Design, Characterization, and Release Kinetics of a Hybrid Hydrogel Drug Delivery System for Sustained Hormone Therapy
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
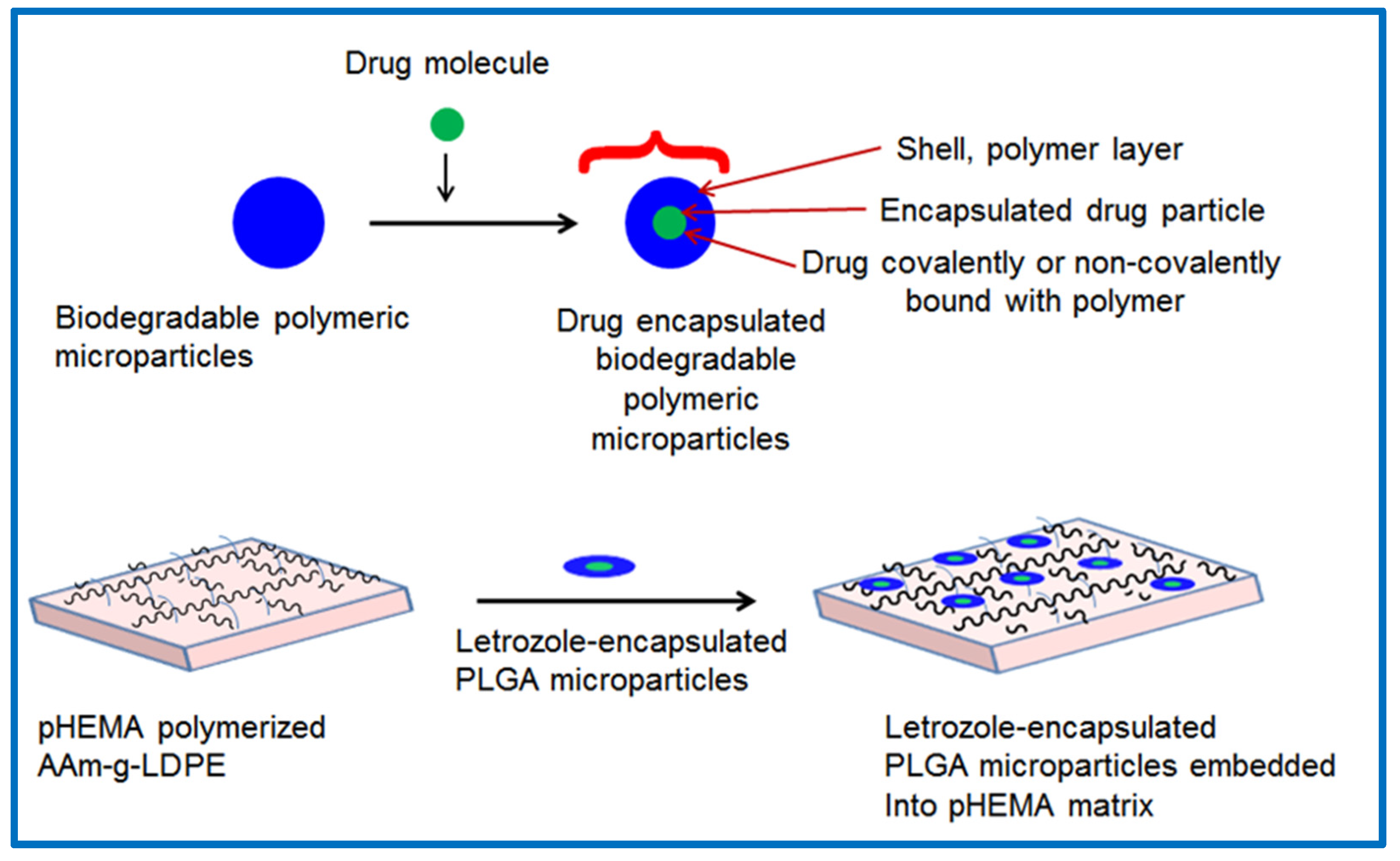
2.2. Preparation of Letrozole-Encapsulated PLGA-pHEMA/AAm–g–LDPE Drug Release System
3. Results and Discussion
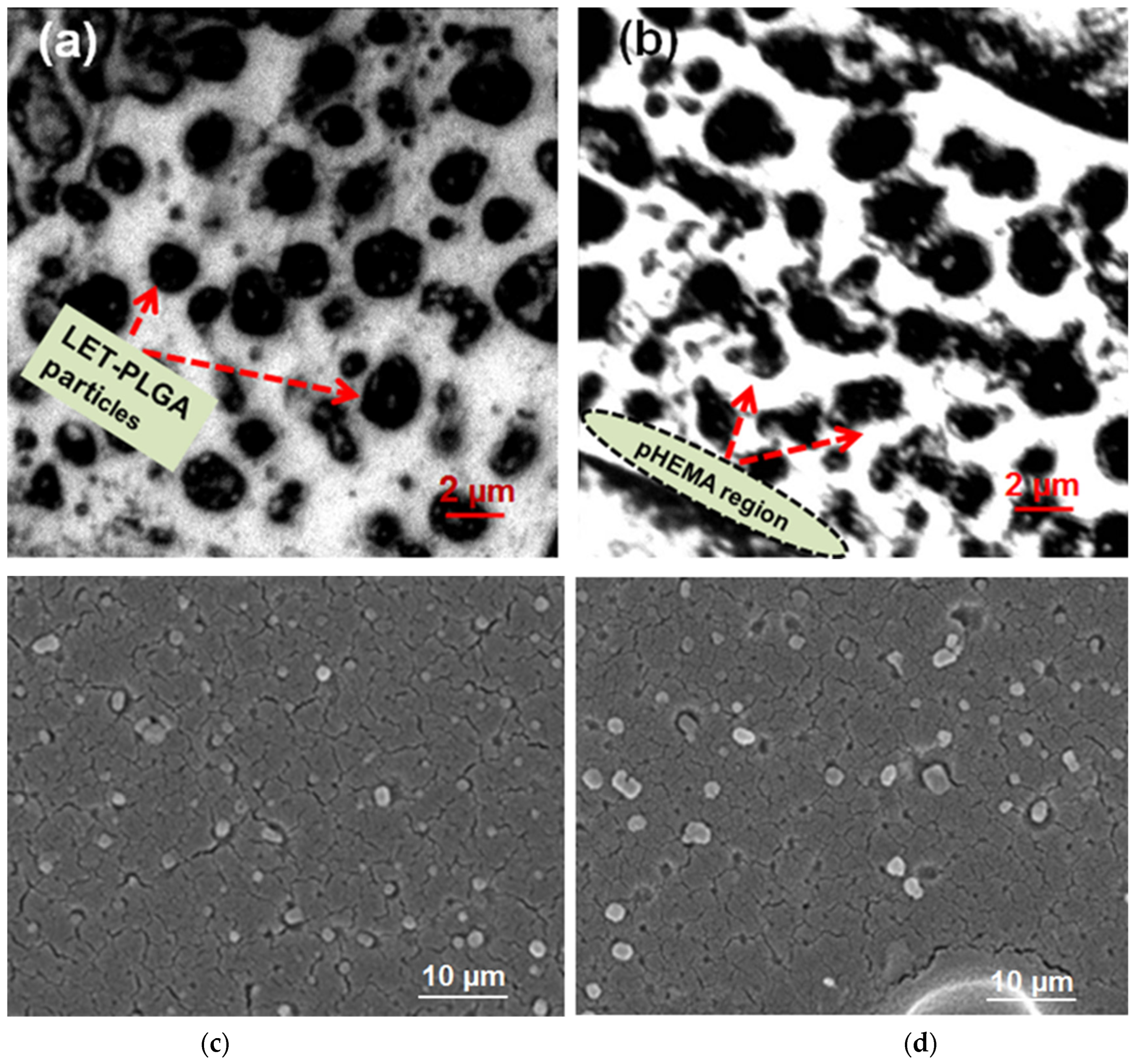
3.1. Analysis of Particle Distribution Within the pHEMA Matrix of the Prepared Release System
3.2. In-Vitro Release Study
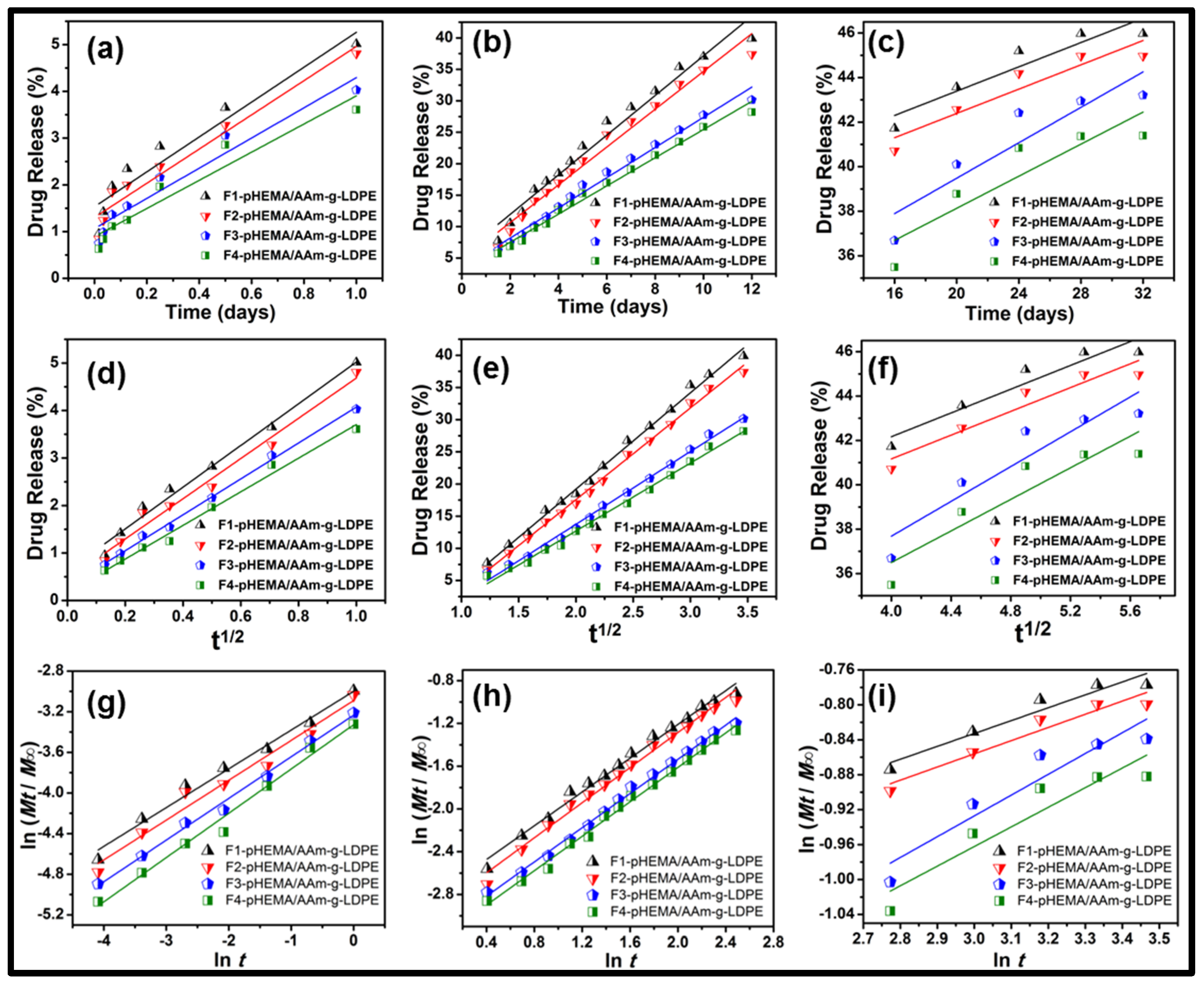
3.3. Release Kinetics Study
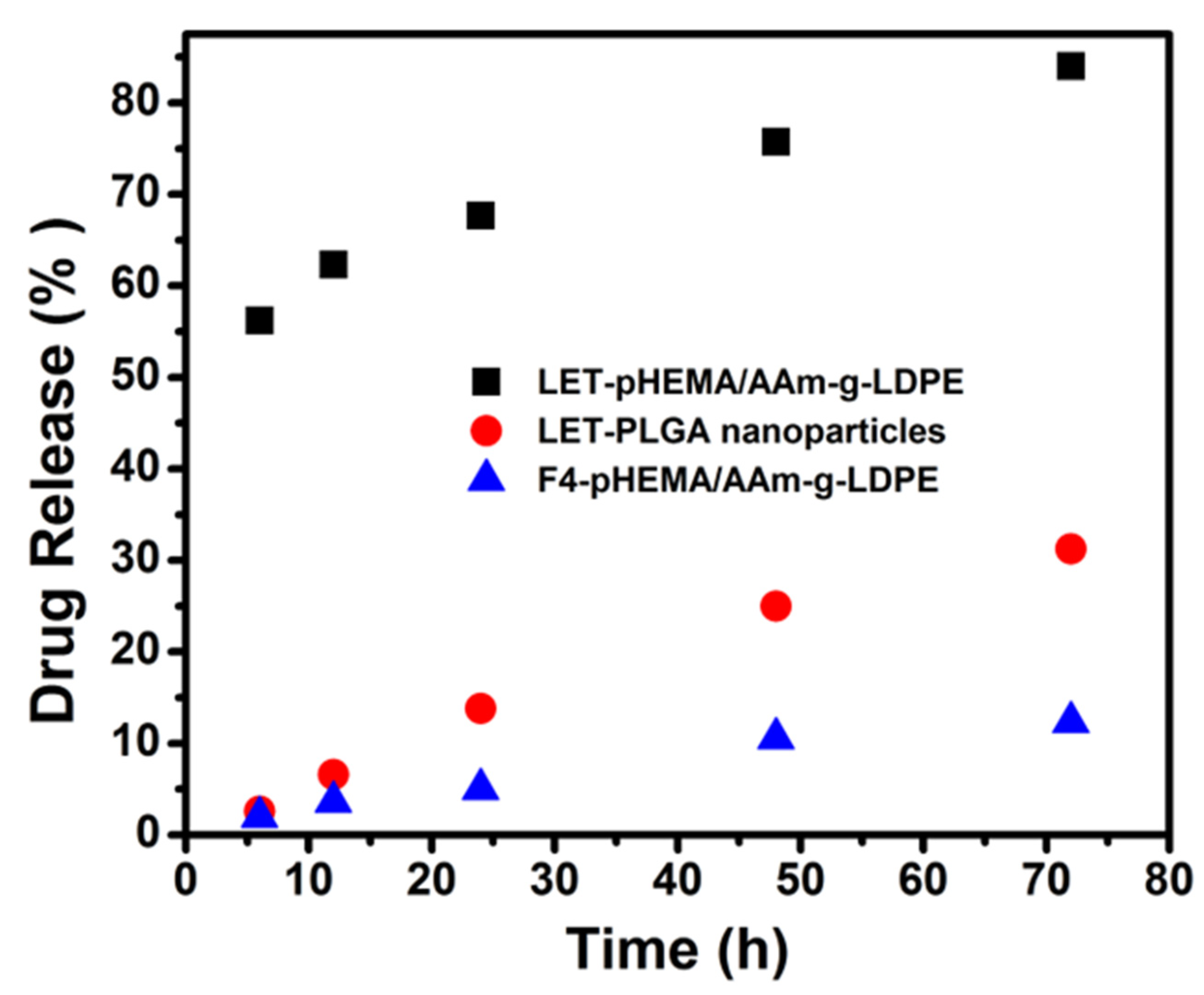
3.4. Comparative Studies on In Vitro Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, G.M. Controlled release oral dosage forms: Some recent advances in matrix type drug delivery systems. J. Med. Sci. 2001, 1, 350–354. [Google Scholar] [CrossRef]
- Reddy, K.R. Controlled-release, pegylation, liposomal formulations: New mechanisms in the delivery of injectable drugs. Ann. Pharmacother. 2000, 34, 915–923. [Google Scholar]
- Patel, D.K.; Jung, E.; Priya, S.; Won, S.Y.; Han, S.S. Recent advances in biopolymer-based hydrogels and their potential biomedical applications. Carbohydr. Polym. 2024, 323, 121408. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Park, K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006, 58, 387–401. [Google Scholar] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Panchagnula, R. Design of controlled release delivery systems using a modified pharmacokinetic approach: A case study for drugs having a short elimination half-life and a narrow therapeutic index. Int. J. Pharm. 2003, 261, 27–41. [Google Scholar] [CrossRef]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S.; et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008, 26, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Polikarpov, N.; Appelhans, D.; Welzel, P.; Kaufmann, A.; Dhanapal, P.; Bellmann, C.; Voit, B. Tailoring uptake and release of ATP by dendritic glycopolymer/PNIPAAm hydrogel hybrids: First approaches towards multicompartment release systems. New J. Chem. 2012, 36, 438–451. [Google Scholar]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar]
- Jiang, G.; Qiu, W.; DeLuca, P.P. Preparation and in vitro/in vivo evaluation of insulin-loaded poly(acryloyl-hydroxyethyl starch)-PLGA composite microspheres. Pharm. Res. 2003, 20, 452–459. [Google Scholar]
- Cheng, S.; Yang, J.; Song, J.; Cao, X.; Zhou, B.; Yang, L.; Li, C.; Wang, Y. A motion-responsive injectable lubricative hydrogel for efficient Achilles tendon adhesion prevention. Mater. Today Bio 2025, 30, 101458. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, U.; Sura, R.; Papadimitrakopoulos, F.; Burgess, D.J. PLGA/PVA hydrogel composites for long-term inflammation control following sc implantation. Int. J. Pharm. 2010, 384, 78–86. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. Accelerated in vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings. Int. J. Pharm. 2012, 422, 341–348. [Google Scholar] [CrossRef]
- Liu, X.; Nakamura, K.; Lowman, A.M. Composite hydrogels for sustained release of therapeutic agents. Soft Mater. 2003, 1, 393–408. [Google Scholar] [CrossRef]
- Hu, Y.; Hollinger, J.O.; Marra, K.G. Controlled release from coated polymer microparticles embedded in tissue-engineered scaffolds. J. Drug Target. 2001, 9, 431–438. [Google Scholar] [CrossRef]
- Meese, T.M.; Hu, Y.; Nowak, R.W.; Marra, K.G. Surface studies of coated polymer microspheres and protein release from tissue-engineered scaffolds. J. Biomater. Sci. Polym. Ed. 2002, 13, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Mathiowitz, E.; Langer, R.S.; Warshawsky, A.; Edelman, E. Polymer Composite for Controlled Release or Membrane Formation. U.S. Patent 4,898,734, 16 February 1990. [Google Scholar]
- Brannon-Peppas, L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int. J. Pharm. 1995, 116, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.M.; Chen, P.P.; Cheng, L.; Zhou, W.; Tang, W.X.; Chen, Z.W.; Ke, C.M. Controlled release of insulin from PLGA nanoparticles embedded within PVA hydrogels. J. Mater. Sci. Mater. Med. 2007, 18, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doostmohammadi, M.; Ameri, A.; Mohammadinejad, R.; Dehghannoudeh, N.; Banat, I.M.; Ohadi, M.; Dehghannoudeh, G. Hydrogels for Peptide Hormones Delivery: Therapeutic and Tissue Engineering Applications. Drug Des. Dev. Ther. 2019, 13, 3405–3418. [Google Scholar] [CrossRef]
- Mehraji, S.; Saadatmand, M.; Eskandari, M. Production of letrozole-loaded alginate oxide-gelatin microgels using microfluidic systems for drug delivery applications. Int. J. Biol. Macromol. 2024, 263 Pt 1, 129685. [Google Scholar] [CrossRef]
- Siddiqa, A.J.; Shrivastava, N.K.; Mohsin, M.E.A.; Abidi, M.H.; Sharaf, M.A.F.; Shaikh, T.A. In Vitro Release and Degradation Study of Letrozole-Loaded Poly(Lactic-co-Glycolic Acid) Microparticles. JOM 2021, 73, 450–459. [Google Scholar] [CrossRef]
- Knebel, W.; Quader, H.; Wijnaendts-van-Resandt, R.; Engelhardt, H. Application of confocal laser scanning microscopy in plant biology. Ber. Bunsenges. Phys. Chem. 1989, 93, 380–386. [Google Scholar] [CrossRef]
- Wu, Y.; Yue, X.; Zhang, Y.; Yu, N.; Ge, C.; Liu, R.; Duan, Z.; Gao, L.; Zang, X.; Sun, X.; et al. Dual-sided centripetal microgrooved poly (D,L-lactide-co-caprolactone) disk encased in immune-regulating hydrogels for enhanced bone regeneration. Mater. Today Bio 2025, 30, 101436. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Galeska, I.; Kim, T.K.; Patil, S.D.; Bhardwaj, U.; Chattopadhyay, D.; Papadimitrakopoulos, F.; Burgess, D.J. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005, 7, E231–E240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, P.; Guo, Y.; Wang, W.; Fan, G.; Teng, D. Selective Enrichment vs Dynamic Recovery of Ga(III) Using Functionalized Hydrogels: A Comparative Study of Polypropylene Hydroxamic Acid and Polyacrylic Acid Hydrogels. ACS Sustain. Chem. Eng. 2025, 13, 2141–2153. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]



| Formulation | Zero Order | Higuchi | Korsmeyer−Peppas | ||||
|---|---|---|---|---|---|---|---|
| R2 | K0 | R2 | KH | R2 | Km | n | |
| (a) | |||||||
| F1 | 0.908 | 1.532 ± 0.045 | 0.986 | 0.626 ± 0.032 | 0.983 | −2.299 ± 0.058 | 0.38 ± 0.02 |
| F2 | 0.940 | 1.303 ± 0.038 | 0.978 | 0.448 ± 0.025 | 0.971 | −3.089 ± 0.063 | 0.39 ± 0.02 |
| F3 | 0.931 | 1.065 ± 0.041 | 0.996 | 0.291 ± 0.027 | 0.993 | −3.233 ± 0.059 | 0.41 ± 0.03 |
| F4 | 0.916 | 0.892 ± 0.036 | 0.987 | 0.165 ± 0.019 | 0.985 | −3.327 ± 0.061 | 0.43 ± 0.03 |
| (b) | |||||||
| F1 | 0.972 | 5.641 ± 0.122 | 0.995 | −10.736 ± 0.287 | 0.989 | −2.789 ± 0.089 | 0.78 ± 0.04 |
| F2 | 0.976 | 4.633 ± 0.118 | 0.996 | −10.935 ± 0.294 | 0.989 | −2.926 ± 0.091 | 0.82 ± 0.04 |
| F3 | 0.986 | 3.381 ± 0.102 | 0.994 | −8.907 ± 0.276 | 0.996 | −3.125 ± 0.087 | 0.80 ± 0.03 |
| F4 | 0.987 | 3.033 ± 0.098 | 0.993 | −8.405 ± 0.264 | 0.994 | −3.221 ± 0.085 | 0.80 ± 0.03 |
| (c) | |||||||
| F1 | 0.849 | 37.938 ± 1.245 | 0.888 | 31.457 ± 1.137 | 0.916 | −1.275 ± 0.057 | 0.14 ± 0.02 |
| F2 | 0.849 | 36.938 ± 1.221 | 0.888 | 30.457 ± 1.112 | 0.916 | −1.308 ± 0.059 | 0.15 ± 0.02 |
| F3 | 0.786 | 31.532 ± 1.089 | 0.833 | 21.998 ± 0.987 | 0.863 | −1.641 ± 0.062 | 0.23 ± 0.03 |
| F4 | 0.752 | 30.935 ± 1.065 | 0.803 | 22.269 ± 0.954 | 0.837 | −1.636 ± 0.061 | 0.22 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsin, M.E.A.; Siddiqa, A.J.; Mousa, S.; Shrivastava, N.K. Design, Characterization, and Release Kinetics of a Hybrid Hydrogel Drug Delivery System for Sustained Hormone Therapy. Polymers 2025, 17, 999. https://doi.org/10.3390/polym17080999
Mohsin MEA, Siddiqa AJ, Mousa S, Shrivastava NK. Design, Characterization, and Release Kinetics of a Hybrid Hydrogel Drug Delivery System for Sustained Hormone Therapy. Polymers. 2025; 17(8):999. https://doi.org/10.3390/polym17080999
Chicago/Turabian StyleMohsin, Mohammed E. Ali, Akhtar Jahan Siddiqa, Suleiman Mousa, and Nilesh Kumar Shrivastava. 2025. "Design, Characterization, and Release Kinetics of a Hybrid Hydrogel Drug Delivery System for Sustained Hormone Therapy" Polymers 17, no. 8: 999. https://doi.org/10.3390/polym17080999
APA StyleMohsin, M. E. A., Siddiqa, A. J., Mousa, S., & Shrivastava, N. K. (2025). Design, Characterization, and Release Kinetics of a Hybrid Hydrogel Drug Delivery System for Sustained Hormone Therapy. Polymers, 17(8), 999. https://doi.org/10.3390/polym17080999






