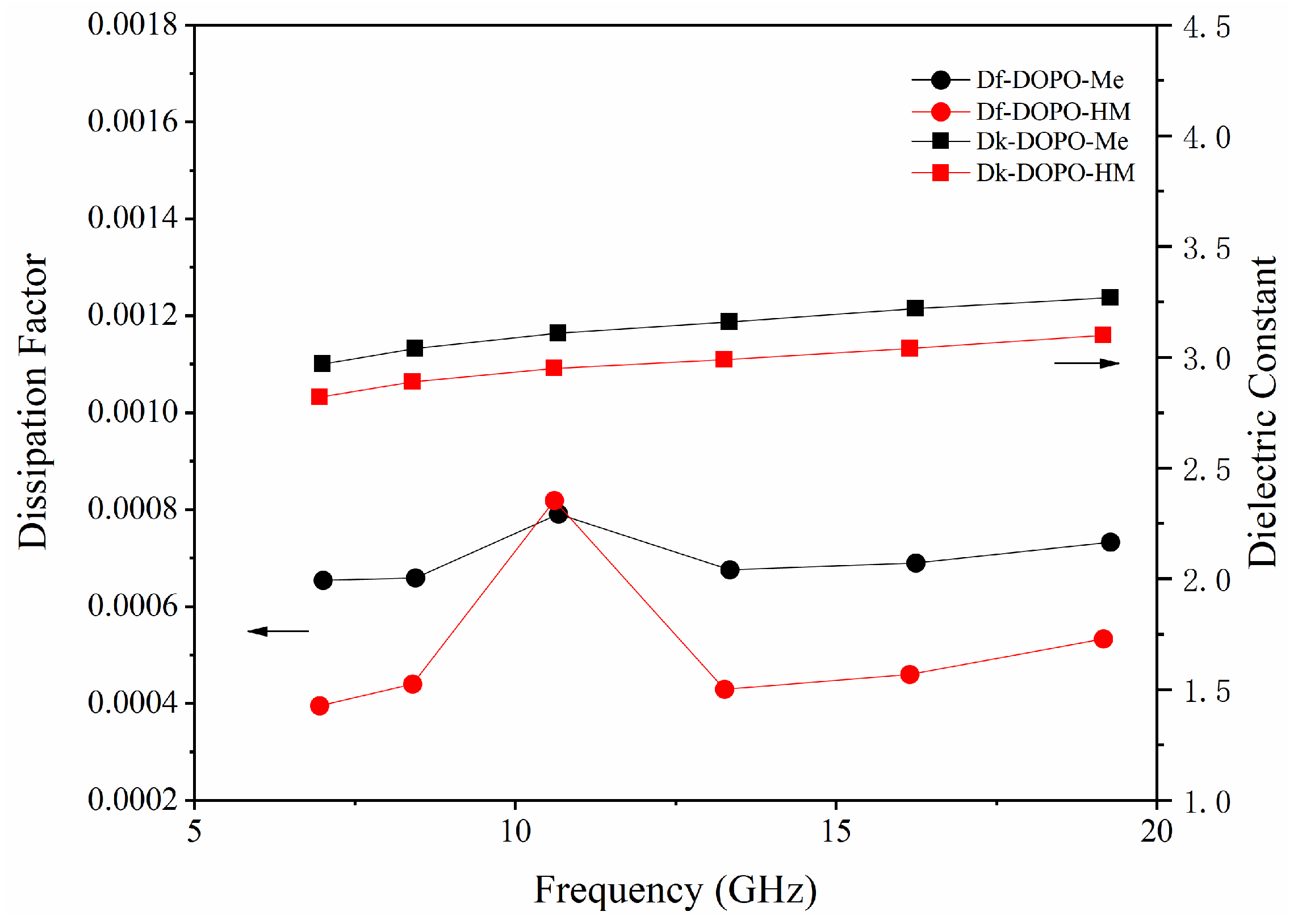
3.1. Effect of OH on Df and Dk of Flame Retardants
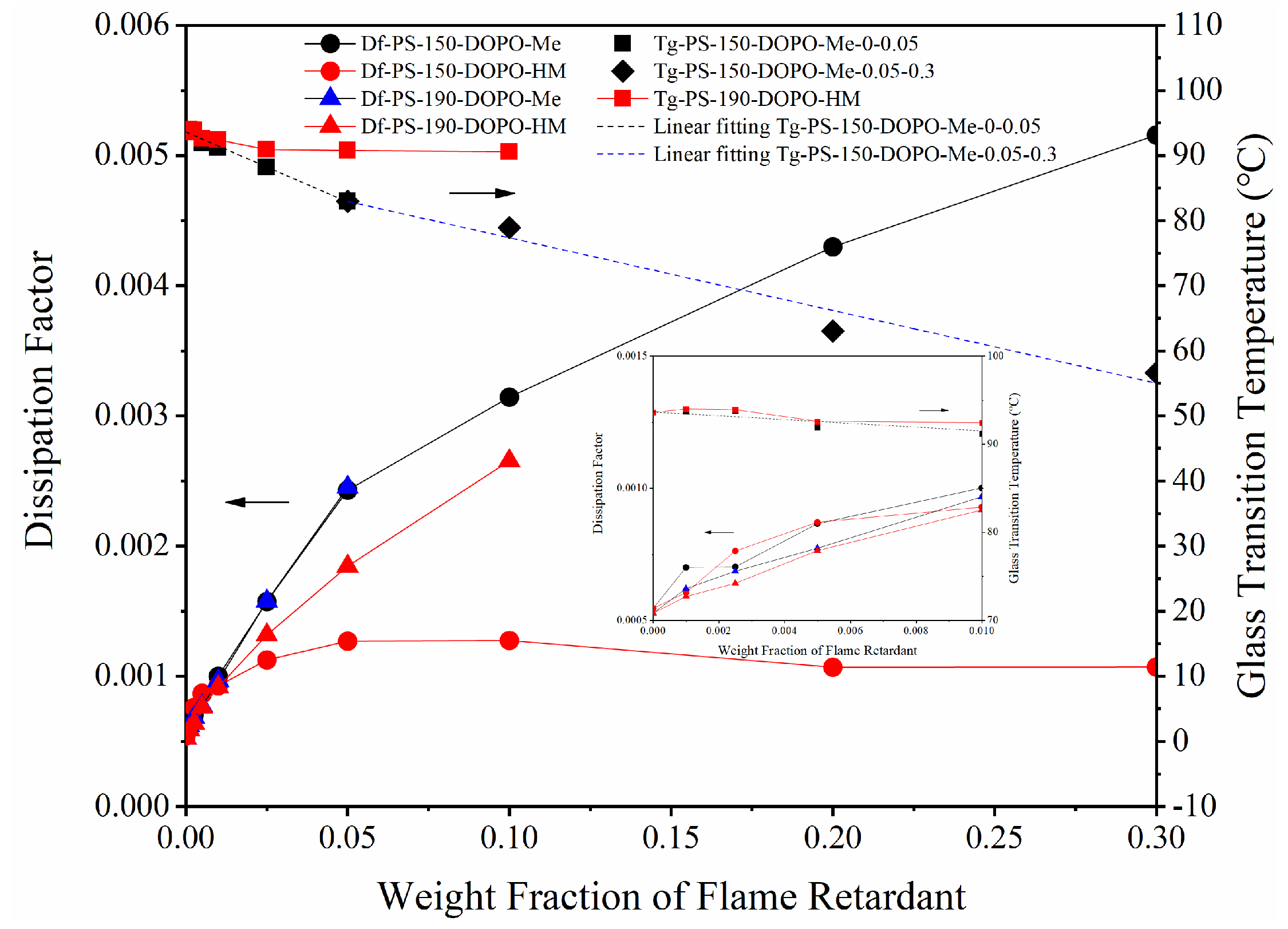
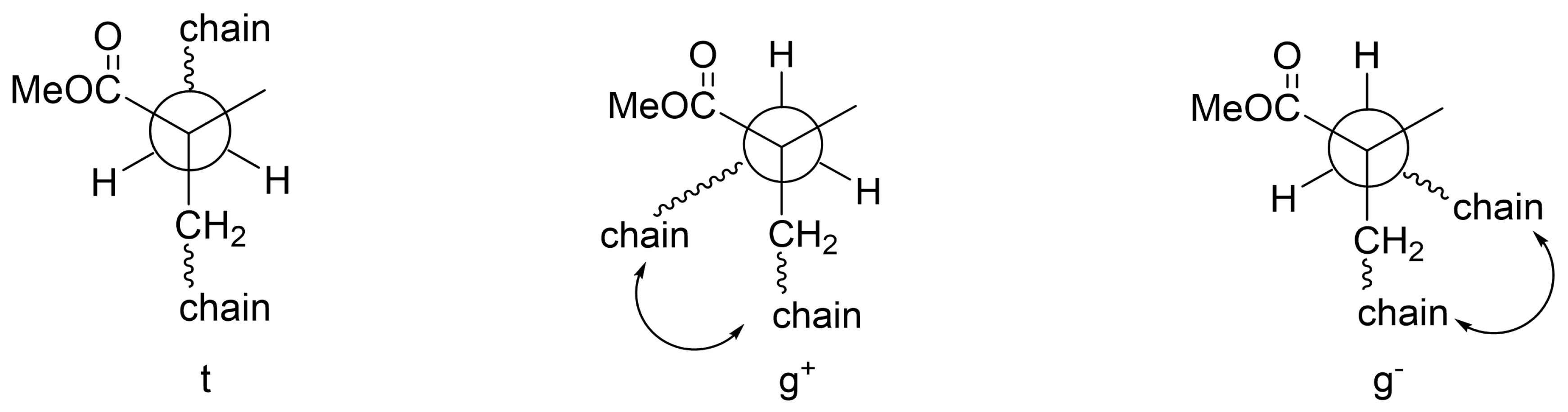
3.2. Effect of OH on Df of PS/FR
3.3. Effect of OH on Df of PMMA/FR
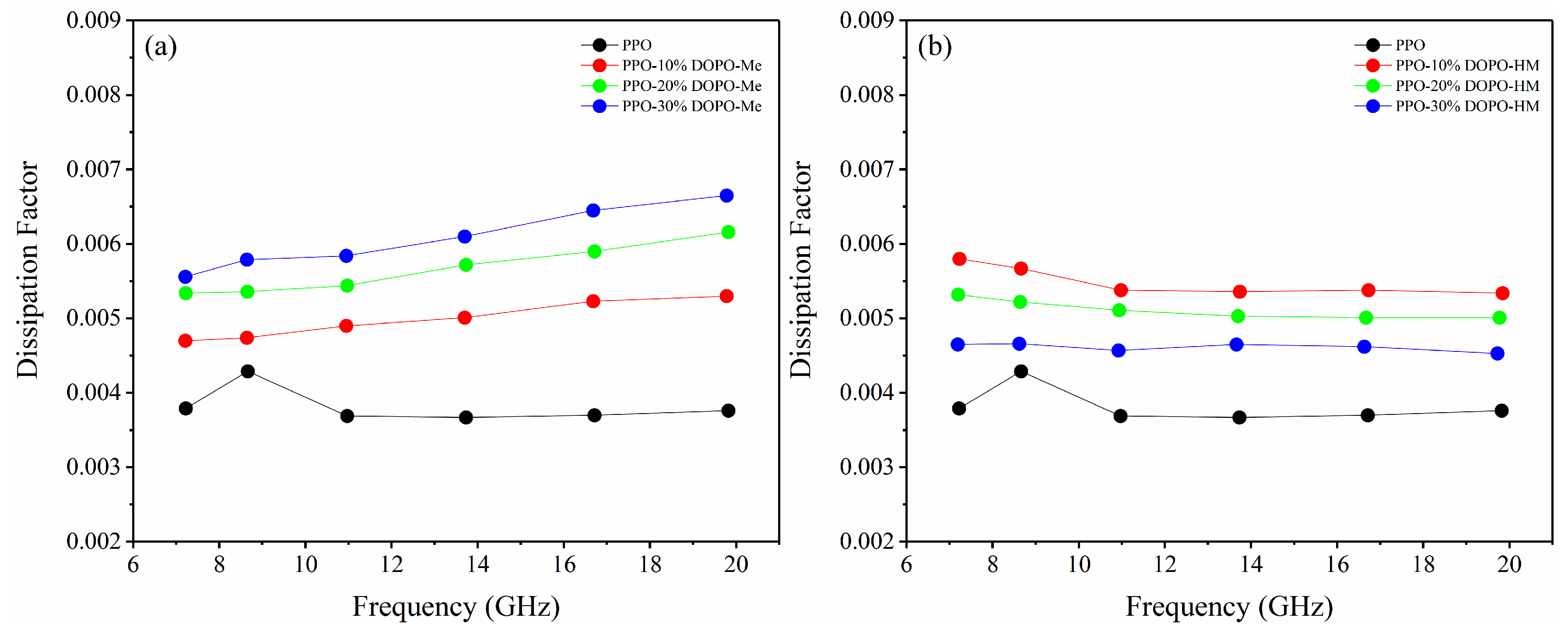
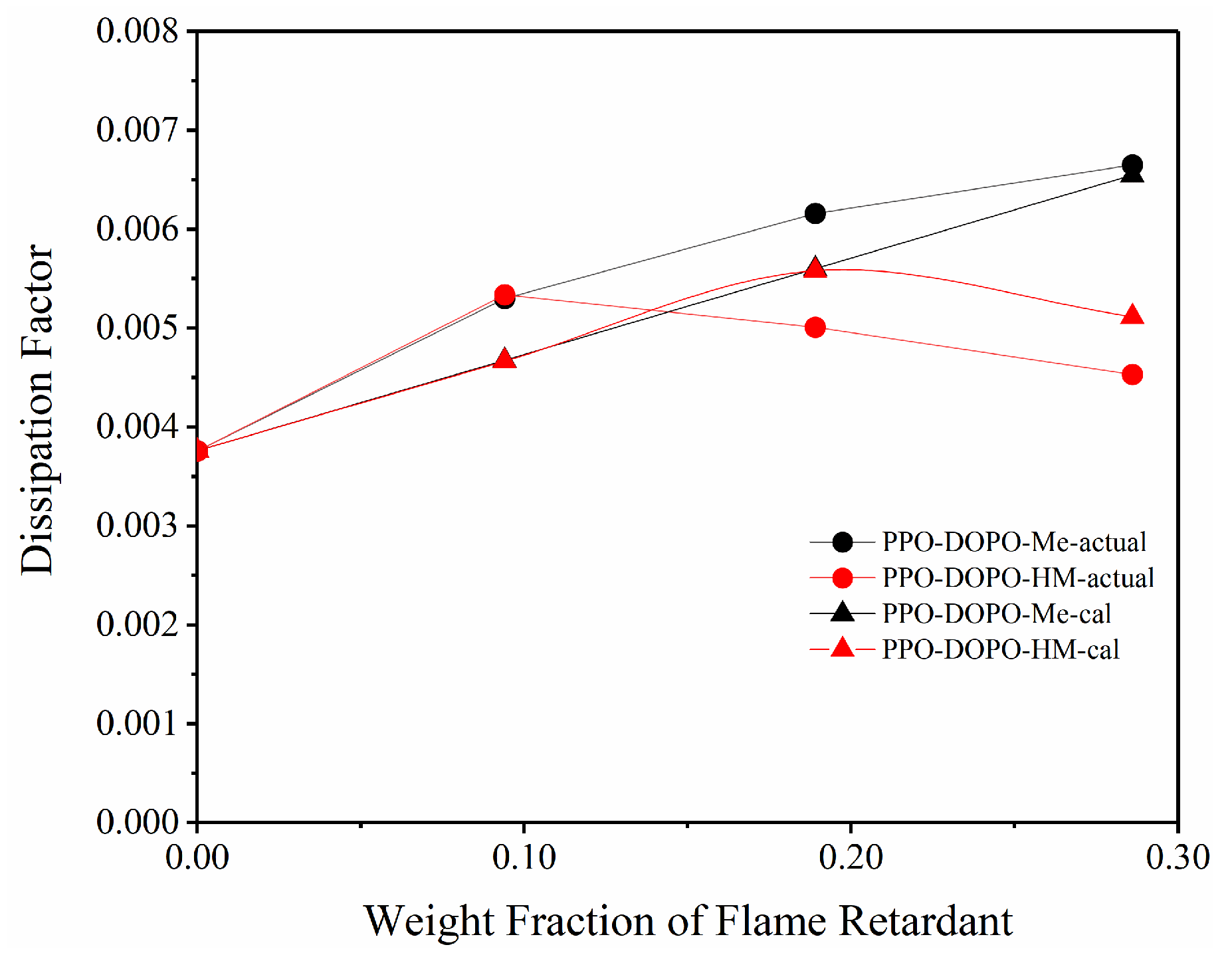
3.4. Effect of OH on Dfs of PPO/FR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Peng, Z.; Ji, J.; Wu, Y.; Chen, Z.; Huang, H.; Liu, S.; Zhao, J. Fluorinated low molecular weight poly(phenylene oxide): Synthesis, characterization, and application in epoxy resin toward improved thermal and dielectric properties. Eur. Polym. J. 2021, 157, 110674. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, Y.; Zhang, X.; Liu, L.; Zhang, H. Synthesis and properties of cured epoxy mixed resin systems modified by polyphenylene oxide for production of high–-frequency copper clad laminates. Polym. Compos. 2017, 39, E2334–E2345. [Google Scholar] [CrossRef]
- Zhuo, D.; Gu, A.; Liang, G.; Hu, J.-T.; Yuan, L. Preparation and properties of hollow silica tubes/cyanate ester hybrids for high-frequency copper-clad laminates. J. Mater. Sci. 2010, 46, 1571–1580. [Google Scholar] [CrossRef]
- Li, K.; Yang, L.; Yang, L.; He, L.; Du, J.; Li, X. Low dielectric polyimide microsphere/polyimide composite films based on porous polyimide microsphere. Polym. Eng. Sci. 2024, 64, 5166–5175. [Google Scholar] [CrossRef]
- Qu, C.; Shan, L.; Zhang, G.; Sun, R. Preparation and dielectric properties research of a novel kind of intrinsic silane-containing polyimide. Polymer 2023, 285, 126361. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Wang, B.; Su, H.; Fan, W.; Jing, X. Facile preparation of recyclable cyclic polyolefin/polystyrene vitrimers with low dielectric loss based on semi-interpenetrating polymer networks for high-frequency copper-clad laminates. Polymer 2021, 233, 124214. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Nie, Y.; Fang, X.; Chen, G. Composite films with low dielectric constant and dielectric loss factor at high frequency prepared from polyimide and polytetrafluoroethylene. Polym. Eng. Sci. 2022, 62, 4226–4234. [Google Scholar] [CrossRef]
- Hou, J.; Fang, L.; Huang, G.; Dai, M.; Liu, F.; Wang, C.; Li, M.; Zhang, H.; Sun, J.; Fang, Q. Low-Dielectric polymers derived from biomass. ACS Appl. Polym. Mater. 2021, 3, 2835–2848. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Hsu, S.-W.; Wang, C.-S. Low dielectric and flame-retardant properties of thermosetting redistributed poly(phenylene oxide). J. Vinyl Add. Tech. 2009, 15, 54–59. [Google Scholar] [CrossRef]
- Long, L.; Chang, Q.; He, W.; Xiang, Y.; Qin, S.; Yin, J.; Yu, J. Effects of bridged DOPO derivatives on the thermal stability and flame retardant properties of poly(lactic acid). Polym. Degrad. Stab. 2017, 139, 55–66. [Google Scholar] [CrossRef]
- Long, L.; Yin, J.; He, W.; Qin, S.; Yu, J. Influence of a Phenethyl-Bridged DOPO Derivative on the Flame Retardancy, Thermal Properties, and Mechanical Properties of Poly(lactic acid). Ind. Eng. Chem. Res. 2016, 55, 10803–10812. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Tang, G.; Tao, Y.; Wu, Q.; Shen, H.; Sun, J.; Deng, D.; Liu, X. DOPO-based derivatives with different phosphorous oxidation states as highly efficient flame retardants for epoxy resins. Polym. Bull. 2023, 81, 6131–6148. [Google Scholar] [CrossRef]
- Li, Y.; Tian, C.; Cheng, G.; Li, C.; Wang, Z. Facile synthesis of Bis-Diphenylphosphine oxide as a flame retardant for epoxy resins. Polymers 2024, 16, 2635. [Google Scholar] [CrossRef]
- Luo, C.; Tang, G.; Zhang, J. Thermosetting Resin Composition (US 2020/062889 A1). 2020. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2020062889A1 (accessed on 21 November 2021).
- Hu, Z.-L.; Hsieh, C.-Y.; Chen, X.-F.; Xiong, X. Phosphaphenanthrene-Based Compound and Related Preparation Method and Application (US 9,896,551 B2). 2018. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DUS9896551B2 (accessed on 21 November 2021).
- Hu, Z.-L.; Hsieh, C.-Y. Resin Composition (US 10,626,250 B2). 2020. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DUS10626250B2 (accessed on 21 November 2021).
- Dang, Z.-M.; Yuan, J.-K.; Zha, J.-W.; Zhou, T.; Li, S.-T.; Hu, G.-H. Fundamentals, processes and applications of high-permittivity polymer-matrix composites. Prog. Mater. Sci. 2011, 57, 660–723. [Google Scholar] [CrossRef]
- Pudovik, A.N.; Konovalova, I.V. Addition Reactions of Esters of Phosphorus(III) Acids with Unsaturated Systems. Synthesis 1979, 1979, 81–96. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Neganova, E.G.; Veits, Y.A. Arylation of 6H-dibenzo[c,e][1,2λ5]oxaphosphinine 6-oxide. Russ. J. Org. Chem. 2004, 40, 1782–1786. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y.; Liu, L.; Jia, H.; Zang, Y.; Zu, L.; Lan, T.; Wang, J. Synthesis and characterization of a novel DOPO-Based Flame Retardant intermediate and its flame retardancy as a polystyrene intrinsic flame retardant. ACS Omega 2023, 8, 48825–48842. [Google Scholar] [CrossRef]
- Bennett, E.L.; Calisir, I.; Yang, X.; Huang, Y.; Xiao, J. Correlation of Dielectric Properties with Structure and H-Bonding for Liquids. J. Phys. Chem. C 2023, 127, 18669–18677. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, C.; Chen, K.; Niu, T.; Wang, J.; Yang, Y. Hydroxyl-group decreased dielectric loss coupled with 3D-BN network enhanced high thermal conductivity epoxy composite for high voltage-high frequency conditions. Compos. Sci. Technol. 2023, 234, 109934. [Google Scholar] [CrossRef]
- Misra, M.; Agarwal, M.; Sinkovits, D.W.; Kumar, S.K.; Wang, C.; Pilania, G.; Ramprasad, R.; Weiss, R.A.; Yuan, X.; Chung, T.C.M. Enhanced Polymeric Dielectrics through Incorporation of Hydroxyl Groups. Macromolecules 2014, 47, 1122–1129. [Google Scholar] [CrossRef]
- Islam, M.Z.; Fu, Y.; Deb, H.; Hasan, M.K.; Dong, Y.; Shi, S. Polymer-based low dielectric constant and loss materials for high-speed communication network: Dielectric constants and challenges. Eur. Polym. J. 2023, 200, 112543. [Google Scholar] [CrossRef]
- Doulut, S.; Demont, P.; Lacabanne, C. Influence of Tacticity on the α Retardation Mode in Amorphous Poly(methyl methacrylate). Macromolecules 2000, 33, 3425–3430. [Google Scholar] [CrossRef]
- Bakr, A.M.; Darwish, A.; Azab, A.A.; Awady, M.E.E.; Hamed, A.A.; Elzwawy, A. Structural, dielectric, and antimicrobial evaluation of PMMA/CeO2 for optoelectronic devices. Sci. Rep. 2024, 14, 2548. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K.; Kulik, A.S.; Beckham, H.W.; Ohlemacher, A.; Pawelzik, U.; Boeffel, C.; Spiess, H.W. Molecular Nature of the.beta. Relaxation in Poly(methyl methacrylate) Investigated by Multidimensional NMR. Macromolecules 1994, 27, 4733–4745. [Google Scholar] [CrossRef]
- Behbahani, A.F.; Allaei, S.M.V.; Motlagh, G.H.; Eslami, H.; Harmandaris, V.A. Structure and dynamics of stereo-regular poly(methyl-methacrylate) melts through atomistic molecular dynamics simulations. Soft Matter 2018, 14, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.S.; Singh, G.; Shankar, R. Structural investigations of poly(methyl methacrylate) by two-dimensional NMR. J. Mol. Struct. 2004, 703, 69–81. [Google Scholar] [CrossRef]
- O’Reilly, J.M.; Mosher, R.A. Conformational energies of stereoregular poly(methyl methacrylate) by Fourier transform infrared spectroscopy. Macromolecules 1981, 14, 602–608. [Google Scholar] [CrossRef]
- Patra, N.; Ramesh, P.; Ramanakumar, N. Oleic acid-induced changes in the dynamic viscoelastic behavior of poly(methyl methacrylate) polymer film. Colloids Surf. 2024, 701, 134889. [Google Scholar] [CrossRef]
- Ma, X.; Sauer, J.A.; Hara, M. Influence of plasticizers on poly(methyl methacrylate) ionomers. Polymer 1997, 38, 4425–4431. [Google Scholar] [CrossRef]
- Jain, P.; Thakor, S.; Joshi, A.; Chauhan, K.V.; Vaja, C.R. Machine learning-driven analysis of dielectric response in polymethyl methacrylate nanocomposites reinforced with multi-walled carbon nanotubes. J. Mater. Sci. Mater. Electron. 2024, 35, 1419. [Google Scholar] [CrossRef]
- Shi, S.-C.; Chen, C.; Zhu, J.-L.; Li, Y.; Meng, X.; Huang, H.-D.; Li, Z.-M. Environmentally friendly regenerated cellulose films with improved dielectric properties via manipulating the hydrogen bonding network. Appl. Phys. Lett. 2021, 119, 022903. [Google Scholar] [CrossRef]


| Sample | Mixing Temperature (°C) | Pressing Temperature (°C) | Fast Cooling | Slow Cooling |
|---|---|---|---|---|
| PS-150-DOPO-Me | 150 | 150 | Y | |
| PS-150-DOPO-HM | 150 | 150 | Y | |
| PS-190-DOPO-Me | 190 | 190 | Y | |
| PS-190-DOPO-HM | 190 | 190 | Y | |
| PMMA-170-slow | 160 | 170 | Y | |
| PMMA-170-quick | 160 | 170 | Y | |
| PMMA-190-quick | 160 | 190 | Y | |
| PMMA-170-DOPO-Me-quick | 160 | 170 | Y | |
| PMMA-170-DOPO-HM-quick | 160 | 170 | Y | |
| PMMA-190-DOPO-HM-quick | 160 | 190 | Y |
| Sample | PPO (g) | DVB (g) | DCP (g) | DOPO-Me (g) | DOPO-HM (g) |
|---|---|---|---|---|---|
| PPO | 20 | 1 | 0.4 | ||
| PPO-DOPO-Me | |||||
| 10% | 18 | 0.9 | 0.36 | 2 | |
| 20% | 16 | 0.8 | 0.32 | 4 | |
| 30% | 14 | 0.7 | 0.28 | 6 | |
| PPO-DOPO-HM | |||||
| 10% | 18 | 0.9 | 0.36 | 2 | |
| 20% | 16 | 0.8 | 0.32 | 4 | |
| 30% | 14 | 0.7 | 0.28 | 6 |
| Sample | Df at 20 GHz | |||
|---|---|---|---|---|
| DOPO-Me | DOPO-HM | |||
| crystal | amorphous | crystal | amorphous | |
| from FR | 0.000733 | 0.000533 | ||
| from PS/FR | 0.0129 | 0.0185 | ||
| from PMMA/FR | 0.0135 | 0.0168 | ||
| Sample | T1 | Df at 20 GHz | Tg (°C) |
|---|---|---|---|
| PMMA-170-slow | 24.6 | 0.003254 | 110.7 |
| PMMA-170-quick | 27.0 | 0.004110 | 113.4 |
| PMMA-190-quick | 29.8 | 0.004287 | 116.1 |
| PMMA-170-DOPO-Me-quick | |||
| 0% | 27.0 | 0.004110 | 113.4 |
| 0.25% | 25.1 | 0.003670 | 112.3 |
| 0.5% | 24.5 | 0.003502 | 111.9 |
| 1% | 22.1 | 0.003644 | 109.8 |
| 5% | 28.1 | 0.004008 | 101.8 |
| 10% | 28.4 | 0.004688 | 93.7 |
| 20% | / | 0.005223 | 76.1 |
| PMMA-170-DOPO-HM-quick | |||
| 0% | 27.0 | 0.004110 | 113.4 |
| 0.1% | / | 0.004097 | 115.0 |
| 0.25% | 29.6 | 0.003840 | 114.0 |
| 0.5% | 30.2 | 0.004321 | 111.9 |
| 1% | 29.8 | 0.004140 | 110.8 |
| 5% | / | 0.004761 | 103.7 |
| 10% | 28.9 | 0.005580 | 92.6 |
| 20% | / | 0.006626 | 84.6 |
| PMMA-190-DOPO-HM-quick | |||
| 0% | 29.8 | 0.004287 | 116.1 |
| 0.25% | 27.6 | 0.003175 | 113.6 |
| 0.5% | 27.1 | 0.003082 | 113.0 |
| 1% | 30.3 | 0.002638 | 112.8 |
| 5% | 29.1 | 0.004026 | 102.6 |
| 10% | / | 0.005801 | 93.0 |
| 20% | / | 0.006577 | 84.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, P.; Yao, Q.; Cao, W.; Sun, J.; Zhao, Y. Effects of Organophosphorus Flame Retardants on the Dissipation Factor of Flame-Retardant Polymers. Polymers 2025, 17, 1254. https://doi.org/10.3390/polym17091254
Jin P, Yao Q, Cao W, Sun J, Zhao Y. Effects of Organophosphorus Flame Retardants on the Dissipation Factor of Flame-Retardant Polymers. Polymers. 2025; 17(9):1254. https://doi.org/10.3390/polym17091254
Chicago/Turabian StyleJin, Peng, Qiang Yao, Weihong Cao, Jinhao Sun, and Yueying Zhao. 2025. "Effects of Organophosphorus Flame Retardants on the Dissipation Factor of Flame-Retardant Polymers" Polymers 17, no. 9: 1254. https://doi.org/10.3390/polym17091254
APA StyleJin, P., Yao, Q., Cao, W., Sun, J., & Zhao, Y. (2025). Effects of Organophosphorus Flame Retardants on the Dissipation Factor of Flame-Retardant Polymers. Polymers, 17(9), 1254. https://doi.org/10.3390/polym17091254





