Green Processes for Chitin and Chitosan Production from Insects: Current State, Challenges, and Opportunities
Abstract
1. Introduction
2. Chitin Composition and Variability Across Insect Species and Residues
| Species | Part | Dry Matter a [%] | Chitin Yield a [%] | Chitosan Yield a [%] | DD Chitosan a [%] | Info | Ref. |
|---|---|---|---|---|---|---|---|
| Hermetia Illucens (Black Soldier Fly, BSF) | Larvae | 29.5 ± 0.3 | (1.) 9.5 ± 0.6 (2.) 7.8 ± 0.3 | n.s. | 89 ** | Process: Conventional chemical extraction and deacetylation. Methods for chitin/chitosan determination: (1.) Gravimetric determination of chitin based on dry matter. (2.) Chitin determination based on a calculation including glucosamine concentration via UPLC-MS/MS and acetate concentration via HPLC-RID. Method proposed by D’Hondt et al. (2020) [75]. Further info: Cocoon chitin was more crystalline (94%) compared to sheddings (89%). * Second attempt with extensive deproteinization process led to 26.6%. ** After prolonged deacetylation time of 3 h (for a time of 1 h, sheddings showed significantly lower deacetylation reactivity with degree of deacetylation (DD) of 50%). | [40] |
| Prepupae | 51.5 ± 1.1 | (1.) 9.1 ± 0.02 (2.) 10.9 ± 0.7 | |||||
| Pupae | 60.9 ± 0.9 | (1.) 10.3 ± 0.7 (2.) 10.7 ± 0.1 | |||||
| Shedding | 92.3 ± 0.5 | (1.) 31.1 ± 0.3 * (2.) 23.7 ± 1.9 | |||||
| Cocoon | 94.2 ± 0.9 | (1.) 23.8 ± 1.5 (2.) 22.4 ± 0.9 | |||||
| Adults | 60.5 ± 3.1 | (1.) 5.6 ± 0.4 (2.) 8.4 ± 1.9 | |||||
| BSF | Larvae | n.s. | 3.06 | n.s. | n.s. | Process: Conventional chemical extraction. No chitosan preparation. Methods for chitin determination: Gravimetric calculation of chitin based on dry weight. Further info: Chitin crystallinity index (CrI) varied in different life stages with 33.09%, 35.14%, 68.44%, and 87.92% for larvae, prepupa, puparium, and adult. Different surface morphologies of chitin observed between life stages. | [80] |
| Prepupae | n.s. | 3.1 | n.s. | n.s. | |||
| Puparium | n.s. | 14.1 | n.s. | n.s. | |||
| Adults | n.s. | 2.9 | n.s. | n.s. | |||
| BSF | Pupae Exuviae (BSFE) | n.s. | 9 | n.s. | n.s. | Process: Conventional chemical extraction. No chitosan preparation. Methods for chitin determination: Gravimetric determination of chitin based on dry weight. Further info: Degree of acetylation (DA) affirmed that Pupae Exuviae (115%) b chitin demonstrated higher purity than Dead Imago (86%) chitin. CrI of chitin varied between Imago and Pupae Exuviae with 25.5% and 49.4%, respectively. BSFE nonporous. BSFI chitin indicates mesoporosity. | [81] |
| Dead Imago (BSFI) | n.s. | 23 | n.s. | n.s. | |||
| BSF | Larval Exoskeletons | n.s. | (1.) 35.7 ± 0.6 (2.) 31 | 13–47 | 34–72 | Process: Larval exoskeletons from protein production. Optimized chemical extraction. Heterogenous and homogeneous chemical deacetylation. Two setups: (1.) small scale and (2.) 10 L-scale. Methods for chitin/chitosan determination: Chitin content measured as acid detergent fiber (ADF) subtracting the acid detergent lignin (ADL) according to Hahn et al. (2018) [76]. Chitosan yield based on dried chitin mass. Further info: At lower temperatures, deacetylation led to chitosan with lower DD and a solution with higher viscosity. But, in comparison to the chitosan obtained at higher temperatures, the chitosan yield was much lower. | [63] |
| BSF | Pupal Shell | n.s. | 12.4 | n.s. | 81.5 | Process: Microbial fermentation for chitin extraction. Conventional chemical deacetylation. Methods for chitin/chitosan determination: No info on chitin/chitosan determination. Further info: CrI extracted from BSF pupal shell (52.8%) and chitosan (55.4%) corresponded to lower crystalline index values. Chitin and chitosan samples displayed even surface structure with nonporous morphology and ordered hexagonal microfibrils. | [82] |
| BSF | Pupal Exuviae | n.s. | (1.) 7.78 ± 0.68 to 11.85 ± 1.16 (2.) 10.18 ± 0.42 | (1.) n.s. (2.) 6.58 | n.s. | Process: Two methods: (1.) Biological chitin extraction with different bacteria species. (2.) Conventional chemical extraction. Conventional chemical deacetylation. Methods for chitin/chitosan determination: Gravimetric calculation of chitin based on dry weight. Chitosan yields based on dried chitin mass. Further info: Low chitosan yield may have been the result of sample loss during deacetylation. Biologically extracted chitin exhibited irregular surfaces with abundant porous fibers. Chitin obtained via chemical method had a smooth surface with repeating circular and hexagonal elements. | [83] |
| BSF | Instar 3 | n.s. | (1.) 7.23 ± 0.33 (2.) 10.2 ± 0.83 | n.s. | n.s. | Process: Insects fed with two different organic wastes: (1.) Fruit waste. (2.) Vegetable waste. Conventional chemical extraction. No chitosan preparation. Methods for chitin determination: Gravimetric determination based on dry weight. Further info: CrI varied in life stages (Instar 3, Instar 4, Instar 5, Prepupa, Pupa) and with feed when fruit waste fed as follows: 51.16%, 58.49%, 75.03%, 71.08%, 59.62%, and, when vegetable waste was fed as follows: 62.09%, 57.39%, 51.48%, 75.89%, 71.39%. Samples displayed an uneven and substantial surface structure consisting of pentagonal and hexagonal units, along with microfiber for all extracted samples with a significant surface diversity. | [84] |
| Instar 4 | n.s. | (1.) 11.01 ± 0.46 (2.) 9.49 ± 0.11 | n.s. | n.s. | |||
| Instar 5 | n.s. | (1.) 9.17 ± 0.84 (2.) 9.83 ± 0.19 | n.s. | n.s. | |||
| Prepupa | n.s. | (1.) 11.78 ± 0.13 (2.) 11.78 ± 0.13 | n.s. | n.s. | |||
| Pupa | n.s. | (1.) 6.82 ± 0.36 (2.) 8.66 ± 0.29 | n.s. | n.s. | |||
| BSF | Puparia | n.s. | (1.) 25.39 ± 2.43 (2.) 21.19 ± 5.71 | n.s. | n.s. | Process: Two methods: (1.) Conventional chemical extraction. (2.) ADF and ADL method with H2SO4 and CTAB, according to Hahn et al. (2018) [76]. No chitosan preparation. Methods for chitin determination: (1.) Gravimetric determination based on dry weight. (2.) Acid detergent fiber (ADF) subtracting the acid detergent lignin (ADL) (ADF-ADL) according to Hahn et al. (2018) [76]. Further info: Chitin crystallinity index (CrI) varied in various developmental stages and depending on method: Puparia, Flakes Adult insect for chemical method: 74.1%, 61.1%, and 77.8%; and for ADF-ADL method: 70.8%, 50.0%, and 39.0%. All below, commercial shrimp chitin samples. Flake samples exhibited a recurring pattern like honeycombs; pupae shell chitin showed compact surface structures repeating with circular and hexagonal elements; however, adult insect chitin demonstrated lightly arranged oval elements interspersed with circular structures, featuring repeated fiber arrangements and lacking pores. In the case of chitins isolated using the ADF-ADL method, the Puparia and Flake samples exhibited a greater degree of homogeneity, a light not dense powder, in contrast to the samples acquired through the acid-based method. | [85] |
| Flakes (from oil production) | n.s. | (1.) 20.69 ± 2.47 (2.) 26.78 ± 2.17 | n.s. | n.s. | |||
| Adult | n.s. | (1.) 7.75 ± 0.49 (2.) 7.94 ± 1.92 | n.s. | n.s. | |||
| BSF | Larval Exuviae | n.s. | (1.) 10.9 ± 0.1 (2.) 11.1 ± 0.4 | n.s. | n.s. | Process: Conventional chemical extraction plus acid hydrolysis (for chitin determination). Methods for chitin/chitosan determination: Purity determination with the following method: Quantification of monomers after acidic hydrolysis (glucosamine, N-acetylglucosamine, acetic acid). Two methods were used to quantify glucosamine and N-acetylglucosamine: (1.) LC-ECD and (2.) LC-MS/MS based on D’Hondt et al. (2020) [75]. To quantify acetic acid, LC-UV was employed Chitin determination in a loaded sample via glucosamine, acetate, and acetylglucosamine contents. Gravimetric determination of chitin in insect material based on dry weight corrected with obtained chitin content in analyzed material. Further info: Purity: Chitin content measured in extracted chitin samples: larval exuviae: 68.1 ± 0.7%, puparium: 81.7 ± 1.1%, adult flies 79.4 ± 0.5%; commercial shrimp shell chitin: 89.1 ± 0.1% | [49] |
| Puparium | n.s. | (1.) 18.5 ± 0.4 (2.) 18.5 ± 0.3 | n.s. | n.s. | |||
| Adult Flies | n.s. | (1.) 9.6 ± 0.2 (2.) 9.2 ± 0.3 | n.s. | n.s. | |||
| BSF | Exuviae | 8 | 20 | n.s. | n.s. | Process: Deproteinization via superheated water hydrolysis. Methods for chitin determination Gravimetric determination based on dry weight. Further info: Besides chitin, proteins were acquired in an aqueous solution (as opposed to strong alkaline solution) with further usability. Chitin exhibited a nonporous surface morphology composed of orderly repeated hexagons. | [86] |
| BSF | Larvae | 22.0 ± 0.8 | (1.) 13 ± 0.7 (2.) 10 ± 0.7 | (1.) 25 ± 2.5 (2.) 33 ± 0.4 | (1.) 91 (2.) 92 | Process: Chemical extraction (formic acid, NaOH). Conventional chemical deacetylation. Methods for chitin/chitosan determination: Gravimetric determination for yield, ADF-ADL method for content in raw samples. Chitosan yield based on dried chitin mass. Further info: Chitin content of raw samples: larvae: 12.4 ± 1.7%, pupal exuviae: 25.5 ± 0.5%, and dead adults: 12.8 ± 1%. Bleached and unbleached products were analyzed: (1.) unbleached and (2.) bleached. CrI for unbleached chitin: larvae: 90.0%, pupal exuviae: 67.0%, and adults: 96.0%; and for bleached chitin: larvae: 84.0%, pupal exuviae: 62.0%, adults: 93.0%; and commercial chitin: 98.0%. Adult chitin showed highest surface complexity (including nanometric and micrometric features), decreasing in pupal exuviae and larvae. Bleaching had little effect on larvae and pupal exuviae but removed round particles in adult chitin. Deacetylation reduced fibrillation of chitin, resulting in a rough but more homogeneous chitosan structure compared to chitin. | [60] |
| Pupal Exuviae | 94.0 ± 0.7 | (1.) 31 ± 1.6 (2.) 23 ± 1.9 | (1.) 28 ± 4.5 (2.) 42 ± 1.5 | (1.) 83 (2.) 90 | |||
| Dead Adults | 93.0 ± 0.9 | (1.) 9 ± 0.4 (2.) 6 ± 0.1 | (1.) 27 ± 2.0 (2.) 41 ± 1.0 | (1.) 91 (2.) 93 | |||
| Zophobas morio (superworm) | Cuticle of Larva | n.s. | 11.21 ± 0.55 | 81.36 ± 1.35 | 83.57 ± 0.28 | Process: Conventional chemical extraction and deacetylation. Methods for chitin/chitosan determination: Gravimetric chitin determination based on dry weight. Chitosan yield based on dried chitin mass. Further info: Relative crystallinity index (RCI) for cuticle of larva chitin: 68.0% and chitosan: 66.3%; for cuticle of adult chitin: 89.2% and chitosan: 80.2%. Both chitin samples exhibited surface morphology with greater density featuring occasional pores and a fibrous structure. Chitosan of the cuticle of the adult demonstrated pores. Chitosan of the cuticle of the larva exhibited a dense morphology, devoid of nanofibers, characterized by pores and repetitive hexagon elements. | [87] |
| Cuticle of Adult | n.s. | 20.89 ± 0.14 | 83.42 ± 0.86 | 88.72 ± 1.13 | |||
| Blaptica Dubia (Dubia Roach) | Cuticle of Nymph | n.s. | 19.23 ± 0.60 | 75.07 ± 0.25 | 75.75 ± 0.19 | Process: Conventional chemical extraction and deacetylation. Methods for chitin/chitosan determination: Gravimetric chitin determination based on dry weight. Chitosan yield based on dried chitin mass. Further info: RCI for cuticle of nymph chitin: 80.9% and chitosan: 66.6%; for cuticle of adult chitin: 86.8% and chitosan: 73.9%. Chitin of the adult’s cuticle demonstrated even surface configuration lacking both pores and nanofibers. Chitin of the cuticle of the nymph showed rough surface morphology with fragmented fibers and no pores. Chitosan samples exhibited an uneven morphology, but, in contrast to chitin, they had fewer fibers. | [87] |
| Cuticle of Adult | n.s. | 15.68 ± 0.20 | 75.75 ± 0.45 | 86.33 ± 3.13 | |||
| Tenebrio molitor (Meal-worm) | Cuticle of Larva | n.s. | 13.25 ± 0.63 | 74.93 ± 0.93 | 76.32 ± 0.26 | Process: Conventional chemical extraction. Conventional chemical deacetylation. Methods for chitin/chitosan determination: Gravimetric chitin determination based on dry weight. Chitosan yield based on dried chitin mass. Further info: RCI for cuticle of larva chitin: 71.7% and chitosan: 65.3%; for cuticle of adult chitin: 73.3% and chitosan: 67.6%. Both chitin samples demonstrated uneven surface structures with no pores and disrupted fibers. Chitosan also showed an uneven surface; however, it displayed fewer fibers in contrast to chitin. Chitosan from the adult’s cuticle showed pores. | [87] |
| Cuticle of Adult | n.s. | 15.13 ± 0.78 | 78.96 ± 0.45 | 89.21 ± 0.96 | |||
| Meal-worm | Cuticles | 94.6 ± 0,1 | 70.9 | 31.9 | 53.9 | Process: Enzymatic deproteinization. Skipped demineralization step due to low mineral concentration (ash content 3.7%). Conventional chemical deacetylation. Methods for chitin/chitosan determination: Gravimetric chitin and chitosan determination based on dry weight. Chitosan yields based on dried chitin mass. Further info: DD of chitosan was not low enough. CrI of chitin: 53.7%, CrI of chitosan: 30.1%. Chitosan that originated from mealworm’s cuticles exhibited larger elements of varied shapes. Chitin and chitosan showed rougher morphology and nanofiber structures. | [30] |
| Meal-worm | Larva Protein Extraction Waste | n.s. | n.s. | (1.) ca. 17 * (2.) ca. 22 * | (1.) 82 ± 9.09 (2.) 84 ± 2.94 | Process: Conventional chemical extraction and deacetylation. Skipped demineralization step (inorganic material only 2–3%). Two batches (1.) and (2.). Methods for chitin/chitosan determination: Gravimetric chitin and chitosan determination based on dry weight. Chitosan yield based on dried chitin mass. Further info: Samples from molting stage and adults exhibited increased reflection peak intensities in contrast to larval samples, indicating elevated crystallinity levels. Low molecular weights analyzed for all chitosan samples (600–800 kDa for larva and adult samples, even lower results with high variability for molt samples). * Data estimated from figure [58]. | [65] |
| Waste from Molt | n.s. | n.s. | (1.) ca. 1 * (2.) ca. 4 * | (1.) 83 ± 2.82 (2.) 84 ± 2.16 | |||
| Adult Insects | n.s. | n.s. | (1.) ca. 17 * (2.) ca. 21 * | (1.) 84 ± 4.54 (2.) 81 ± 0.81 | |||
| Meal-worm | Larvae | (1.) 97.7 ± 0.05 (2.) 97.7 ± 0.05 | (1.) 5.3 ± 0.38 (2.) 6.0 ± 0.10 | (1.) 73.9 ± 2.03 (2.) 80.0 ± 0.58 | (1.) 67.4 (2.) 70.9 | Process: Conventional chemical extraction and deacetylation. Two different processing methods: (1.) First, deproteinization, then, demineralization (DEP-DEM). (2.) First, demineralization, then, deproteinization plus decoloring (DEM-DEP). Methods for chitin/chitosan determination: Gravimetric determination of chitin based on dry weight. Chitosan yield based on dried chitin mass. Further info: CrI for larvae chitin: (1.) 48% and (2.) 52%; chitin adult: (1.) 50% and (2.) 56%. DEM-DEP exhibited lower mineral concentrations and increased viscosity in contrast to the first method and was classed more efficient. In contrast to chitin obtained by DEP-DEM treatment (fibrous and uneven surface), chitin acquired through the DEM-DEP process displayed a more even surface morphology and more evident pores. Larger and more abundant pores were analyzed by chitin of the DEM-DEP method. | [88] |
| Adult | (1.) 97.8 ± 0.08 (2.) 97.8 ± 0.15 | (1.) 10.9 ± 0.18 (2.) 14.6 ± 0.15 | (1.) 81.9 ± 1.36 (2.) 87.3 ± 2.21 | (1.) 69.3 (2.) 73.2 | |||
| Meal-worm | Larval Exuviae | n.s. | (1.) 7.9 ± 0.1 (2.) 8.6 ± 0.1 | n.s. | n.s. | Process: Conventional chemical extraction plus acid hydrolysis (for chitin determination). Methods for chitin/chitosan determination: Purity determination with the following method: Quantifying monomers after acidic hydrolysis (glucosamine, N-acetylglucosamine, acetic acid). Two methods were used to quantify glucosamine and N-acetylglucosamine: (1.) LC-ECD and (2.) LC-MS/MS based on the study of [75]. To quantify acetic acid, LC-UV was employed. Chitin determination in loaded sample via glucosamine, acetate, and acetylglucosamine contents. Gravimetric determination of chitin in insect material based on dry weight corrected with obtained chitin content in analyzed material. Further info: Purity: Chitin content of extracted sample: 54.1 ± 1.2%; commercial shrimp shell chitin: 89.1 ± 0.1%. | [49] |
| Bombyx mori (Silkworm) | Pupae | n.s. | 18 | 91 | 66.9 ± 0.2 | Process: Conventional chemical extraction and deacetylation. Methods for chitin/chitosan determination: Gravimetric procedure. No detailed info. Chitosan yields based on chitin mass. Further info: CrI for pupae chitin: 74.5% and for chitosan: 48.4%; for eggshell chitin: 75.2% and for chitosan: 38.1%. In both chitosan samples, consistent “sheet-like” surface characteristics were observed without fibrous morphology. An increased quantity of “particulate matter” was detected in chitosan from pupae. | [45] |
| Egg Shells | n.s. | 6 | 80 | 59.2 ± 0.2 | |||
| Silkworm | Cuticle | n.s. | (1.) 51.93 ± 0.73 (2.) 56.94 ± 4.05 | n.s. | n.s. | Process: Aim of the work was not the extraction of chitin, but the chitin content of cuticle was determined after chemical deproteinization. Determination based on control sample after (1.) 12 days and (2.) 14 days after the start of the study to determine the effect of jellyfish venom on silkworm cuticle. Methods for chitin/chitosan determination: Gravimetric procedure. No detailed info. Further info: Not applicable. | [89] |
| Acheta domesticus (House Cricket) | Exuviae from Various Instar Stages | n.s. | (1.) 9.6 ± 0.2 (2.) 9.9 ± 0.2 | n.s. | n.s. | Process: Conventional chemical extraction plus acid hydrolysis (for chitin determination) Methods for chitin/chitosan determination: Purity determination with the following method: quantification of monomers after acidic hydrolysis (glucosamine, N-acetylglucosamine, and acetic acid). Two methods were used to quantify glucosamine and N-acetylglucosamine: (1.) LC-ECD and (2.) LC-MS/MS based on D’Hondt et al. (2020) [75]. To quantify acetic acid, LC-UV was employed. Chitin determination in loaded sample via glucosamine, acetate, and acetylglucosamine contents. Gravimetric determination of chitin in insect material based on dry weight corrected with obtained chitin content in analyzed material. Further info: Purity: Chitin content of extracted chitin sample: 66.7 ± 0.3%; commercial shrimp shell chitin: 89.1 ± 0.1%. | [42] |
3. Common Chitin Extraction and Chitosan Modification Across Different Biomass Sources
| Parameter | Crustaceans | Insects | Fungi |
|---|---|---|---|
| Availability | Seasonal, influenced by breeding cycles, molting periods, and fishing regulations. | Always available due to controlled farming systems. | Always available from year-round cultivation and agro-industrial byproducts. |
| Chitin/Chitosan Content in Raw Material (% Dry Weight) | Chitin: 6–75 [91,92]. | Chitin: 1.2–60 [91]. | Chitin + Chitosan: 2–42 [91,94]. |
| Chitin Yield after Conventional (conv.) Processing (% of Dry Biomass) | Higher yields due to larger chitin content in raw material (15–40%) [102]. | Generally lower yields from whole-body or larvae compared to crustaceans (5–15%); certain residues (e.g., sheddings, pupae) can yield comparable or higher amounts (up to ~55%) (see Table 1). | Generally lower than conventional sources [103]. |
| Industry Scale | Industry standard. | Underexplored. | Underexplored. |
| Use of Waste Products | Only byproducts such as shrimp shells and crab processing waste are used. | Includes farming byproducts like BSF cocoons, silkworm pupae, mealworm sheddings, exoskeletons, and residues from protein/lipid extraction. | Agro-industrial residues (e.g., mushroom stalks, fruit bodies, mycelium, further biomass). |
| Defatting (conv.) | Not necessary due to low lipid content. | Often required for high-fat insects; not required for specific waste materials (e.g., sheddings). | Not required; fungal biomass has negligible fat content. |
| Demineralization (conv.) and Deproteinization (conv.) | Intensive demineralization due to high mineral content in raw materials. | Highly dependent on raw material. Intensive demineralization is needed for waste products like exuviae and cocoons (high mineral content). | Demineralization is not required due to negligible mineral content. Deproteinization is conducted under milder conditions due to reduced protein complexity [104]. |
| Chitosan Production (via conv. Deacetylation) | Comparable to insects. Milder conditions for β-chitin; some α-chitin require harsher setups. | Comparable to crustaceans. | Typically, milder due to the inherent properties of fungal chitosan; chitosan is already present in some species. |
| Bleaching (conv.) | Well-established and less challenging due to more uniform and predictable pigment profiles. | More demanding due to diverse and complex pigments (e.g., melanins, catechols), requiring more intensive and tailored bleaching protocols. | Depending on raw material. For some, minimal bleaching is needed due to naturally low pigment levels [105]. |
| Alternative Greener Methods | Well-studied with emerging green methods. Needs scaling. | Underexplored, high potential; greener methods need development and scaling. | Underexplored, high potential due to waste-based feedstocks and simpler extraction/modification. |
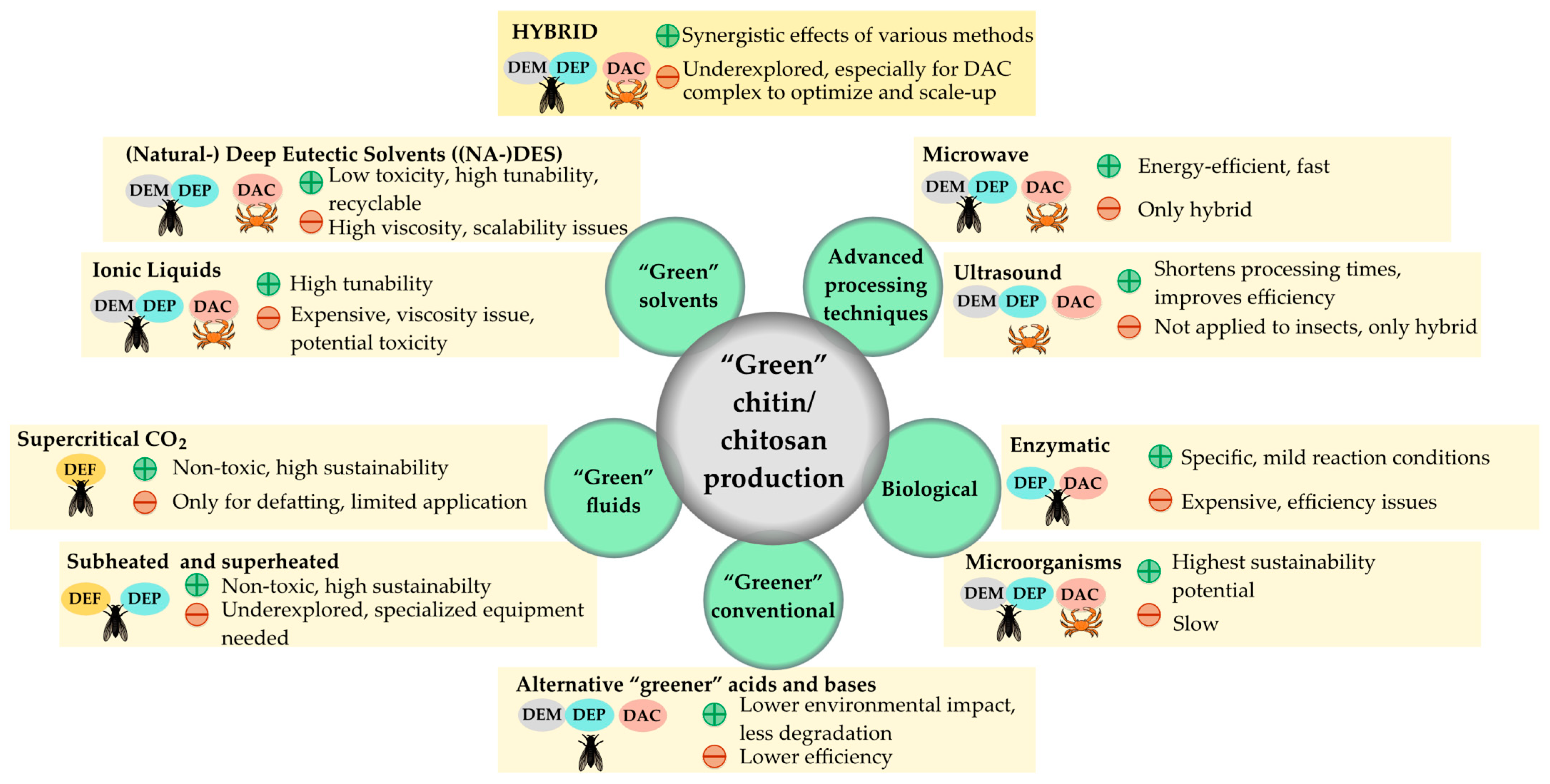
4. Green Chitin Extraction from Insects and Modifications to Chitosan
4.1. Biological and Enzyme-Assisted Methods
4.2. Methods Combined with Microwave Techniques
4.3. Extraction with Supercritical, Superheated, and Subcritical Fluids
4.4. Ionic Liquids (ILs), Deep Eutectic Solvents (DES), Natural Deep Eutectic Solvents (NADESs), and the Role of Artificial Intelligence (AI)
4.5. Emerging Green Processes and Hybrid Methods
| Insect and Material | Process | Deproteinization | Demineralization | Deacetylation | Results | Green Aspects | Ref. |
|---|---|---|---|---|---|---|---|
| Acheta Domesticus (House Cricket) | Enzymatic, microwave, greener acids, fermentation, DES | 1. Papain 24 h, 60 °C. 2. Bromelain 5 h, 60 °C. | 1. MW: 1 M HCl; 500 W, 8 min. 2. Citric Acid 0.5 M, room temp, 2 h. 3. Lactic acid fermentation; 30 °C, 48 h. | Conventional | Demineralization performance: Lowest performance: citric acid. Ferm. with lactic acid promising. Microwave and control (conventional): Best performance, conventional better than lactic acid, worse than MW. Chitin content after deproteinization: “greener” methods’ lowest performance. Conventional (NaOH) more than double. Biological process led to lowest molar mass (depolymerization). Effective large-scale production (2 L) of chitosan with biological method (bromelain + lactic acid) | DF 0 DEM + DEP + DA - B 0 REC 0 | [123] |
| One pot: 1. Fermentation with B. subtilis 5 d, 37 °C. 2. DES (Choline Chloride/Malonic Acid), 80 °C, 3 h. | |||||||
| BSF pupal shell waste | Fermentation | Protease-producing bacteria (Bacillus subtilis and Pseudomonas aeruginosa) 37 °C, 5 d. | Lactic acid-producing bacteria (Lactobacillus plantarum), 37 °C, 5 d. | Conventional | Chemical and biological extraction yielded chitin at 10.18% and 11.85%, respectively. Maximum chitosan yield of 6.58% based on chitin mass. | DF 0 DEM + DEP + DA - B 0 REC 0 | [83] |
| Crab shell | Fermentation + ultrasound-treatment | Lacticaseibacillus paracasei 37 °C, 48 h with low-intensity ultra sound treatment (0.167 W/cm2) for 10 min at 8 h intervals. | n.s. | Improved decalcification (DEC) by 16.72% (DEM rate: 71.77%) and deproteinization by 33.45% (DEP rate: 59.50%). Fermentation time shortened to 48 h. Chitin’s molecular structure preserved; deacetylation degree unchanged when combined with low-intensity ultrasound. | DF 0 DEM + DEP + DA 0 B 0 REC 0 | [125] | |
| Tenebrio molitor (Meal-worm) cuticle | Enzymatic | Alcalase enzyme; pH 8.0, 50 °C, 250 min. | Skipped, since low mineral amount. | Conventional | Enzyme used was efficient (85% DEP efficiency but protein residue: 8.3%) Sufficient reduction justifies use of green process. Rest mineral content: 3.7%. Cuticle-to-chitosan yield of 31.9%. Higher degree of deacetylation needed as DD of chitosan is 53.9%. | DF 0 DEM 0 DEP + DA - B 0 REC 0 | [30] |
| Clanis bilineata (Lined Hawk- moth) larvae skin | Enzymatic | Endo- and exo-peptidases (flavourzyme); 40–60 °C, 8 h. | Conventional. | Conventional | Optimum parameters for outcome at pH 6.5 and 50 °C. Chitosan yield 31.37% based on raw mass. Protein residue in chitosan 0.81%. Only green deproteinization. Rest conventional. | DF 0 DEM - DEP + DA - B 0 REC 0 | [135] |
| BSF prepupae | Enzymatic | (1.) B.·licheniformis protease pH 6.5, 60 °C, 16 h. (2.) Pepsin pH 3.0, 37 °C, 16 h. (3.) Papain 60 °C, pH 7.5, 16 h. (4.) Pancreatin 37 °C, pH 7.8, overnight. | n.s. | n.s. | Hydrolytic activity: highest for B. licheniformis, followed by pancreatin, followed by papain. Pepsin lowest performance. Degree of hydrolysis (DH %): ~6% (B. licheniformis), 17% pepsin, up to ~25% for pancreatin and papain. Chitin yield (~9%) with residues of minerals and non-hydrolyzed proteins. | DF 0 DEM 0 DEP + DA 0 B 0 REC 0 | [128] |
| Caribena versicolor (Tarantula) molts (ecdysis cuticles) | Microwave-assisted | Hybrid: Chemical 2.5 M NaOH+ MW * 750 W 2450 MhZ reached 95 °C, 3 min. | Skipped | n.s. | No fully green bleaching and defatting step, but combination with microwave treatment. Thus, faster and expected to be greener. Chitin content at 19% of the molt by molt dry mass. | DF + DEP + DEM 0 DA 0 B + REC 0 | [79] |
| Crayfish shell waste not insect | Hybrid: MW + DES | 1. Choline chloride/lactic acid (CL). 2. Choline chloride/urea (CU). 3. Choline chloride/glycerol (CG); 80–140 °C 10–40 min with microwave radiation = 300 W with different weight ratios of sample to DES. | n.s. | Extraction yield (YE), chitin yield (YC), and purity: CL: 19.11%/73.22%/97.44%. CG: 53.40%/88.17%/41.99%. CU 49.16%/88.87%/45.97%. Ash content: CL: ca. 0%, CG: 56.48%, CU: 52.41%. Protein content: CL: 2.56%, CG: 1.53%, CU: 1.62%. Recycling of DES. | DF 0 DEP + DEM + DA + B - REC + | [138] | |
| BSF farming waste | Subcritical water extraction | n.s. | n.s. | n.s. | Defatting: Subcritical water extraction of lipids. Optimized conditions (236.8 °C, 10 min, 1 g/100 mL) resulted in a lipid yield of 13.31% by total sample weight. Aqueous phase contains proteins (hydrolyzed, with low molecular masses of 6 kDa). | DF + DEM 0 DEP + DA 0 B 0 REC 0 | [147] |
| BSF exuviae | Superheated water | Superheated water; 150 °C, 1.5, 10, 15, 20 h. | Conventional. | Conventional | Soxhlet extraction with diethyl ether 7% fat, 40% proteins, and 20% chitin. | DF - DEP: + DEM - DA - B 0 REC 0 | [86] |
| Penaeus vannamei (White shrimp) shell not insect | Binary ionic liquids | Different ionic liquids based on [EMIM][Ac] * with [BMIM][Br] * mixed at ratios of 0:1, 1:1, 3:2, 4:1, and 1:0; 110 °C, 24 h. | Citric acid 12% (w/v); 60 °C, 5 h. | n.s. | Optimal chitin quality obtained with IL (3:2) at viscosity, conductivity, and radius of gyration values of 0.16 ± 0.00 Pa s, 0.30 ± 0.01 S/m, and 0.10 ± 0.00 nm, respectively. Chitin yield using IL reached up to 35.72 ± 0.31%, about twice as high as acid–base method (17.50 ± 0.16%). Whiteness index (WI) of chitin extracted by IL (3:2) (94.93 ± 0.09) superior to commercial chitin (93.31 ± 0.11). Surface morphology, secondary structure, and thermal stability comparable to commercially available chitin. | DF 0 DEP + DEM + DA 0 B: + REC 0 | [160] |
| BSF prepupae | Co-solvent | Conventional. | Co-solvent of glycerol and HCl (37% glycerol, 5% HCl); 90 °C, 2 h. | n.s. | Defatting: via Soxhlet and petroleum ether. No info on chitin yield. γ-chitin identified. | DF - DEM + DEP - DA 0 B - REC 0 | [122] |
| BSF exo- skeleton | Enzymatic, “greener” acids, fermentation, SC-CO2, special defatting. | (1.) + (2.) Protease from Bacillus licheniformis; 37 °C, 72 h. | (1.) + (2.) EtOH (for DF), lactic acid, and acetic acid; 25°C, 27h. | n.s. | Defatting via EtOH, optional Pseudomonas fluorescens (lipase production), or SC-CO2. Four methods applied: (1.) DEM+DF then DEP (Chitin: 69.4 ± 1.0%). (2.) DEP first, then DEM+DF (Chitin: 65.4 ± 0.2%, best protein, and lipid removal efficiency: protein decrease 33%, lipid reduction 38.5%). (3.) SC-CO2 + fermentation (Chitin: 69.8 ± 1.2%). (4.) Acetic acid + EtOH then fermentation (Chitin: 72.4 ± 1.6%, highest yield). High chitin yields suggest possible residual impurities as noted by authors. | DF + DEM + DEP + DA 0 B 0 REC 0 | [173] |
| (3.) SC-CO2 (defatting (65 °C, 2 h, 5400 psi)) then Lactobacillus plantarum, Bacillus subtilis, and Pseudomonas fluorescens (30 °C, 5 d). | |||||||
| (4.) L. plantarum, B. subtilis, and P. fluorescens (30 °C, 5 d). | (4.) Acetic acid (50 mL), 70% ethanol (100 mL) (1 day). | ||||||
| BSF prepupae, skimmed | NADES | Different NADESs: HBD (ChCl and betaine) and HBA (DL-lactic acid, n-butyric acid, glycerol, urea and oxalic acid); 50–80 °C, 2 h. | n.s. | Complex results on purity, yield, and DD. Lacking link between pH of NADESs and yield, purity, or DD of chitin. No correlation between acidity of HBD and chitin’s purity. Relatively high yield for several NADES applications in comparison to conventional method. Degree of deacetylation of chitin from samples relatively high in comparison to commercial sample. Recycling of NADES. | DF 0 DEM + DEP + REC + B 0 DA 0 REC + | [129] | |
| Mealworm exo-skeletons | Fermentation with isolates from mealworm | 1st fermentation: Inoculated TSB with colony-forming units of isolates from mealworm with Serratia marcescens (1 VA) and Serratia liquefaciens (16 VB); 25 °C, 5 d. Alternative 2nd fermentation: Lactobacillus plantarum (DSM 20 174); 30 °C, 7 d. | n.s. | Two varieties, S. marcescens (1 VA) and S. liquefaciens (16 VB), demonstrated best protease activity, reaching 96.78 U/mL and 97.34 U/mL, respectively. Demineralization of around 94% for all processes. Chitin Yields (dry weight): 1 VA: 28.2%; 16 VB: 28.6%; 1 VA+ L. plantarum: 18.6%; 16 VB + L. plantarum: 17.2%. Discolored product with residues of catechol compounds, sclerotin-like proteins, and pigments. | DF 0 DEM + DEP + DA 0 B 0 REC 0 | [134] | |
| Litopenaeus Vannamei (Shrimp) shell powder not insect | DES (+MW) | 1. N-methyl urea, N-methylacetamide, and acetic acid (1:1:3), different mass ratios of shrimp shell powder to DES (1:10, 1:20; 1:30), MW, 3–11 min. 2. N-methylurea, N-methylacetamide, and acetic acid (1:1:3), different mass ratios of shrimp shell powder to DES (1.10, 1:20; 1:30), RT, 6–48 h. | n.s. | Innovative DES with notably lower melting point (−16.82 °C) and viscosity (7.38 mPa·s; 25 °C) alongside high extraction efficiency at room temperature. Demineralization rate up to 99.07%. Deproteinization rate up to 92.67%. DD: 7.89% for conventional method; 6.77% for DES method at room temperature. 6.39% for DES method combined with MW. DD for conventional method’s chitin higher since NaOH used with intense deacetylation effects at elevated temperatures demonstrated robust recyclability, with DES showing consistent viscosity over repeated cycles. After 10 cycles, demineralization and deproteinization rates stayed at 89.78% and 86.84%, respectively. | DF 0 DEM + DEP + DA 0 B 0 REC + | [164] | |
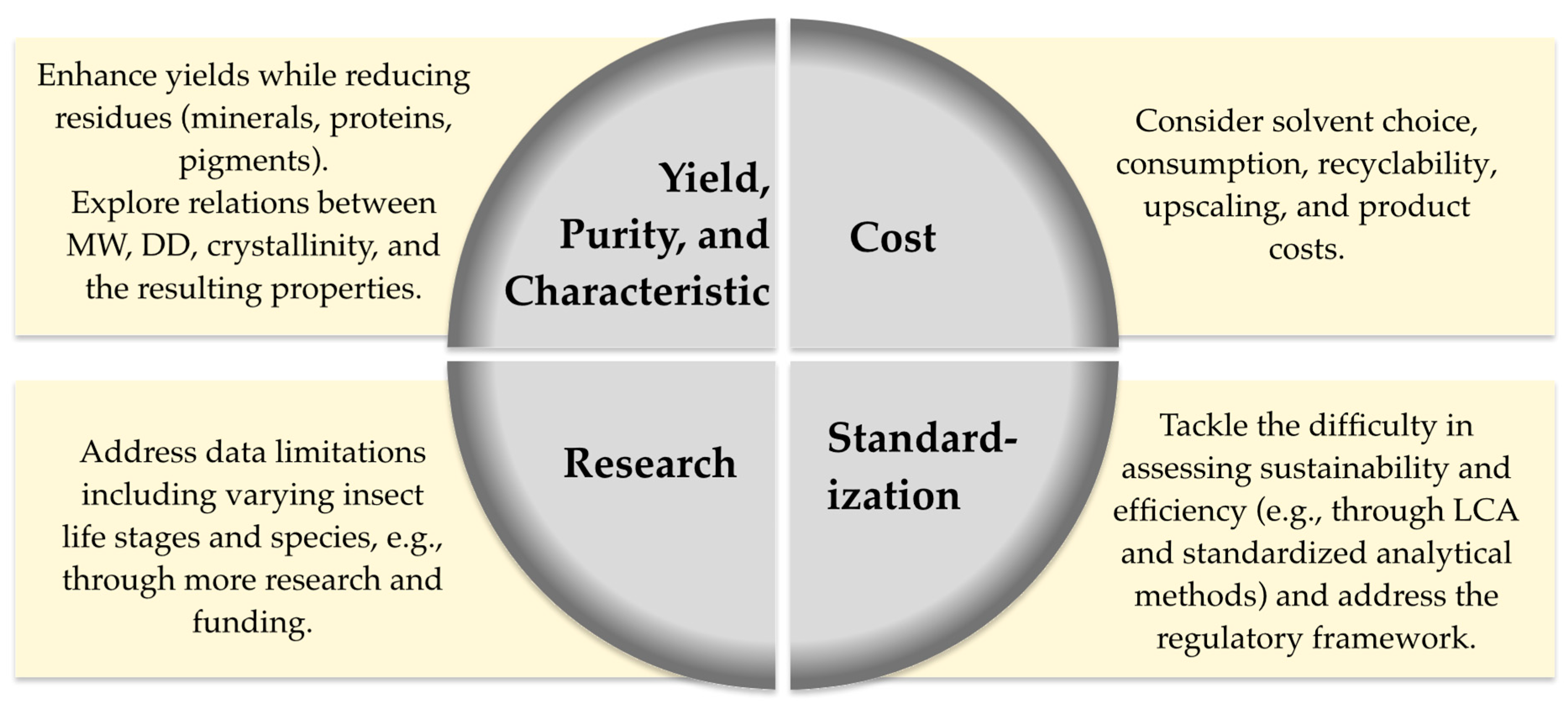
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.S.; Ciais, P.; Tubiello, F.N.; Smith, P.; Campbell, N.; Jain, A.K. Global Greenhouse Gas Emissions from Animal-Based Foods Are Twice Those of Plant-Based Foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Setti, A.F.F.; Azeiteiro, U.M.; Lokupitiya, E.; Donkor, F.K.; Etim, N.A.N.A.; Matandirotya, N.; Olooto, F.M.; Sharifi, A.; Nagy, G.J.; et al. An Overview of the Interactions between Food Production and Climate Change. Sci. Total Environ. 2022, 838, 156438. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Alves, M.M.; Santos, C.F.; Ribeiro, I.A.C.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L. Biodegradable chitosan films with ZnO nanoparticles synthesized using food industry by-products—Production and characterization. Coatings 2021, 11, 646. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Castanheira, I.; Loizzo, M.R.; Fernando, A.L.; Silva, A.S. A new insight on cardoon: Exploring new uses besides cheese making with a view to zero waste. Foods 2020, 9, 564. [Google Scholar] [CrossRef]
- Rodrigues, C.; Paula, C.D.D.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef]
- Nations, U. World Population Prospects 2022: Ten Key Messages; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022. [Google Scholar]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and Supply of High-Quality Food Protein for Human Consumption: Sustainability, Challenges, and Innovations. Ann. N. Y Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef]
- Medek, D.E.; Schwartz, J.; Myers, S.S. Estimated Effects of Future Atmospheric CO2 Concentrations on Protein Intake and the Risk of Protein Deficiency by Country and Region. Environ. Health Perspect. 2017, 125, 087002. [Google Scholar] [CrossRef]
- Fanzo, J.; Hunter, D.; Borelli, T.; Mattei, F. Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health; Taylor and Francis: Oxfordshire, UK, 2013; ISBN 9780203127261. [Google Scholar]
- Van Huis, A.; Rumpold, B.; Maya, C.; Roos, N. Nutritional Qualities and Enhancement of Edible Insects. Annu. Rev. Nutr. 2021, 41, 551–576. [Google Scholar] [CrossRef]
- van Huis, A. Prospects of Insects as Food and Feed. Org. Agric. 2021, 11, 301–308. [Google Scholar] [CrossRef]
- Looy, H.; Dunkel, F.V.; Wood, J.R. How Then Shall We Eat? Insect-Eating Attitudes and Sustainable Foodways. Agric. Hum. Values 2014, 31, 131–141. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Dias, C.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Could Consumption of Insects, Cultured Meat or Imitation Meat Reduce Global Agricultural Land Use? Glob. Food Secur. 2017, 15, 22–32. [Google Scholar] [CrossRef]
- Halloran, A.; Roos, N.; Eilenberg, J.; Cerutti, A.; Bruun, S. Life Cycle Assessment of Edible Insects for Food Protein: A Review. Agron. Sustain. Dev. 2016, 36, 57. [Google Scholar] [CrossRef] [PubMed]
- Lundy, M.E.; Parrella, M.P. Crickets Are Not a Free Lunch: Protein Capture from Scalable Organic Side-Streams via High-Density Populations of Acheta Domesticus. PLoS ONE 2015, 10, e0118785. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J. Bioconversion Potential of Agro-Industrial Byproducts by Tenebrio Molitor—Long-Term Results. Insects 2022, 13, 810. [Google Scholar] [CrossRef]
- van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth Performance and Feed Conversion Efficiency of Three Edible Mealworm Species (Coleoptera: Tenebrionidae) on Diets Composed of Organic by-Products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- Davison, C.; Michie, C.; Tachtatzis, C.; Andonovic, I.; Bowen, J.; Duthie, C.A. Feed Conversion Ratio (FCR) and Performance Group Estimation Based on Predicted Feed Intake for the Optimisation of Beef Production. Sensors 2023, 23, 4621. [Google Scholar] [CrossRef]
- Kee, P.E.; Cheng, Y.S.; Chang, J.S.; Yim, H.S.; Tan, J.C.Y.; Lam, S.S.; Lan, J.C.W.; Ng, H.S.; Khoo, K.S. Insect Biorefinery: A Circular Economy Concept for Biowaste Conversion to Value-Added Products. Environ. Res. 2023, 221, 115284. [Google Scholar] [CrossRef]
- Hawkey, K.J.; Lopez-Viso, C.; Brameld, J.M.; Parr, T.; Salter, A.M. Insects: A Potential Source of Protein and Other Nutrients for Feed and Food. Annu. Rev. Anim. Biosci. 2021, 9, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Liceaga, A.M.; Eleazar Aguilar-Toalá, J.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Insects as an Alternative Protein Source. Annu. Rev. Food Sci. Technol. 2022, 13, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Tabassum-Abbasi; Abbasi, T.; Abbasi, S.A. Reducing the Global Environmental Impact of Livestock Production: The Minilivestock Option. J. Clean. Prod. 2016, 112, 1754–1766. [Google Scholar] [CrossRef]
- Ordoñez-Araque, R.; Quishpillo-Miranda, N.; Ramos-Guerrero, L. Edible Insects for Humans and Animals: Nutritional Composition and an Option for Mitigating Environmental Damage. Insects 2022, 13, 944. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food Applications of Chitin and Chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In vitro bioactivity of novel chitosan bionanocomposites incorporated with different essential oils. Ind. Crops Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Han, X.; Heinonen, M. Development of Ultra-High Performance Liquid Chromatographic and Fluorescent Method for the Analysis of Insect Chitin. Food Chem. 2021, 334, 127577. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Quadro Oreste, E.; Leão Gouveia Costa, H.; Martín López, H.; Dias Medeiros Saad, C.; Prentice, C. Extraction, Physicochemical Characterization, and Morphological Properties of Chitin and Chitosan from Cuticles of Edible Insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef]
- Henriques, B.S.; Garcia, E.S.; Azambuja, P.; Genta, F.A. Determination of Chitin Content in Insects: An Alternate Method Based on Calcofluor Staining. Front. Physiol. 2020, 11, 117. [Google Scholar] [CrossRef]
- Kumar, M.; Vivekanand, V.; Pareek, N. Insect Chitin and Chitosan: Structure, Properties, Production, and Implementation Prospective. In Natural Materials and Products from Insects: Chemistry and Applications; Springer: Cham, Switzerland, 2020; pp. 51–66. ISBN 978-3-030-36610-0. [Google Scholar]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L. Chitosan composites in packaging industry-current trends and future challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin, Chitosan and Their Melanin Complexes from Insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Almeida, K.M.; Augusto, A.S.; Vieira, E.T.; Fernando, A.L.; Souza, V.G.L. Application of Biocomposite Films of Chitosan/Natural Active Compounds for Shelf Life Extension of Fresh Poultry Meat. J. Compos. Sci. 2022, 6, 342. [Google Scholar] [CrossRef]
- Vieira, H.; Lestre, G.M.; Solstad, R.G.; Cabral, A.E.; Botelho, A.; Helbig, C.; Coppola, D.; de Pascale, D.; Robbens, J.; Raes, K.; et al. Current and Expected Trends for the Marine Chitin/Chitosan and Collagen Value Chains. Mar. Drugs 2023, 21, 605. [Google Scholar] [CrossRef] [PubMed]
- Global Industry Analysts, Inc. Chitin and Chitosan Derivatives: Global Strategic Business Report; Global Industry Analysts, Inc.: Dublin, Ireland, 2023. [Google Scholar]
- van Huis, A. Edible Insects: Challenges and Prospects. Entomol. Res. 2022, 52, 161–177. [Google Scholar] [CrossRef]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of Chitin Extracted from Black Soldier Fly in Different Life Stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin Metabolism in Insects: Structure, Function and Regulation of Chitin Synthases and Chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Andersen, S.O. Biochemistry of Insect Cuticle. Annu. Rev. Entomol. 1979, 24, 29–59. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitin Nanostructures in Living Organisms. In Chitin: Formation and Diagenesis; Springer: Dordrecht, The Netherlands, 2011; pp. 1–34. ISBN 978-90-481-9684-5. [Google Scholar]
- Battisti, A.; Holm, G.; Fagrell, B.; Larsson, S. Urticating Hairs in Arthropods: Their Nature and Medical Significance. Annu. Rev. Entomol. 2011, 56, 203–220. [Google Scholar] [CrossRef]
- Battampara, P.; Nimisha Sathish, T.; Reddy, R.; Guna, V.; Nagananda, G.S.; Reddy, N.; Ramesha, B.S.; Maharaddi, V.H.; Rao, A.P.; Ravikumar, H.N.; et al. Properties of Chitin and Chitosan Extracted from Silkworm Pupae and Egg Shells. Int. J. Biol. Macromol. 2020, 161, 1296–1304. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T. Description of a New Surface Morphology for Chitin Extracted from Wings of Cockroach (Periplaneta americana). Int. J. Biol. Macromol. 2015, 75, 7–12. [Google Scholar] [CrossRef]
- Rudall, K.M.; Kenchington, W. The Chitin System. Biol. Rev. 1973, 48, 597–633. [Google Scholar] [CrossRef]
- Roze, M.; Gorb, S.N.; Zeimet, T.; Krings, W. Mandible Composition and Properties in Two Selected Praying Mantises (Insecta, Mantodea). Anat. Rec. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nurfikari, A.; de Boer, W. Chitin Determination in Residual Streams Derived From Insect Production by LC-ECD and LC-MS/MS Methods. Front. Sustain. Food Syst. 2021, 5, 795694. [Google Scholar] [CrossRef]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; LaJeunesse, D. SEM Characterization of Anatomical Variation in Chitin Organization in Insect and Arthropod Cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Lelešius, E.; Nagrockaite, R.; Sargin, I.; Arslan, G.; Mol, A.; Baran, T.; Can, E.; Bitim, B. Differentiations of Chitin Content and Surface Morphologies of Chitins Extracted from Male and Female Grasshopper Species. PLoS ONE 2015, 10, e0115531. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and Its Derivatives: Structural Properties and Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M.; Bouligand, Y. Chitin-Protein Molecular Organization in Arthropod. In Chitin in Nature and Technology; Springer: Boston, MA, USA, 1986; pp. 29–35. ISBN 978-1-4613-2167-5. [Google Scholar]
- Terkula Iber, B.; Kasan, A.; Torsabo, D.; Omuwa, J.W. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2021, 10, 1097–1123. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On Chemistry of γ-Chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Sulthan, R.; Sambhudevan, S.; Greeshma, S.; Gayathri, S.; Anagha, D.A.; Niranjan, B.; Gayathri, K.; Unni, V.V. Extraction of β-Chitin Using Deep Eutectic Solvents for Biomedical Applications. Mater. Today Proc. 2023, 94, 44–48. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Methacanon, P.; Prasitsilp, M.; Pothsree, T.; Pattaraarchachai, J. Heterogeneous N-Deacetylation of Squid Chitin in Alkaline Solution. Carbohydr. Polym. 2003, 52, 119–123. [Google Scholar] [CrossRef]
- Romany, A.; Payne, G.F.; Shen, J. Mechanism of the Temperature-Dependent Self-Assembly and Polymorphism of Chitin. Chem. Mater. 2023, 35, 6472–6481. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of Chitin and Chitosan Derived from Hermetia Illucens, a Further Step in a Circular Economy Process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Rong, J.; Lin, Y.; Sui, Z.; Wang, S.; Wei, X.; Xiao, J.; Huang, D. Amorphous Calcium Phosphate in the Pupal Cuticle of Bactrocera Dorsalis Hendel (Diptera: Tephritidae): A New Discovery for Reconsidering the Mineralization of the Insect Cuticle. J. Insect Physiol. 2019, 119, 103964. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia illucens) Larvae, Prepupae, and Pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; von Seggern, N.; Falabella, P.; Salvia, R.; Thomä, J.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Stegbauer, L.; et al. Purification of Chitin from Pupal Exuviae of the Black Soldier Fly. Waste Biomass Valorization 2022, 13, 1993–2008. [Google Scholar] [CrossRef]
- Khatami, N.; Guerrero, P.; Martín, P.; Quintela, E.; Ramos, V.; Saa, L.; Cortajarena, A.L.; de la Caba, K.; Camarero-Espinosa, S.; Abarrategi, A. Valorization of Biological Waste from Insect-Based Food Industry: Assessment of Chitin and Chitosan Potential. Carbohydr. Polym. 2024, 324, 121529. [Google Scholar] [CrossRef]
- Huet, G.; Hadad, C.; Husson, E.; Laclef, S.; Lambertyn, V.; Araya Farias, M.; Jamali, A.; Courty, M.; Alayoubi, R.; Gosselin, I.; et al. Straightforward Extraction and Selective Bioconversion of High Purity Chitin from Bombyx Eri Larva: Toward an Integrated Insect Biorefinery. Carbohydr. Polym. 2020, 228, 115382. [Google Scholar] [CrossRef]
- Wang, J.; Teng, C.; Yan, L. Applications of Deep Eutectic Solvents in the Extraction, Dissolution, and Functional Materials of Chitin: Research Progress and Prospects. Green. Chem. 2022, 24, 552–564. [Google Scholar] [CrossRef]
- Rathke, T.D.; Hudson, S.M. Review of Chitin and Chitosan as Fiber and Film Formers. J. Macromol. Sci. Part. C Polym. Rev. 1994, 34, 375–437. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification and Chitosan Production from Insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.W. Chitosan-Based Biodegradable Functional Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Fuciños, P.; Pastrana, L.; Fernando, A.L. Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers 2022, 14, 1359. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Current Applications of Bionanocomposites in Food Processing and Packaging. Polymers 2023, 15, 2336. [Google Scholar] [CrossRef]
- Silva, S.J.; Samba, N.; Mendes, J.; Pires, J.R.A.; Rodrigues, C.; Curto, J.; Gomes, A.; Fernando, A.L.; Silva, L. Sustainable Food Packaging with Chitosan Biofilm Reinforced with Nanocellulose and Essential Oils. Macromol 2023, 3, 704–722. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- D’Hondt, E.; Soetemans, L.; Bastiaens, L.; Maesen, M.; Jespers, V.; Van den Bosch, B.; Voorspoels, S.; Elst, K. Simplified Determination of the Content and Average Degree of Acetylation of Chitin in Crude Black Soldier Fly Larvae Samples. Carbohydr. Res. 2020, 488, 107899. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwalla, T.; Zibek, S. New Methods for High-Accuracy Insect Chitin Measurement. J. Sci. Food Agric. 2018, 98, 5069–5073. [Google Scholar] [CrossRef]
- Luparelli, A.V.; Leni, G.; Fuso, A.; Pedrazzani, C.; Palini, S.; Sforza, S.; Caligiani, A. Development of a Quantitative UPLC-ESI/MS Method for the Simultaneous Determination of the Chitin and Protein Content in Insects. Food Anal. Methods 2023, 16, 252–265. [Google Scholar] [CrossRef]
- Kvasnička, F.; Kouřimská, L.; Bleha, R.; Škvorová, P.; Kulma, M.; Rajchl, A. Electrophoretic Determination of Chitin in Insects. J. Chromatogr. A 2023, 1695, 463952. [Google Scholar] [CrossRef] [PubMed]
- Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Schimpf, C.; Rafaja, D.; Brendler, E.; Viehweger, C.; Żółtowska-Aksamitowska, S.; Petrenko, I.; et al. Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena Versicolor Spider Molt Cuticle. Molecules 2019, 24, 3736. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.u.; Feng, W.; Yang, D.; Rehman, R.u.; Cai, M.; Zhang, J.; Yu, Z.; Zheng, L. Physicochemical Structure of Chitin in the Developing Stages of Black Soldier Fly. Int. J. Biol. Macromol. 2020, 149, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, D.; Sarkar, S. Physicochemical Structure Analysis of Chitin Extracted from Pupa Exuviae and Dead Imago of Wild Black Soldier Fly (Hermetia illucens). J. Polym. Environ. 2020, 28, 445–457. [Google Scholar] [CrossRef]
- Lin, Y.S.; Liang, S.H.; Lai, W.L.; Lee, J.X.; Wang, Y.P.; Liu, Y.T.; Wang, S.H.; Lee, M.H. Sustainable Extraction of Chitin from Spent Pupal Shell of Black Soldier Fly. Processes 2021, 9, 976. [Google Scholar] [CrossRef]
- Lagat, M.K.; Were, S.; Ndwigah, F.; Kemboi, V.J.; Kipkoech, C.; Tanga, C.M. Antimicrobial Activity of Chemically and Biologically Treated Chitosan Prepared from Black Soldier Fly (Hermetia illucens) Pupal Shell Waste. Microorganisms 2021, 9, 2417. [Google Scholar] [CrossRef]
- Rampure, S.M.; Velayudhannair, K.; Marimuthu, N. Characteristics of Chitin Extracted from Different Growth Phases of Black Soldier Fly, Hermetia Illucens, Fed with Different Organic Wastes. Int. J. Trop. Insect Sci. 2023, 43, 979–987. [Google Scholar] [CrossRef]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and Physicochemical Properties of Chitin Polymer from Insect Farm Side Stream as a New Source of Renewable Biopolymer. J. Clean. Prod. 2020, 275, 122924. [Google Scholar] [CrossRef]
- Bhavsar, P.S.; Dalla Fontana, G.; Zoccola, M. Sustainable Superheated Water Hydrolysis of Black Soldier Fly Exuviae for Chitin Extraction and Use of the Obtained Chitosan in the Textile Field. ACS Omega 2021, 6, 8884–8893. [Google Scholar] [CrossRef]
- Machado, S.S.N.; da Silva, J.B.A.; Nascimento, R.Q.; Lemos, P.V.F.; Assis, D.d.J.; Marcelino, H.R.; Ferreira, E.d.S.; Cardoso, L.G.; Pereira, J.D.; Santana, J.S.; et al. Insect Residues as an Alternative and Promising Source for the Extraction of Chitin and Chitosan. Int. J. Biol. Macromol. 2024, 254, 127773. [Google Scholar] [CrossRef] [PubMed]
- Chalghaf, M.; Charradi, K.; Ksouri, R.; Alsulami, Q.A.; Jaouani, A.; Keshk, S.M.A.S.; Hayouni, E.A. Physicochemical Characterization of Chitin Extracted by Different Treatment Sequences from an Edible Insect. Int. J. Biol. Macromol. 2023, 253, 127156. [Google Scholar] [CrossRef]
- Yu, H.; Li, R.; Chen, X.; Yue, Y.; Xing, R.; Liu, S.; Li, P. Effect of Venom from the Jellyfish Nemopilema nomurai on the Silkworm Bombyx mori, L. Toxins 2015, 7, 3876–3886. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- Izadi, H.; Asadi, H.; Bemani, M. Chitin: A Comparison between Its Main Sources. Front. Mater. 2025, 12, 1537067. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Ngasotter, S.; Xavier, K.A.M.; Meitei, M.M.; Waikhom, D.; Madhulika; Pathak, J.; Singh, S.K. Crustacean Shell Waste Derived Chitin and Chitin Nanomaterials for Application in Agriculture, Food, and Health—A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100349. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; El Kady, E.M. Current Trends in Fungal Biosynthesis of Chitin and Chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J. Fungal Cell Wall: Structure, Synthesis, and Assembly; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Islam, N.; Hoque, M.; Taharat, S.F. Recent Advances in Extraction of Chitin and Chitosan. World J. Microbiol. Biotechnol. 2022, 39, 28. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Sams, C.E. Chitin and Chitosan Value-Added Products from Mushroom Waste. J. Agric. Food Chem. 2004, 52, 7905–7910. [Google Scholar] [CrossRef]
- Teng, W.L.; Khor, E.; Tan, T.K.; Lim, L.Y.; Tan, S.C. Concurrent Production of Chitin from Shrimp Shells and Fungi. Carbohydr. Res. 2001, 332, 305–316. [Google Scholar] [CrossRef]
- Araújo, D.; Ferreira, I.C.; Torres, C.A.V.; Neves, L.; Freitas, F. Chitinous Polymers: Extraction from Fungal Sources, Characterization and Processing towards Value-Added Applications. J. Chem. Technol. Biotechnol. 2020, 95, 1277–1289. [Google Scholar] [CrossRef]
- Delezuk, J.A.d.M.; Pavinatto, A.; Campana-Filho, S.P. Influence of the Process Parameters on β-Chitin and α-Chitin Extraction: Probing about the Grinding and Particles Size. Mater. Today Proc. 2019, 14, 722–732. [Google Scholar] [CrossRef]
- Abidin, N.A.Z.; Kormin, F.; Abidin, N.A.Z.; Anuar, N.A.F.M.; Bakar, M.F.A. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Hazmi, A.T.; Ahmad, F.B.; Maziati Akmal, M.H.; Md Ralib, A.A.; Binti Ali, F. Fungal Chitosan for Potential Application in Piezoelectric Energy Harvesting: Review on Experimental Procedure of Chitosan Extraction. Alex. Eng. J. 2023, 67, 105–116. [Google Scholar] [CrossRef]
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus Ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. Waste Biomass Valorization 2021, 12, 6139–6153. [Google Scholar] [CrossRef]
- Suthar, M.; Dufossé, L.; Singh, S.K. The Enigmatic World of Fungal Melanin: A Comprehensive Review. J. Fungi 2023, 9, 891. [Google Scholar] [CrossRef]
- Kjartansson, G.T.; Zivanovic, S.; Kristbergsson, K.; Weiss, J. Sonication-Assisted Extraction of Chitin from North Atlantic Shrimps (Pandalus borealis). J. Agric. Food Chem. 2006, 54, 5894–5902. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; How, N.C.; Chandrakrachang, S.; Stevens, W.F. Effect of Chemical Treatment on the Characteristics of Shrimp Chitosan. J. Met. Mater. Miner. 2022, 12, 11–18. [Google Scholar]
- Hahn, T.; Roth, A.; Ji, R.; Schmitt, E.; Zibek, S. Chitosan Production with Larval Exoskeletons Derived from the Insect Protein Production. J. Biotechnol. 2020, 310, 62–67. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent Insights into the Extraction, Characterization, and Bioactivities of Chitin and Chitosan from Insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Honarkar, H.; Barikani, M. Applications of Biopolymers I: Chitosan. Monatsh Chem. 2009, 140, 1403–1420. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in Vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Delezuk, J.A.d.M.; Cardoso, M.B.; Domard, A.; Campana-Filho, S.P. Ultrasound-Assisted Deacetylation of Beta-Chitin: Influence of Processing Parameters. Polym. Int. 2011, 60, 903–909. [Google Scholar] [CrossRef]
- Rege, P.R.; Block, L.H. Chitosan Processing: Influence of Process Parameters during Acidic and Alkaline Hydrolysis and Effect of the Processing Sequence on the Resultant Chitosan’s Properties. Carbohydr. Res. 1999, 321, 235–245. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burked, I.T. Alkaline Residues and the Environment: A Review of Impacts, Management Practices and Opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Zouch, H.; Cabrol, L.; Chifflet, S.; Tedetti, M.; Karray, F.; Zaghden, H.; Sayadi, S.; Quéméneur, M. Effect of Acidic Industrial Effluent Release on Microbial Diversity and Trace Metal Dynamics During Resuspension of Coastal Sediment. Front. Microbiol. 2018, 9, 410248. [Google Scholar] [CrossRef]
- Shkuratov, A.S.; Panackal Shibu, R.; Therasme, O.; Berton, P.; Shamshina, J.L. Sustainable Production of Chitin Nanowhiskers from Crustacean Biomass Using Cost-Effective Ionic Liquids: Strategies to Avoid Byproduct Formation. Sustain. Chem. 2024, 5, 130–148. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and Eco-Friendly Approaches for the Extraction of Chitin and Chitosan: A Review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef]
- Anastas, P.T. Green Chemistry and the Role of Analytical Methodology Development. Crit. Rev. Anal. Chem. 1999, 29, 167–175. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 30. [Google Scholar]
- Le, T.M.; Tran, C.L.; Nguyen, T.X.; Duong, Y.H.P.; Le, P.K.; Tran, V.T. Green Preparation of Chitin and Nanochitin from Black Soldier Fly for Production of Biodegradable Packaging Material. J. Polym. Env. 2023, 31, 3094–3105. [Google Scholar] [CrossRef]
- Psarianos, M.; Ojha, S.; Schneider, R.; Schlüter, O.K. Chitin Isolation and Chitosan Production from House Crickets (Acheta domesticus) by Environmentally Friendly Methods. Molecules 2022, 27, 5005. [Google Scholar] [CrossRef]
- Echeverria, D.; Venditti, R.; Jameel, H.; Yao, Y. A General Life Cycle Assessment Framework for Sustainable Bleaching: A Case Study of Peracetic Acid Bleaching of Wood Pulp. J. Clean. Prod. 2021, 290, 125854. [Google Scholar] [CrossRef]
- Xing, Y.; Aweya, J.J.; Jin, R.; Lin, R.; Weng, W.; Zhang, Y.; Deng, S.; Yang, S. Low-Intensity Ultrasound Combines Synergistically with Lacticaseibacillus Paracasei Fermentation to Enhance Chitin Extraction from Crab Shells. Lebensm. Wiss. Technol. 2023, 179, 114651. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of Black Soldier Fly Prepupae and Systematic Approaches for Extraction and Fractionation of Proteins, Lipids and Chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Yan, T.; Wang, X.; Huang, J.; Kuang, Z.; Ye, M.; Pan, M. Selectivity of Deproteinization and Demineralization Using Natural Deep Eutectic Solvents for Production of Insect Chitin (Hermetia illucens). Carbohydr. Polym. 2019, 225, 115255. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 481174. [Google Scholar] [CrossRef]
- Maled, S.B.; Bhat, A.R.; Hegde, S.; Sivamani, Y.; Muthuraman, A.; Elayaperumal, S. Enzyme-Assisted Extraction. In Bioactive Extraction and Application in Food and Nutraceutical Industries. Methods and Protocols in Food Science; Sarkar, T., Pati, S., Eds.; Humana: New York, NY, USA, 2024; pp. 173–200. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin Extraction from Shrimp Shell Using Enzymatic Treatment. Antitumor, Antioxidant and Antimicrobial Activities of Chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Paes da Silva, F.K.; Brück, D.W.; Brück, W.M. Isolation of Proteolytic Bacteria from Mealworm (Tenebrio molitor) Exoskeletons to Produce Chitinous Material. FEMS Microbiol. Lett. 2017, 364, 177. [Google Scholar] [CrossRef]
- Wu, S. Preparation of Chitosan from Clanis Bilineata Larvae Skin Using Enzymatic Methods. Carbohydr. Polym. 2011, 83, 1008–1010. [Google Scholar] [CrossRef]
- Rakshit, S.; Pal, K.; Mondal, S.; Jana, A.; Mondal, K.C.; Halder, S.K. Extraction of Chitosan from Biologically-Derived Chitin by Bacterial Chitin Deacetylase: Process Optimization and Product Quality Assessment. Int. J. Biol. Macromol. 2023, 244, 125389. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds; Chemat, F., Ed.; Springer: Boston, MA, USA, 2012; pp. 15–52. ISBN 978-1-4614-4830-3. [Google Scholar]
- Li, Z.; Li, M.C.; Liu, C.; Liu, X.; Lu, Y.; Zhou, G.; Liu, C.; Mei, C. Microwave-Assisted Deep Eutectic Solvent Extraction of Chitin from Crayfish Shell Wastes for 3D Printable Inks. Ind. Crops Prod. 2023, 194, 116325. [Google Scholar] [CrossRef]
- Dong, W.; Tang, J.; Cropotova, J.; Sun, D.W.; Tiwari, B.K. Green Technologies for Bio-Refinery in Marine Crustacean Shell Valorisation from Chitin Perspective. Trends Food Sci. Technol. 2024, 150, 104580. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Safavizadeh, V.; Yousefi, M.; Hosseini, S.M. A Short Review of Supercritical Fluid Extraction of Plant Extracts. J. Food Meas. Charact. 2024, 18, 3651–3664. [Google Scholar] [CrossRef]
- Cunico, L.P.; Turner, C. Supercritical Fluids and Gas-Expanded Liquids. In The Application of Green Solvents in Separation Processes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–214. ISBN 9780128054437. [Google Scholar]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Mäkinen, O.E.; Rommi, K.; Heiniö, R.L.; Holopainen-Mantila, U.; Hokkanen, S.; Hakala, T.K.; Nordlund, E. Biochemical and Sensory Characteristics of the Cricket and Mealworm Fractions from Supercritical Carbon Dioxide Extraction and Air Classification. Eur. Food Res. Technol. 2018, 244, 19–29. [Google Scholar] [CrossRef]
- Vian, M.; Breil, C.; Vernes, L.; Chaabani, E.; Chemat, F. Green Solvents for Sample Preparation in Analytical Chemistry. Curr. Opin. Green. Sustain. Chem. 2017, 5, 44–48. [Google Scholar] [CrossRef]
- Smith, R.M. Superheated Water: The Ultimate Green Solvent for Separation Science. Anal. Bioanal. Chem. 2006, 385, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical Water as Reaction Environment: Fundamentals of Hydrothermal Biomass Transformation. ChemSusChem 2011, 4, 566–579. [Google Scholar] [CrossRef]
- Okoro, O.V.; Preat, V.; Karimi, K.; Nie, L.; Debaste, F.; Shavandi, A. Optimizing the Subcritical Water Valorization of Insect (Hermetia illucens L.) Farming Waste for Biodiesel Production. Chem. Eng. Res. Des. 2023, 196, 413–426. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Revisiting Greenness of Ionic Liquids and Deep Eutectic Solvents. Green. Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Amde, M.; Liu, J.F.; Pang, L. Environmental Application, Fate, Effects, and Concerns of Ionic Liquids: A Review. Environ. Sci. Technol. 2015, 49, 12611–12627. [Google Scholar] [CrossRef]
- Sulthan, R.; Reghunadhan, A.; Sambhudevan, S. A New Era of Chitin Synthesis and Dissolution Using Deep Eutectic Solvents- Comparison with Ionic Liquids. J. Mol. Liq. 2023, 380, 121794. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-Chitin in Deep Eutectic Solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Hong, S.; Lian, H.; Mei, C.; Lee, J.; Wu, Q.; Hubbe, M.A.; Li, M.C. Recent Advances in Extraction and Processing of Chitin Using Deep Eutectic Solvents. Chem. Eng. J. 2022, 446, 136953. [Google Scholar] [CrossRef]
- Costa, S.P.F.; Azevedo, A.M.O.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S. Environmental Impact of Ionic Liquids: Recent Advances in (Eco)Toxicology and (Bio)Degradability. ChemSusChem 2017, 10, 2321–2347. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Clark, K.D. Ionic Liquids as Tunable Materials in (Bio)Analytical Chemistry. Anal. Bioanal. Chem. 2018, 410, 4565–4566. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, R.; Zhu, Y.; Yang, P.; Lin, Z.; Wang, Z.; Cong, W. Effectively Inhibiting the Degradation of Chitin during Extraction from Crustacean Waste via a Novel Deep Eutectic Solvent Aqueous Solution. Process Biochem. 2022, 121, 142–151. [Google Scholar] [CrossRef]
- Li, K.; Fang, H.; Duan, X.; Deng, D. Efficient Uptake of NH3 by Dual Active Sites NH4SCN-Imidazole Deep Eutectic Solvents with Low Viscosity. J. Mol. Liq. 2021, 339, 116724. [Google Scholar] [CrossRef]
- Wineinger, H.B.; Kelly, A.; Shamshina, J.L.; Rogers, R.D. Farmed Jumbo Shrimp Molts: An Ionic Liquid Strategy to Increase Chitin Yield per Animal While Controlling Molecular Weight. Green. Chem. 2020, 22, 6001–6007. [Google Scholar] [CrossRef]
- Dong, Q.; Qiu, W.; Li, L.; Tao, N.; Liang Wang, A.; Deng, S.; Jin, Y. Extraction of Chitin from White Shrimp (Penaeus vannamei) Shells Using Binary Ionic Liquid Mixtures. J. Ind. Eng. Chem. 2023, 120, 529–541. [Google Scholar] [CrossRef]
- Feng, M.; Lu, X.; Zhang, J.; Li, Y.; Shi, C.; Lu, L.; Zhang, S. Direct Conversion of Shrimp Shells to O -Acylated Chitin with Antibacterial and Anti-Tumor Effects by Natural Deep Eutectic Solvents. Green. Chem. 2019, 21, 87–98. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile Acid Base Sustainable Solvent for Fast Extraction of Various Molecular Weight Chitin from Lobster Shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-Pot Production of Chitin with High Purity from Lobster Shells Using Choline Chloride–Malonic Acid Deep Eutectic Solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, Y.; Luo, J.; Mao, X.; Huang, W.C. Efficient Room-Temperature Chitin Extraction Using a Novel Ternary Deep Eutectic Solvent with Improved Molecular Mobility and Enhanced Recyclability. ACS Sustain. Chem. Eng. 2024, 12, 751–759. [Google Scholar] [CrossRef]
- Vicente, F.A.; Huš, M.; Likozar, B.; Novak, U. Chitin Deacetylation Using Deep Eutectic Solvents: Ab Initio-Supported Process Optimization. ACS Sustain. Chem. Eng. 2021, 9, 3874–3886. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Yu, J.; Wei, Q.; Ren, X. Study on the Deacetylation and Mechanism of Chitin in Natural Deep Eutectic Solvent. Int. J. Biol. Macromol. 2024, 255, 127698. [Google Scholar] [CrossRef]
- Mccarthy, J. What Is Artificial Intelligence. 2007. Available online: https://www-formal.stanford.edu/jmc/whatisai.pdf (accessed on 2 April 2025).
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef]
- Malashin, I.; Martysyuk, D.; Tynchenko, V.; Gantimurov, A.; Semikolenov, A.; Nelyub, V.; Borodulin, A. Machine Learning-Based Process Optimization in Biopolymer Manufacturing: A Review. Polymers 2024, 16, 3368. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, F.; Xu, G.; Liu, X.; Zhang, Y.; Sun, J.; Yao, B.; Huang, H.; Wu, N.; Tian, J. A Novel Thermophilic Chitinase Directly Mined from the Marine Metagenome Using the Deep Learning Tool Preoptem. Bioresour. Bioprocess. 2022, 9, 54. [Google Scholar] [CrossRef]
- Capaldi, G.; Binello, A.; Aimone, C.; Mantegna, S.; Grillo, G.; Cravotto, G. New Trends in Extraction-Process Intensification: Hybrid and Sequential Green Technologies. Ind. Crops Prod. 2024, 209, 117906. [Google Scholar] [CrossRef]
- Fomo, G.; Madzimbamuto, T.N.; Ojumu, T.V. Applications of Nonconventional Green Extraction Technologies in Process Industries: Challenges, Limitations and Perspectives. Sustainability 2020, 12, 5244. [Google Scholar] [CrossRef]
- Tan, Y.N.; Chin, Y.L.; Chen, W.N. Comparison of Sustainable Lipid and Protein Removal Methods for the Isolation of Insect Chitin from Black Soldier Fly Exoskeleton. ACS Food Sci. Technol. 2021, 1, 698–706. [Google Scholar] [CrossRef]
- Fernández-Ronco, M.P.; De Lucas, A.; Rodríguez, J.F.; García, M.T.; Gracia, I. New Considerations in the Economic Evaluation of Supercritical Processes: Separation of Bioactive Compounds from Multicomponent Mixtures. J. Supercrit. Fluids 2013, 79, 345–355. [Google Scholar] [CrossRef]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food Waste Valorisation and Circular Economy Concepts in Insect Production and Processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Kiehbadroudinezhad, M.; Hosseinzadeh-Bandbafha, H.; Varjani, S.; Wang, Y.; Peng, W.; Pan, J.; Aghbashlo, M.; Tabatabaei, M. Marine Shell-Based Biorefinery: A Sustainable Solution for Aquaculture Waste Valorization. Renew. Energy 2023, 206, 623–634. [Google Scholar] [CrossRef]
- Vicente, F.A.; Hren, R.; Novak, U.; Čuček, L.; Likozar, B.; Vujanović, A. Energy Demand Distribution and Environmental Impact Assessment of Chitosan Production from Shrimp Shells. Renew. Sustain. Energy Rev. 2024, 192, 114204. [Google Scholar] [CrossRef]
- Bashiri, B.; Bashiri, B.; Tappi, A.C.D.A.S.; Rocculi, S.; Kaleda, P.; And Vilu, A.; De Aguiar, A.C.; Pinheiro, S.; Tappi, S.; Rocculi, P.; et al. Life Cycle Assessment of Laboratory-Scale Chitosan Production: Comparison of High-Pressure Processing-Assisted and Conventional Methods. Proc. Est. Acad. Sci. 2025, 74, 1–14. [Google Scholar] [CrossRef]
- Olatunji, O. Chitin. In Aquatic Biopolymers; Springer: Cham, Switzerland, 2020; pp. 31–65. ISBN 978-3-030-34709-3. [Google Scholar]
- Moreira, D.; Pires, J.C.M. Atmospheric CO2 Capture by Algae: Negative Carbon Dioxide Emission Path. Bioresour. Technol. 2016, 215, 371–379. [Google Scholar] [CrossRef]
- European Commission. A New Circular Economy Action Plan: For a Cleaner and More Competitive Europe (COM/2020/98 Final). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52020DC0098 (accessed on 10 April 2025).
- European Commission. Product Environmental Footprint (PEF) Methodology. Available online: https://eplca.jrc.ec.europa.eu/EnvironmentalFootprint.html (accessed on 10 April 2025).
- European Parliament and Council Regulation (EU). 2020/852 of the European Parliament and of the Council on the Establishment of a Framework to Facilitate Sustainable Investment. Available online: https://eur-lex.europa.eu/eli/reg/2020/852/oj/eng (accessed on 10 April 2025).
- Scaffardi, L.; Formici, G. Novel Foods and Edible Insects in the European Union: An Interdisciplinary Analysis; Springer International Publishing: Cham, Switzerland, 2022; ISBN 9783031134944. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mersmann, L.; Souza, V.G.L.; Fernando, A.L. Green Processes for Chitin and Chitosan Production from Insects: Current State, Challenges, and Opportunities. Polymers 2025, 17, 1185. https://doi.org/10.3390/polym17091185
Mersmann L, Souza VGL, Fernando AL. Green Processes for Chitin and Chitosan Production from Insects: Current State, Challenges, and Opportunities. Polymers. 2025; 17(9):1185. https://doi.org/10.3390/polym17091185
Chicago/Turabian StyleMersmann, Lisa, Victor Gomes Lauriano Souza, and Ana Luísa Fernando. 2025. "Green Processes for Chitin and Chitosan Production from Insects: Current State, Challenges, and Opportunities" Polymers 17, no. 9: 1185. https://doi.org/10.3390/polym17091185
APA StyleMersmann, L., Souza, V. G. L., & Fernando, A. L. (2025). Green Processes for Chitin and Chitosan Production from Insects: Current State, Challenges, and Opportunities. Polymers, 17(9), 1185. https://doi.org/10.3390/polym17091185









