Interactions and Curing Dynamics Between UV-Triggered Epoxy Acrylate Binder, Curing Agents and Photoinitiators
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of UV Curing Pastes and UV-Cured Sample
2.3. Curing of Epoxy Acrylate-Based Binder
2.4. Characterization
2.4.1. UV Curing
2.4.2. Thermal Properties
2.4.3. Curing Properties
2.4.4. Mechanical Properties
2.4.5. Morphology
3. Results and Discussion
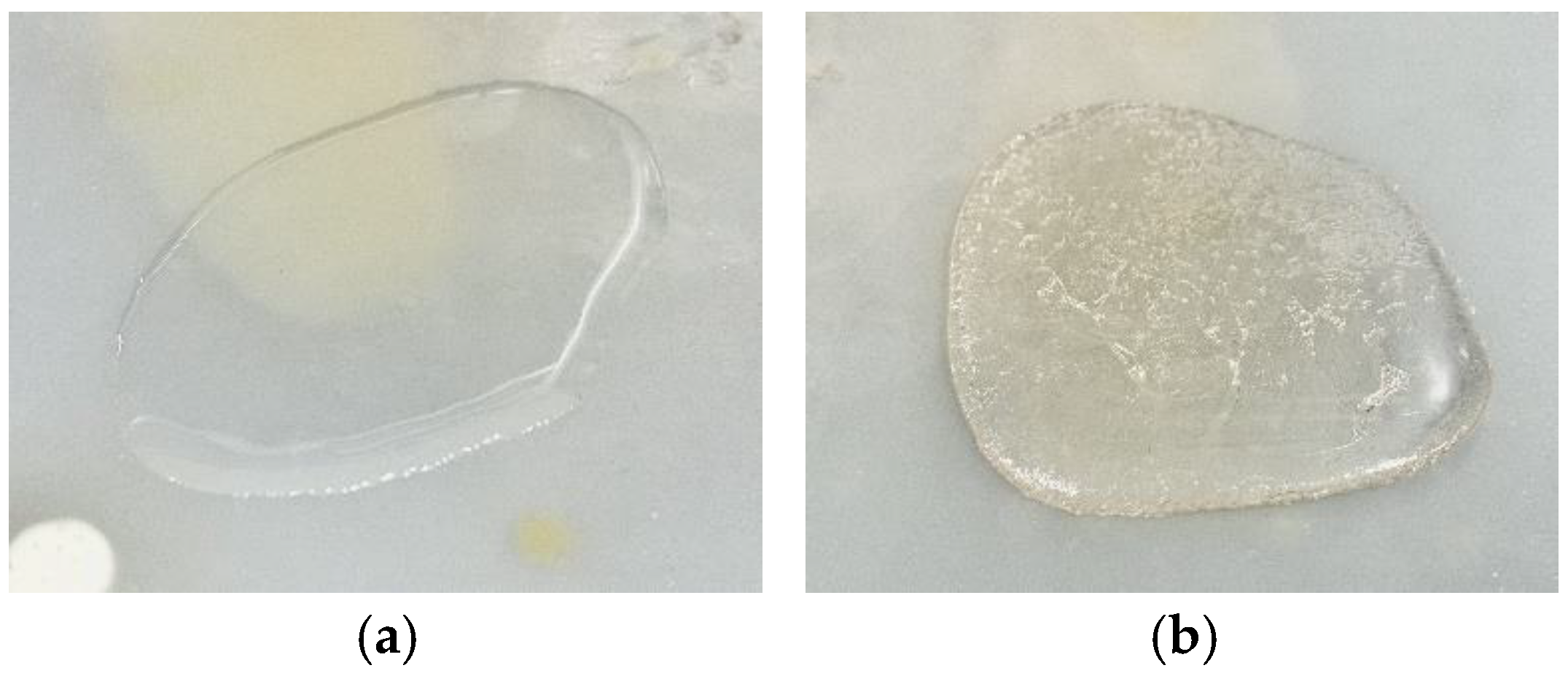
3.1. Fabrication of UV Curing Pastes and Cured UV Sample
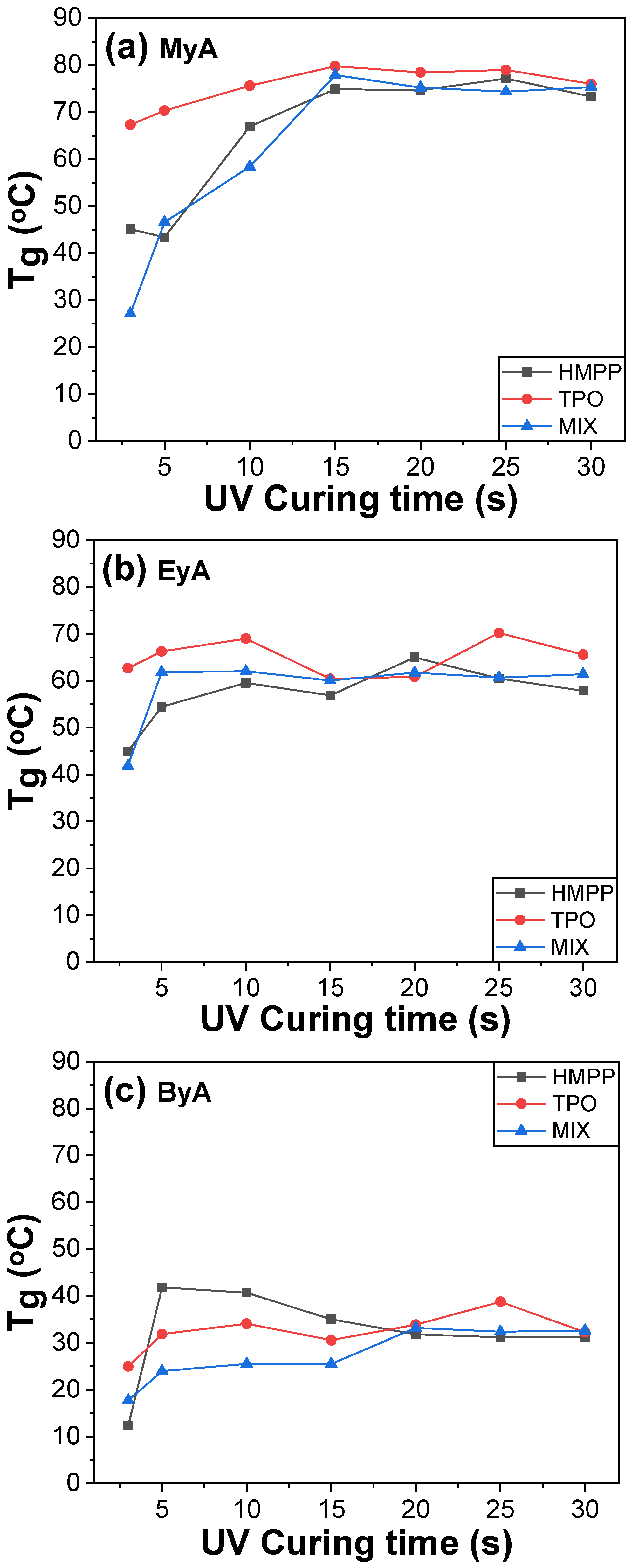
3.2. Thermal Properties of UV Paste
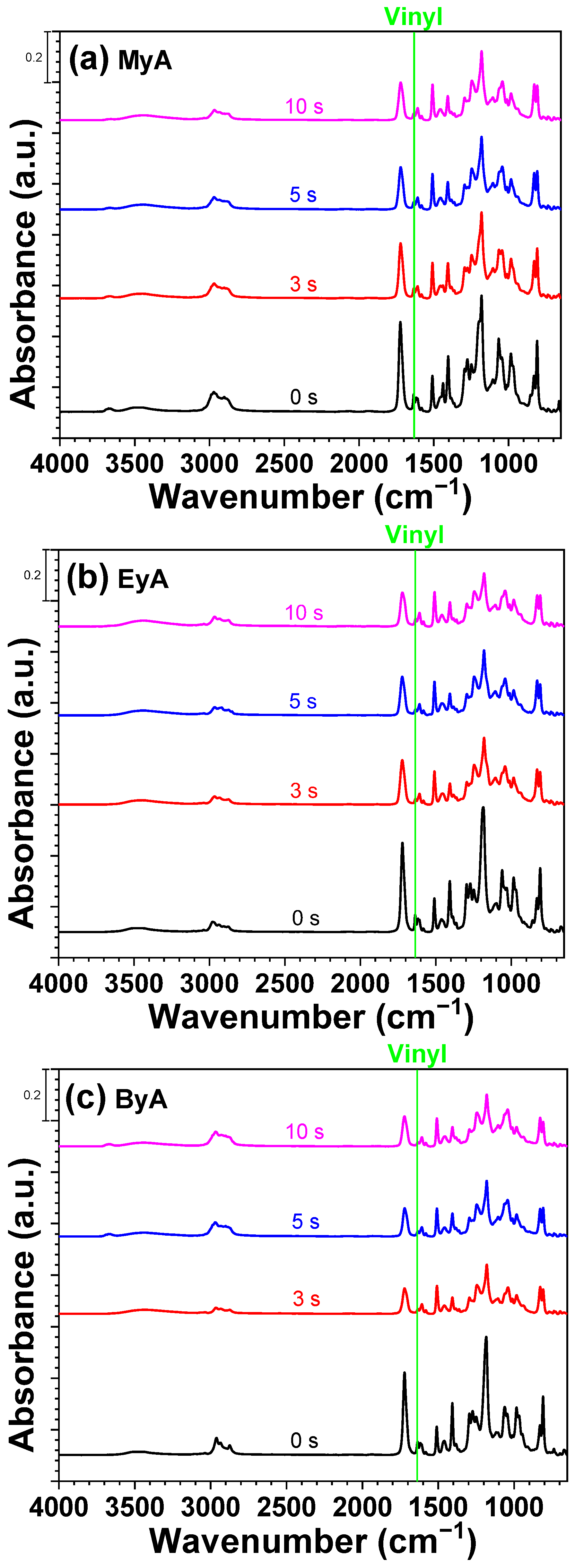
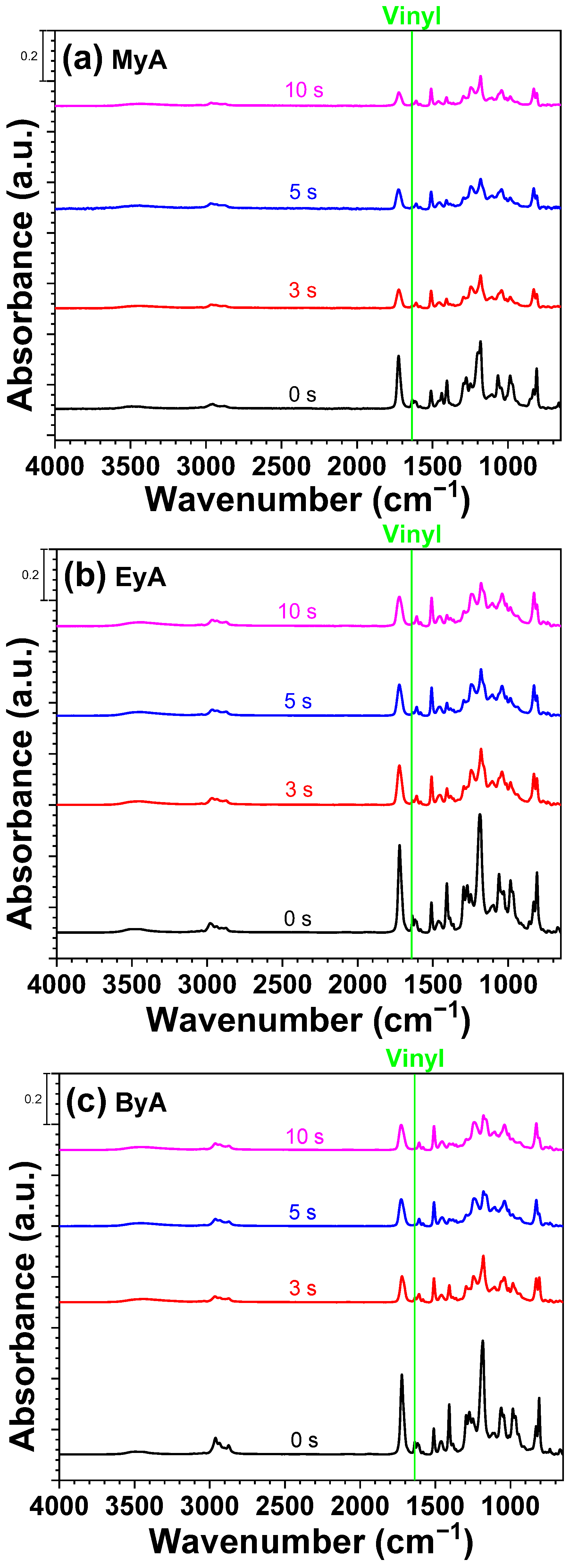
3.3. FT-IR Analysis of UV Paste as a Function of Curing Time
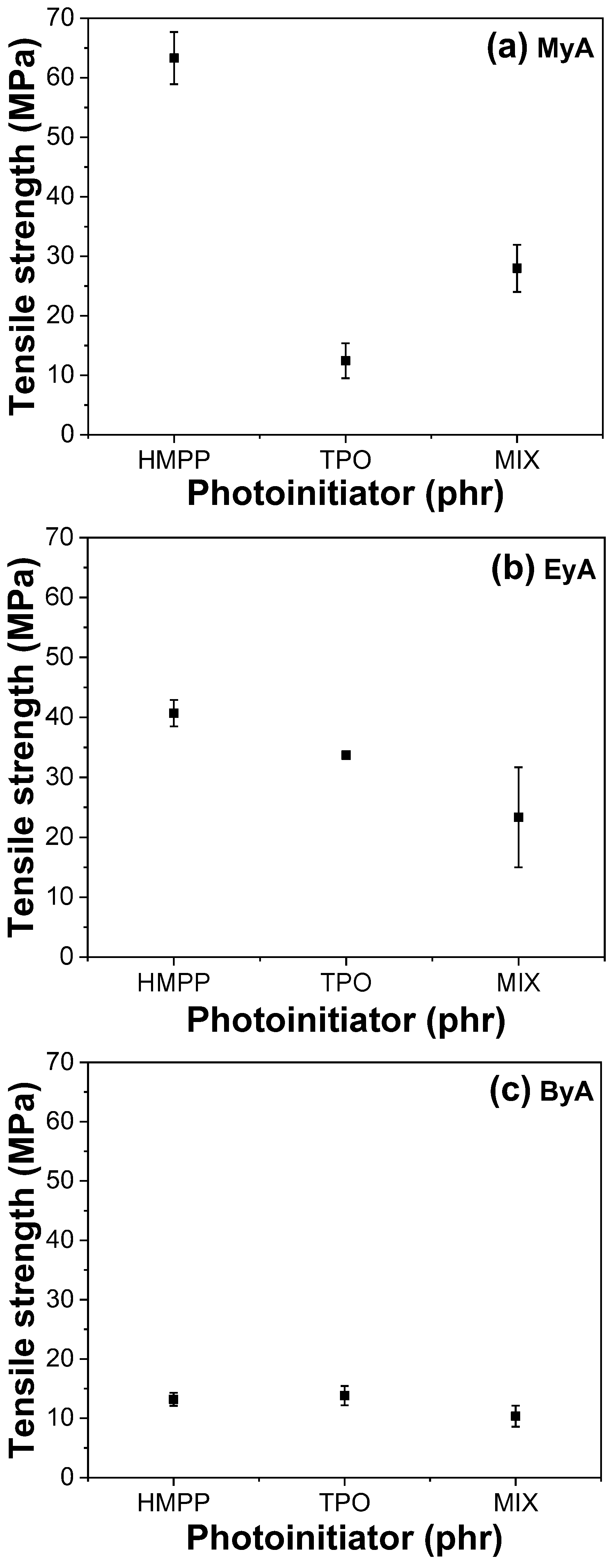
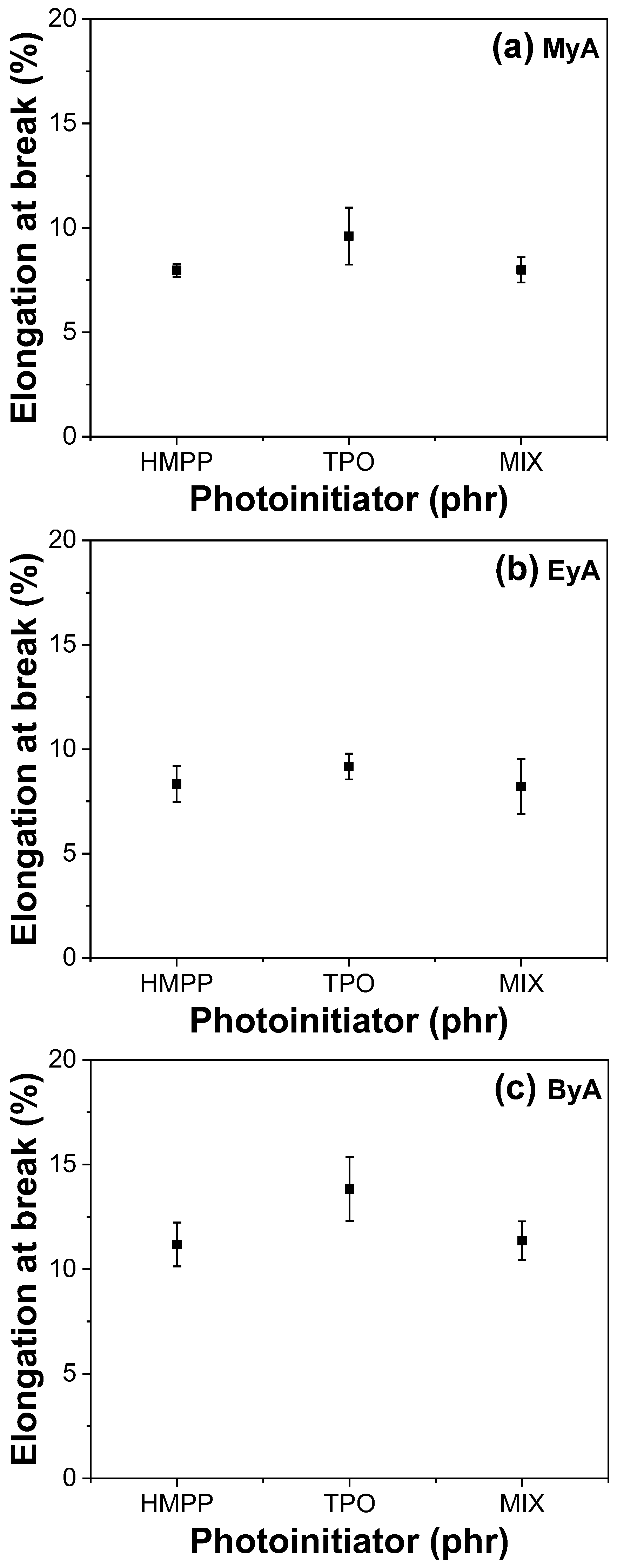
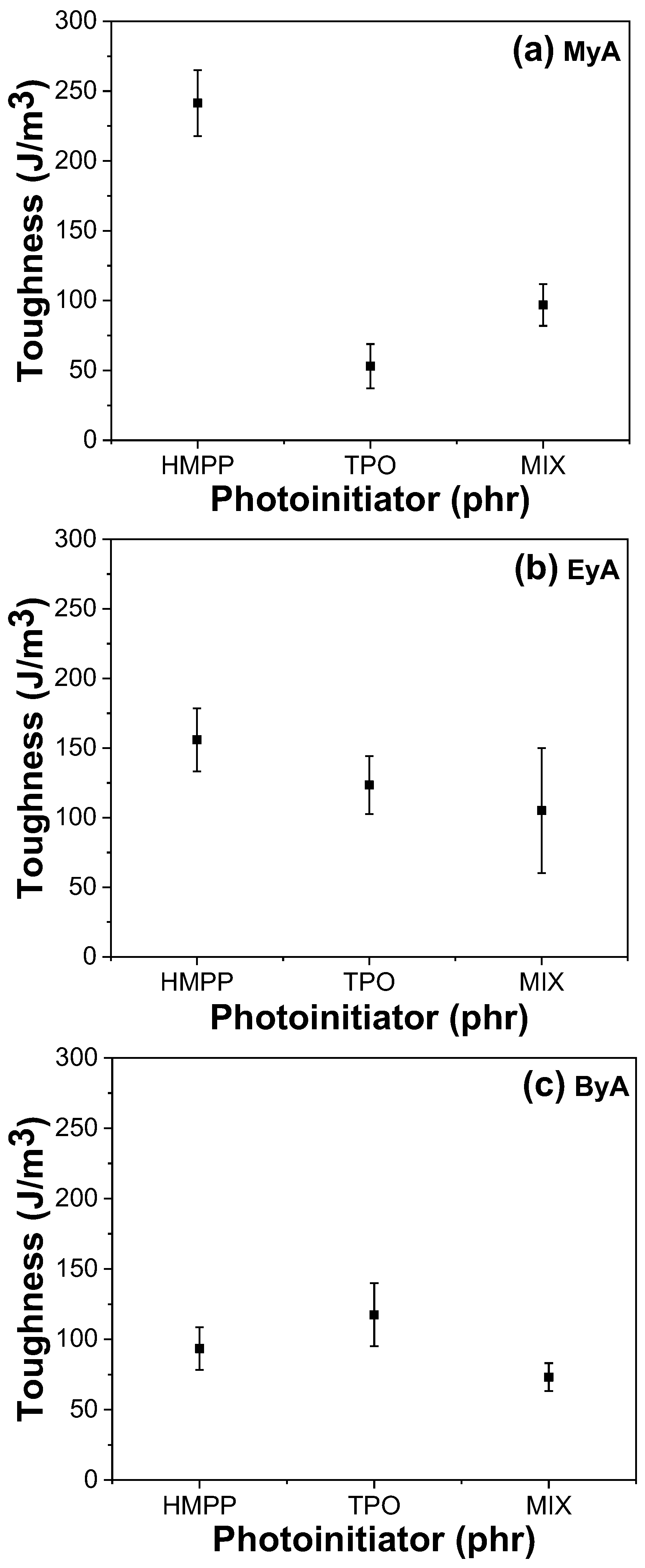
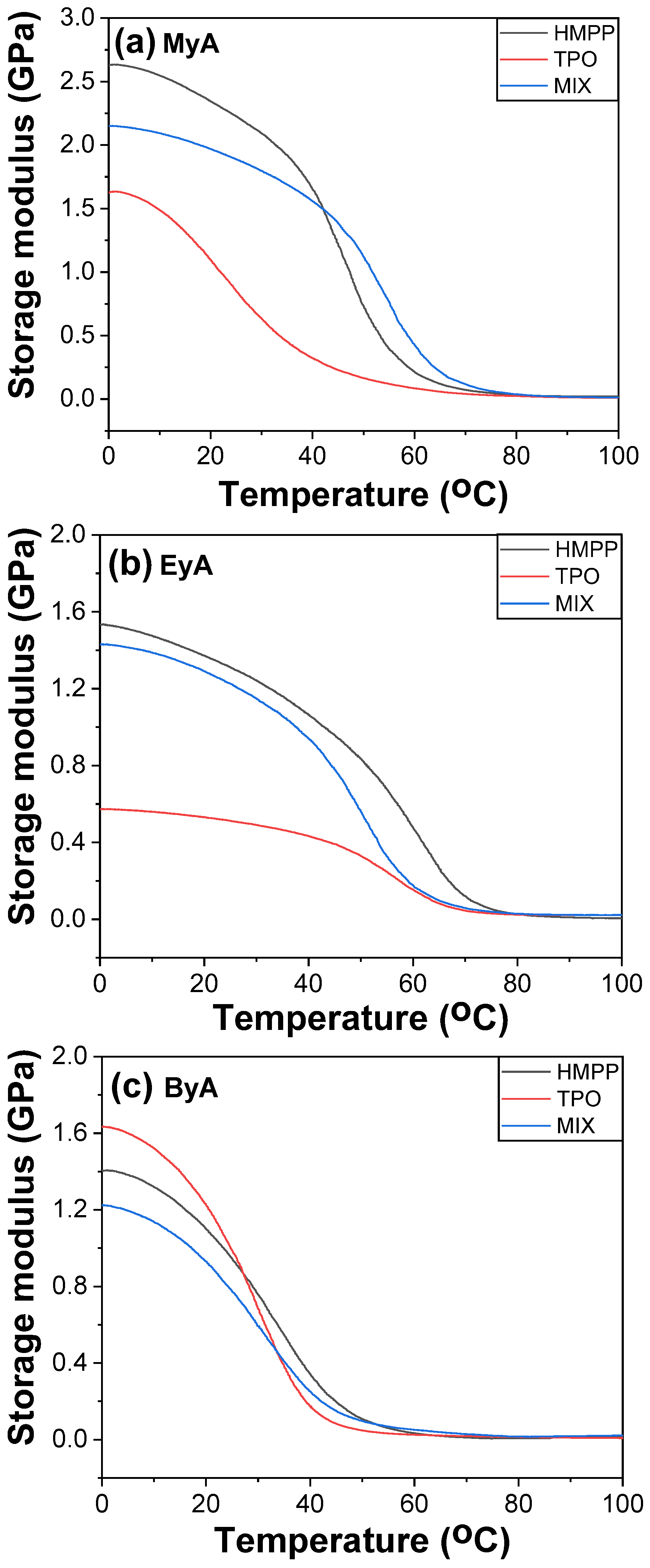
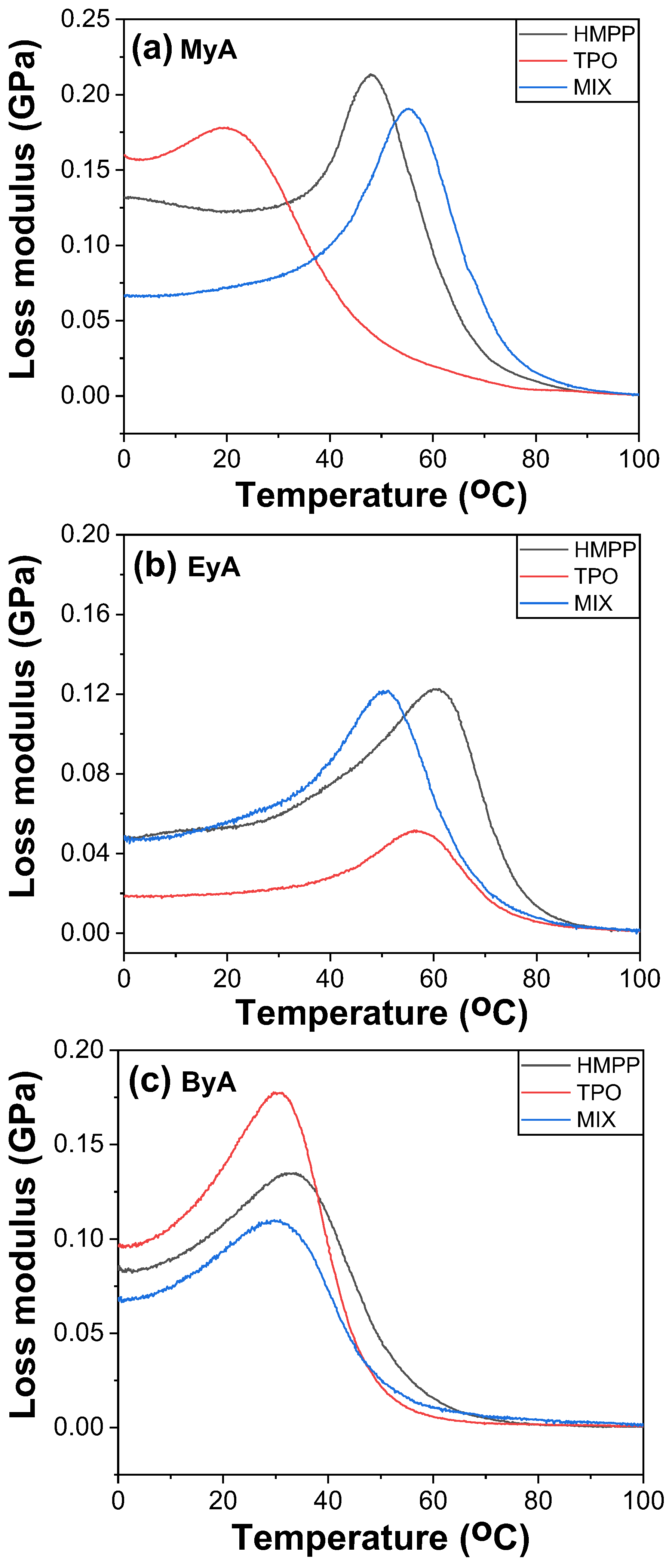
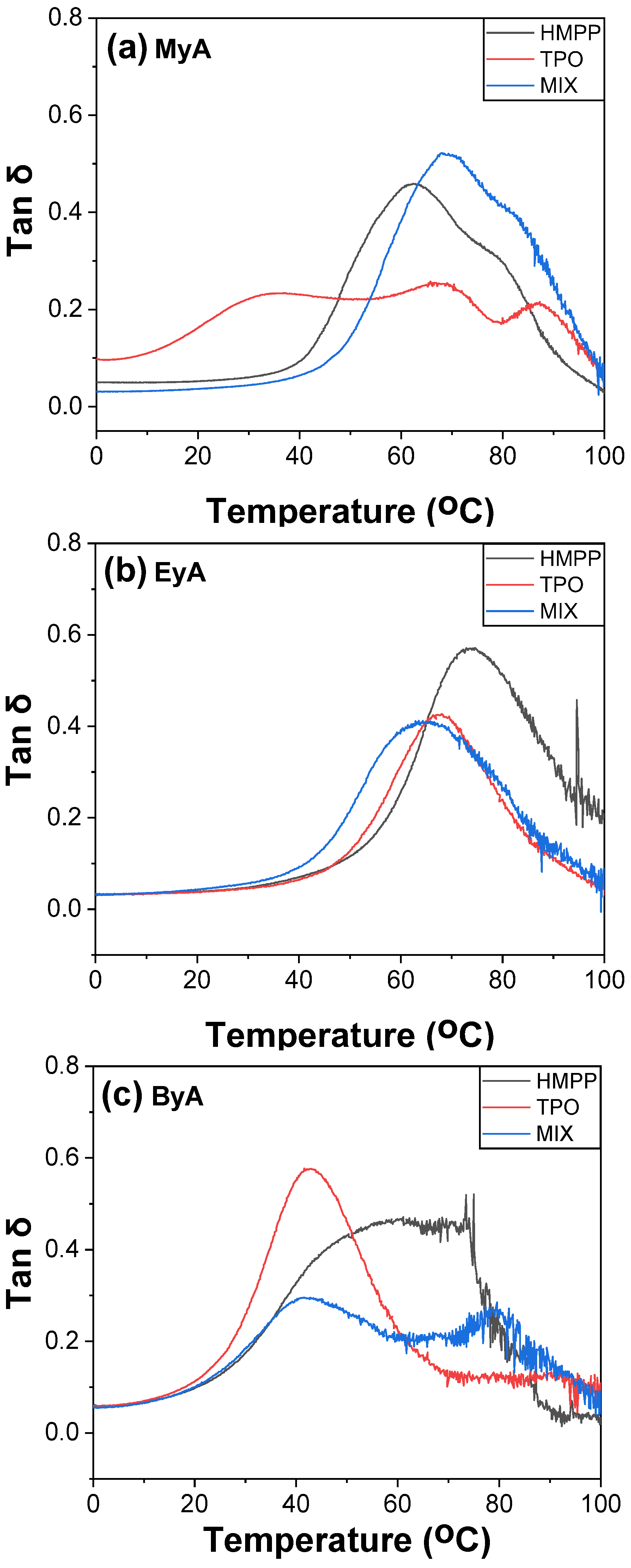
3.4. Mechanical Properties of Cured UV Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kwon, Y.; Lee, S.; Kim, J.; Jun, J.; Jeon, W.; Park, Y.; Kim, H.-J.; Gierschner, J.; Lee, J.; Kim, Y.; et al. Ultraviolet Light Blocking Optically Clear Adhesives for Foldable Displays via Highly Efficient Visible-Light Curing. Nat. Commun. 2024, 15, 2829. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, S.; Lee, J. Comprehensive Review on Post-Polymerization Modification of Hydroxyl-Terminated Polybutadiene (HTPB). Elastomers Compos. 2024, 59, 108–120. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, C.; Xia, Y.; Wang, C.; Sang, X.; Fang, H.; Wang, N. UV/Thermal Dual-Cured MWCNTs Composites for Pipeline Rehabilitation: Mechanical Properties and Damage Analysis. Constr. Build. Mater. 2024, 450, 138602. [Google Scholar] [CrossRef]
- Güler, O.; Er, Y.; Hekimoğlu, G.; Ustaoglu, A.; Sarı, A.; Subaşı, S.; Maraşlı, M.; Gencel, O. Production and Assessment of UV-Cured Resin Coated Stearyl Alcohol/Expanded Graphite as Novel Shape-Stable Composite Phase Change Material for Thermal Energy Storage. Appl. Therm. Eng. 2024, 247, 123105. [Google Scholar] [CrossRef]
- Kim, C.S.; Jang, J.; Im, H.-G.; Yoon, S.; Kang, D.J. Preparation and Performance of Alumina/Epoxy-Siloxane Composites: A Comparative Study on Thermal- and Photo-Curing Process. Heliyon 2024, 10, e27580. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mater. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Ehrhardt, D.; Van Durme, K.; Jansen, J.F.; Van Mele, B.; Van den Brande, N. Self-Healing UV-Curable Polymer Network with Reversible Diels-Alder Bonds for Applications in Ambient Conditions. Polymer 2020, 203, 122762. Available online: https://www.sciencedirect.com/science/article/pii/S0032386120305929?casa_token=WNAhYjdgxCkAAAAA:smcn3dnLLzR4yKTztRAMQK-G5R01iWkBHhJaMJDIVImws4BIcfmyrNyQiri1uEAk9AjdDyapSWM (accessed on 13 November 2024). [CrossRef]
- Huang, J.; Yuan, T.; Ye, X.; Man, L.; Zhou, C.; Hu, Y.; Zhang, C.; Yang, Z. Study on the UV Curing Behavior of Tung Oil: Mechanism, Curing Activity and Film-Forming Property. Ind. Crops Prod. 2018, 112, 61–69. [Google Scholar] [CrossRef]
- Lin, J.; Wang, S.; Jin, H.; Wang, R.; Cui, S.; Yang, Y.; Wang, J.; Huang, G. Preparation of Si-O-C-Based Precursor Ceramics for Photo-Curing 3D Printing: Selection of Silicone Prepolymer System and Photoinitiator. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Bhogi, A.; Srinivas, B.; Papolu, P.; Konakanchi, R.; Jaya Prakash, K.; Shareefuddin, M.; Kistaiah, P. Theoretical Investigation on Electronic, Optical and Phonon Properties of Compound Semiconductors Suitable for Photovoltaic Device Applications. J. Indian Chem. Soc. 2024, 101, 101285. [Google Scholar] [CrossRef]
- Li, T.; Li, P.; Sun, R.; Yu, S. Polymer-Based Nanocomposites in Semiconductor Packaging. IET Nanodielectr. 2023, 6, 147–158. [Google Scholar] [CrossRef]
- Jiang, J.; Pan, Z.; Yuan, J.; Shan, J.; Chen, C.; Li, S.; HaiXu; Chen, Y.; Zhuang, Q.; Ju, Z.; et al. Zincophilic Polymer Semiconductor as Multifunctional Protective Layer Enables Dendrite-Free Zinc Metal Anodes. Chem. Eng. J. 2023, 452, 139335. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, R.; He, Y.; Zhang, M.; Tian, F.; Wang, L.; Zhao, Y.; Huang, W. 3D Printed Epoxy/Acrylate Hybrid Polymers with Excellent Mechanical and Shape Memory Properties via UV and Thermal Cationic Dual-Curing Mechanism. Addit. Manuf. 2024, 79, 103904. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, H.; Yuan, K.; Zhang, G.; Xiao, C.; Wang, C.; Zong, L.; Wang, J.; Jian, X. Construction of Fluorinated Hyperbranched Polyaryletherketone-Based UV-Cured Films with Low Dielectric and Enhanced Mechanical Properties. Polymer 2024, 299, 126902. [Google Scholar] [CrossRef]
- Park, S.-H.; Shin, J.-A.; Park, H.-H.; Yi, G.Y.; Chung, K.-J.; Park, H.-D.; Kim, K.-B.; Lee, I.-S. Exposure to Volatile Organic Compounds and Possibility of Exposure to By-Product Volatile Organic Compounds in Photolithography Processes in Semiconductor Manufacturing Factories. Saf. Health Work 2011, 2, 210–217. [Google Scholar] [CrossRef]
- Balcerak, A.; Kabatc-Borcz, J.; Czech, Z.; Bartkowiak, M. Latest Advances in Highly Efficient Dye-Based Photoinitiating Systems for Radical Polymerization. Polymers 2023, 15, 1148. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Yu, S.; Ma, D.; Han, Y. Micropatterning and Transferring of Polymeric Semiconductor Thin Films by Hot Lift-off and Polymer Bonding Lithography in Fabrication of Organic Field Effect Transistors (OFETs) on Flexible Substrate. Appl. Surf. Sci. 2011, 257, 9264–9268. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, X.; Zhang, T.; Huang, Y. Recent Advances in UV/Thermal Curing Silicone Polymers. Chem. Eng. J. 2022, 435, 134843. [Google Scholar] [CrossRef]
- Patil, R.S.; Thomas, J.; Patil, M.; John, J. To Shed Light on the UV Curable Coating Technology: Current State of the Art and Perspectives. J. Compos. Sci. 2023, 7, 513. [Google Scholar] [CrossRef]
- Ni, X.; Luo, J.; Liu, R.; Liu, X. Facile Fabrication of Flexible UV-Cured Polyelectrolyte-Based Coatings for Humidity Sensing. Sens. Actuators B Chem. 2021, 329, 129149. [Google Scholar] [CrossRef]
- Shi, Q.; Cai, Y.; Zhuang, C.; Lin, B.; Wu, D.; Zeng, R.; Bao, W. A Robust Hybridizable Discontinuous Galerkin Scheme with Harmonic Averaging Technique for Steady State of Real-World Semiconductor Devices. J. Comput. Phys. 2024, 519, 113422. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, X.; Liu, F.; Zhu, S. Gate Voltage-Controlled Spin-Rectifier Diode Based on Janus Transition Metal Nitride MXene with Spin Gapless Semiconductor. Diam. Relat. Mater. 2024, 141, 110641. [Google Scholar] [CrossRef]
- Du, Y.; Jochem, K.S.; Thakral, N.; McCormick, A.V.; Francis, L.F. Roll-to-Roll Micromolding of UV Curable Coatings. J. Coat. Technol. Res. 2021, 18, 627–639. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lim, D.-H.; Kim, H.-J.; Park, D.-S.; Sung, I.-K. UV- and Thermal-Curing Behaviors of Dual-Curable Adhesives Based on Epoxy Acrylate Oligomers. Int. J. Adhes. Adhes. 2009, 29, 710–717. [Google Scholar] [CrossRef]
- Ghasemi, A.; Azzouz, R.; Laipple, G.; Kabak, K.E.; Heavey, C. Optimizing Capacity Allocation in Semiconductor Manufacturing Photolithography Area—Case Study: Robert Bosch. J. Manuf. Syst. 2020, 54, 123–137. [Google Scholar] [CrossRef]
- Czachor-Jadacka, D.; Pilch-Pitera, B. Progress in Development of UV Curable Powder Coatings. Prog. Org. Coat. 2021, 158, 106355. [Google Scholar] [CrossRef]
- Hermann, A.; Giljean, S.; Pac, M.-J.; Marsiquet, C.; Burr, D.; Landry, V. Physico-Mechanical Characterisation of Basecoats for Tailored UV-Cured Multilayered Wood Coating Systems. Prog. Org. Coat. 2023, 182, 107673. [Google Scholar] [CrossRef]
- Ke, X.; Liang, H.; Xiong, L.; Huang, S.; Zhu, M. Synthesis, Curing Process and Thermal Reversible Mechanism of UV Curable Polyurethane Based on Diels-Alder Structure. Prog. Org. Coat. 2016, 100, 63–69. [Google Scholar] [CrossRef]
- Jančovičová, V.; Mikula, M.; Havlínová, B.; Jakubíková, Z. Influence of UV-Curing Conditions on Polymerization Kinetics and Gloss of Urethane Acrylate Coatings. Prog. Org. Coat. 2013, 76, 432–438. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hong, S.; Sun, H.; Kim, M.G.; Choi, K.; Cho, J.; Choi, H.K.; Koo, J.C.; Moon, H.; Byun, D.; et al. UV-Curing Kinetics and Performance Development of in Situ Curable 3D Printing Materials. Eur. Polym. J. 2017, 93, 140–147. Available online: https://www.sciencedirect.com/science/article/pii/S0014305717303348?casa_token=apEeBU_EmTcAAAAA:boINW2fkPuNSkZ0mCj7YWlO0qmynsdsGZERQJlzJwub96OoNmKKp4X3K4fMrw0Xt8DgvdFsqqms (accessed on 15 November 2024). [CrossRef]
- Chattopadhyay, D.K.; Panda, S.S.; Raju, K.V.S.N. Thermal and Mechanical Properties of Epoxy Acrylate/Methacrylates UV Cured Coatings. Prog. Org. Coat. 2005, 54, 10–19. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Do Truc, V.; Nguyen, T.A.; Tran, D.L. Cationic UV-Cured Epoxy Coating with Inhibitor-Loaded Nanoparticles: Photoinitiated Cationic Polymerization, Mechanical Properties and Corrosion Protection of Steel. J. Coat. Technol. Res. 2024, 21, 1113–1124. [Google Scholar] [CrossRef]
- Kim, D.-E.; Kang, S.-H.; Lee, S.-H. Thermal Stability of Phenylphosphonic Acid Modified Polyurethanes. Elastomers Compos. 2023, 58, 70–80. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic Study of the Oxidation Resistance of Phosphorus-Containing Activated Carbons. Carbon 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Lee, S.-W.; Park, J.-W.; Park, C.-H.; Lim, D.-H.; Kim, H.-J.; Song, J.-Y.; Lee, J.-H. UV-Curing and Thermal Stability of Dual Curable Urethane Epoxy Adhesives for Temporary Bonding in 3D Multi-Chip Package Process. Int. J. Adhes. Adhes. 2013, 44, 138–143. [Google Scholar] [CrossRef]
- Sun, G.; Wu, X.; Liu, R. A Comprehensive Investigation of Acrylates Photopolymerization Shrinkage Stress from Micro and Macro Perspectives by Real Time MIR-Photo-Rheology. Prog. Org. Coat. 2021, 155, 106229. [Google Scholar] [CrossRef]
- Yavitt, B.M.; Wiegart, L.; Salatto, D.; Huang, Z.; Endoh, M.K.; Poeller, S.; Petrash, S.; Koga, T. Structural Dynamics in UV Curable Resins Resolved by In Situ 3D Printing X-Ray Photon Correlation Spectroscopy. ACS Appl. Polym. Mater. 2020, 2, 4096–4108. [Google Scholar] [CrossRef]
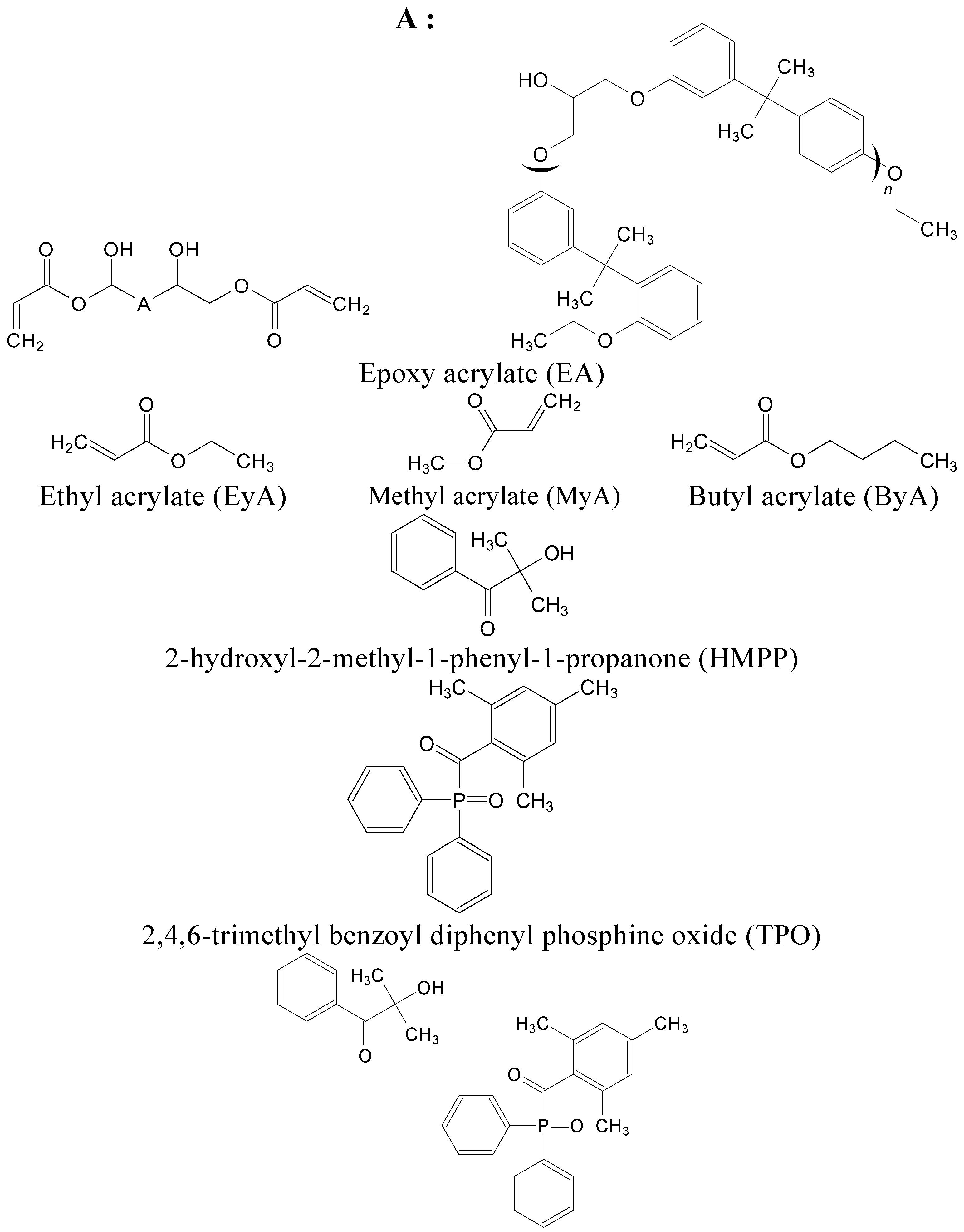





| Name | EA | Curing Agent (1:1 Equivalent Ratio) | Photoinitiator (HMPP/TPO/MIX) |
|---|---|---|---|
| ByA | 5 g | 4.05 g | 0.09 g |
| MyA | 2.72 g | 0.08 g | |
| EyA | 3.17 g | 0.08 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-m.; Jang, S.; Jang, K.-S. Interactions and Curing Dynamics Between UV-Triggered Epoxy Acrylate Binder, Curing Agents and Photoinitiators. Polymers 2025, 17, 1252. https://doi.org/10.3390/polym17091252
Choi J-m, Jang S, Jang K-S. Interactions and Curing Dynamics Between UV-Triggered Epoxy Acrylate Binder, Curing Agents and Photoinitiators. Polymers. 2025; 17(9):1252. https://doi.org/10.3390/polym17091252
Chicago/Turabian StyleChoi, Ji-min, Sang Jang, and Keon-Soo Jang. 2025. "Interactions and Curing Dynamics Between UV-Triggered Epoxy Acrylate Binder, Curing Agents and Photoinitiators" Polymers 17, no. 9: 1252. https://doi.org/10.3390/polym17091252
APA StyleChoi, J.-m., Jang, S., & Jang, K.-S. (2025). Interactions and Curing Dynamics Between UV-Triggered Epoxy Acrylate Binder, Curing Agents and Photoinitiators. Polymers, 17(9), 1252. https://doi.org/10.3390/polym17091252







