Polyvinylidene Fluoride (PVDF) and Nanoclay Composites’ Mixed-Matrix Membranes: Exploring Structure, Properties, and Performance Relationships
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
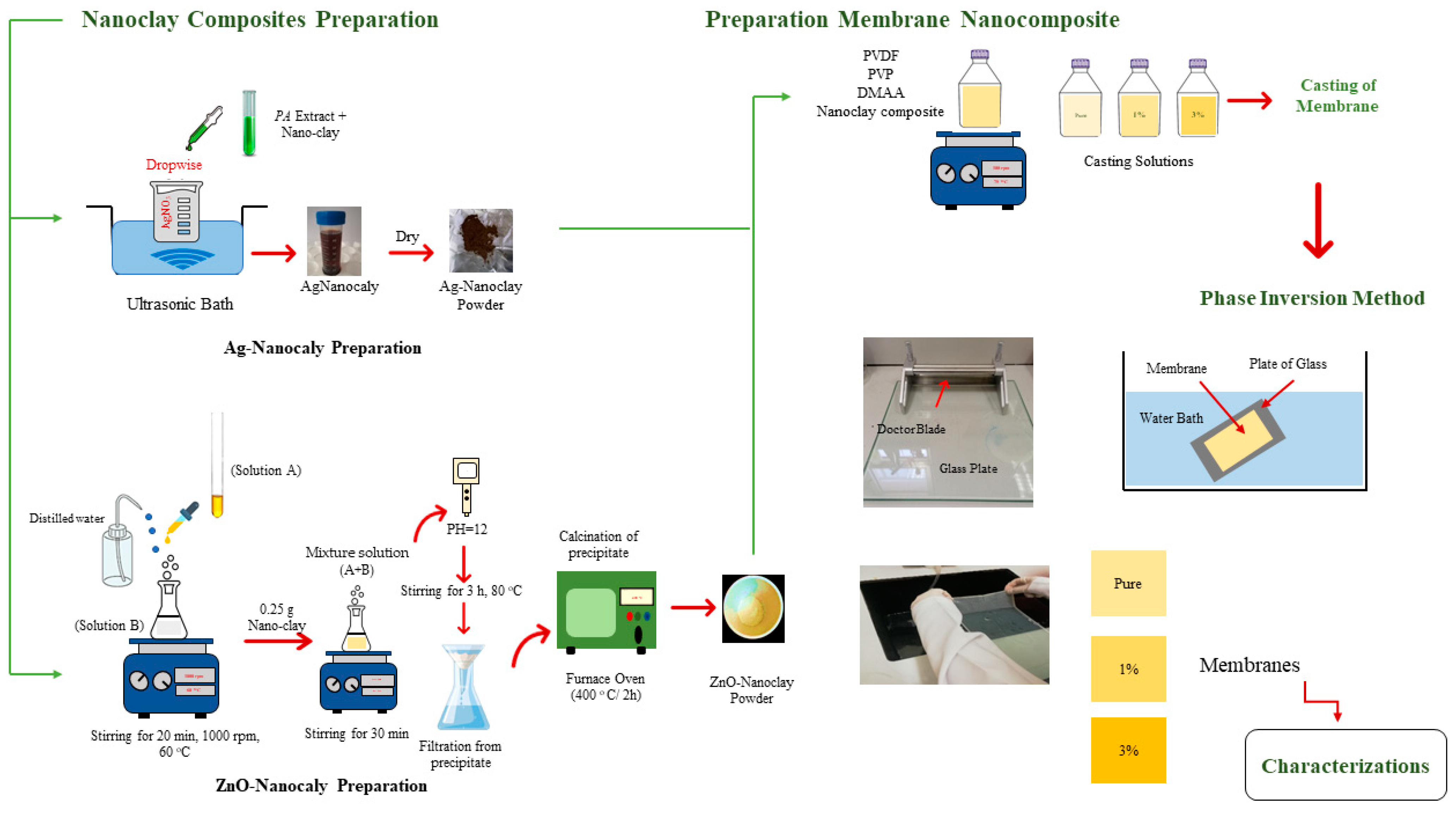
2.2.1. Membrane Preparation
2.2.2. Characterization of Nanoclay Composites
2.2.3. Characterization of PVDF Membranes
Structure and Surface Properties
Mechanical Properties
Antibacterial Properties
Barrier Properties
3. Results
3.1. Nanoclay Composite Characterization
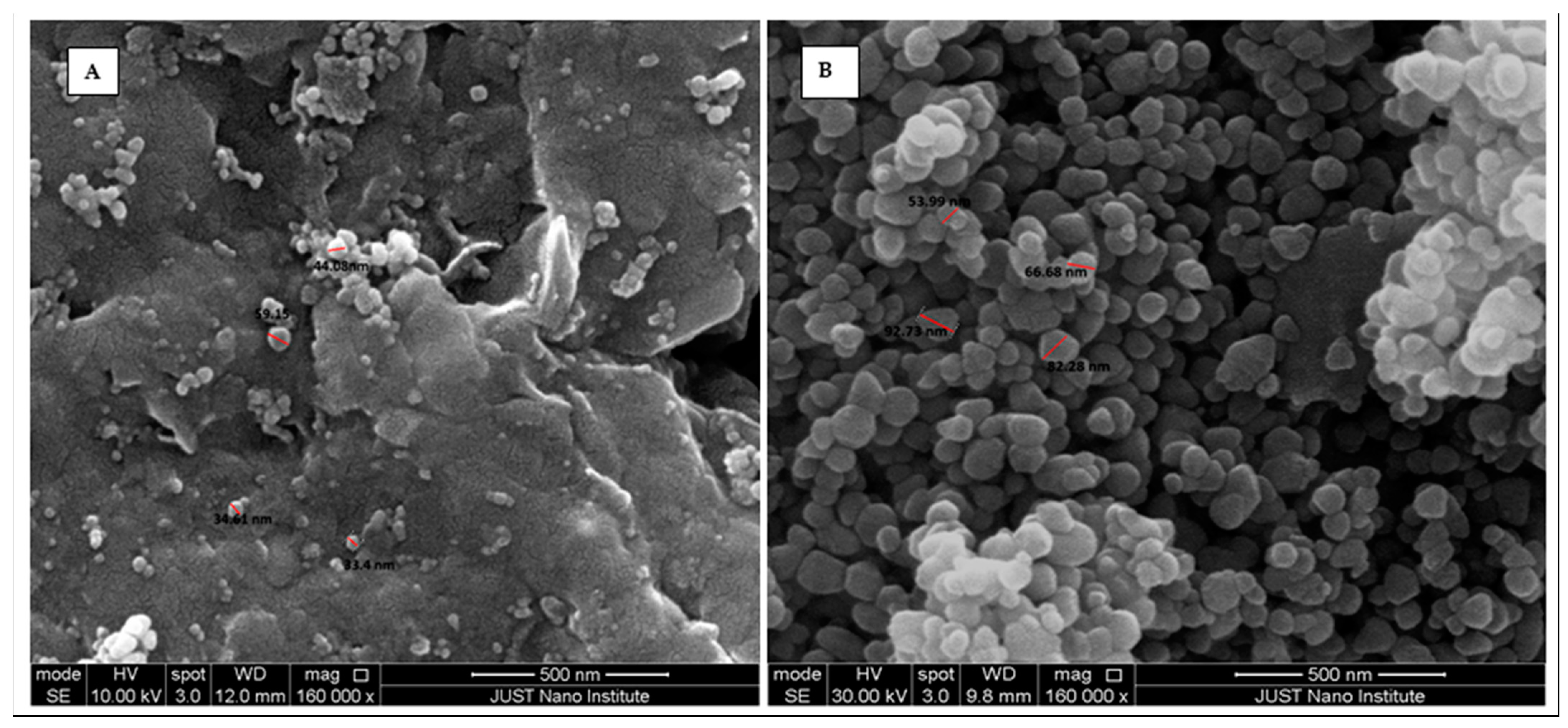
3.1.1. SEM
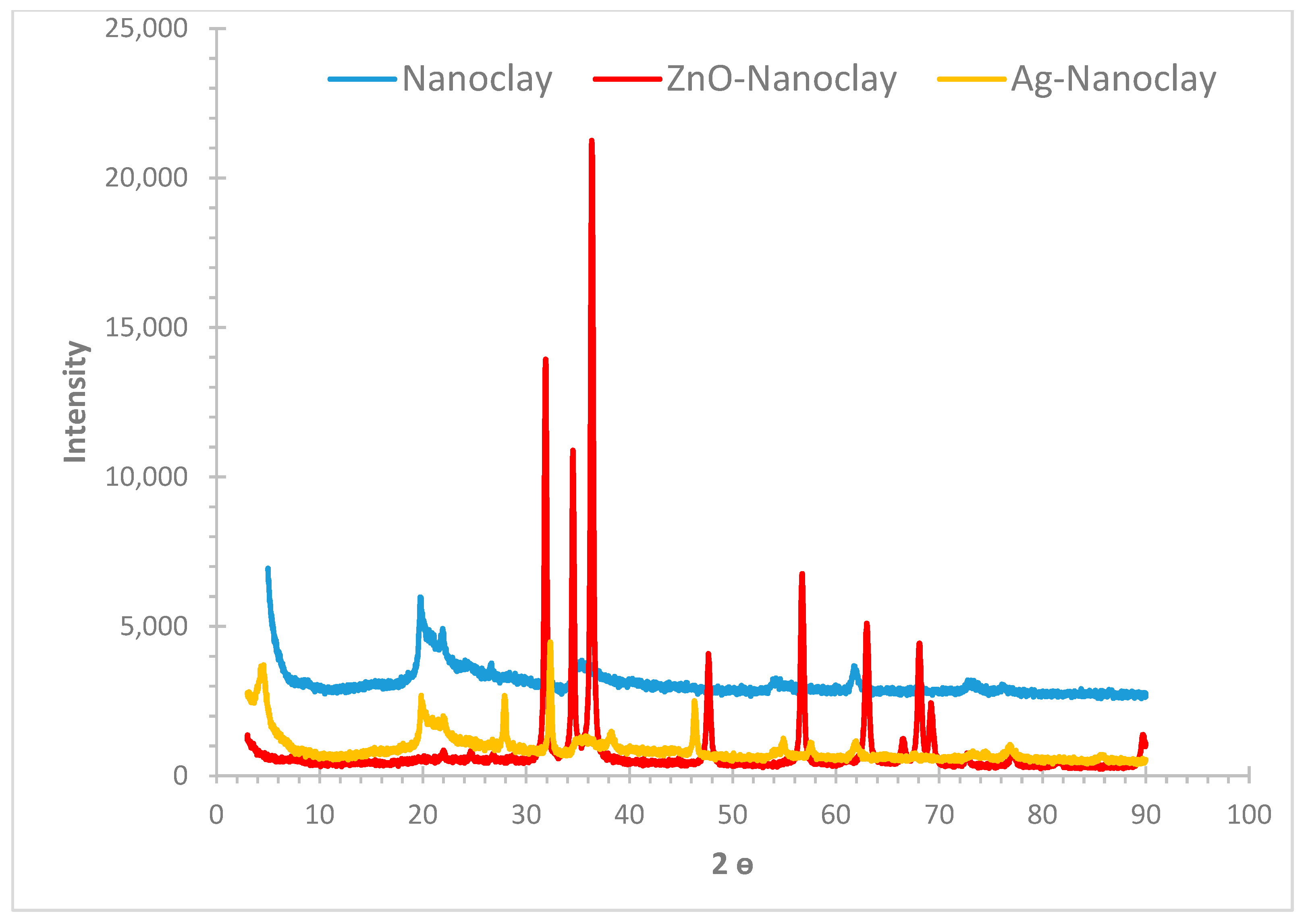
3.1.2. XRD
3.2. Membrane Characterization
3.2.1. Structure and Surface Properties
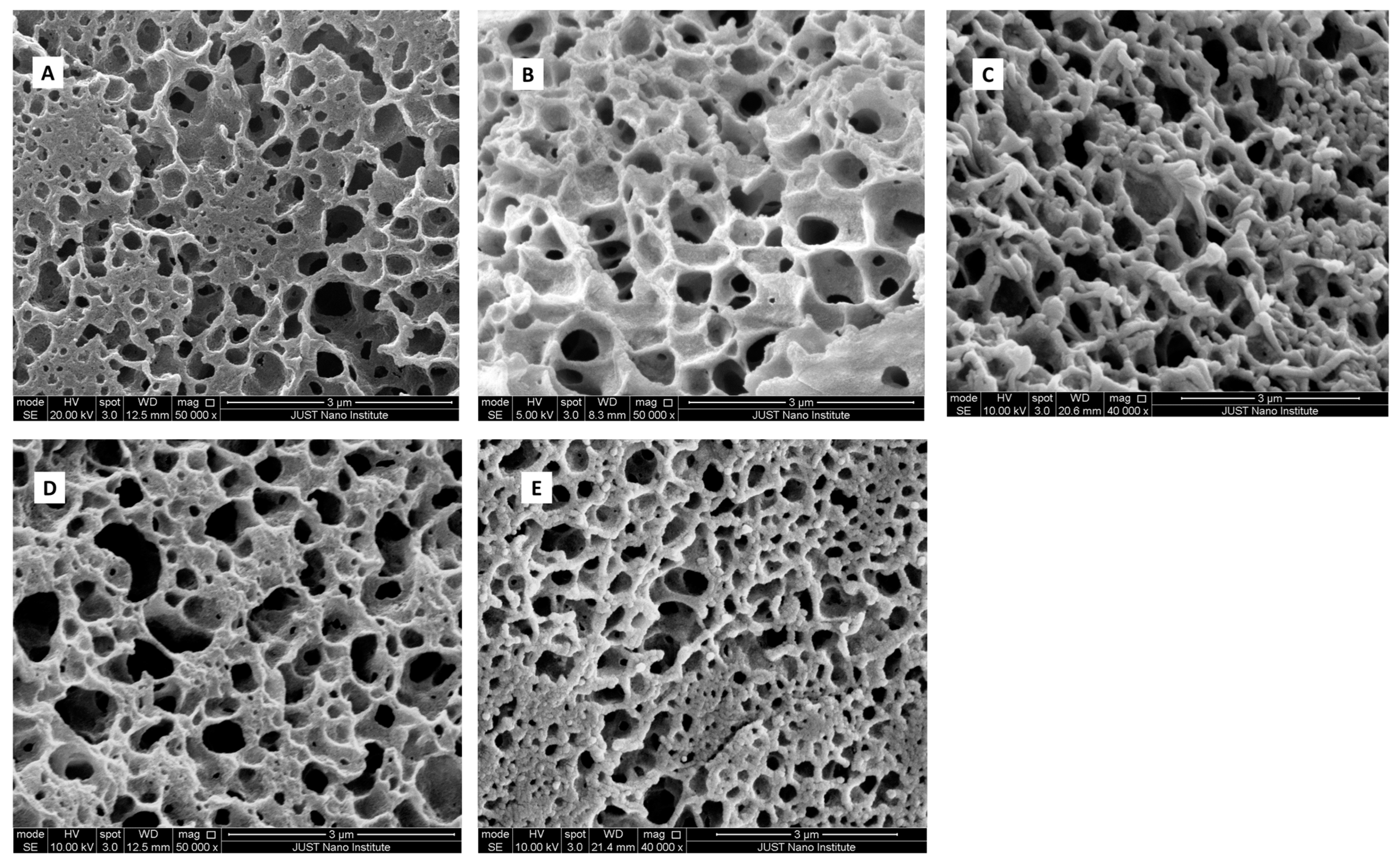
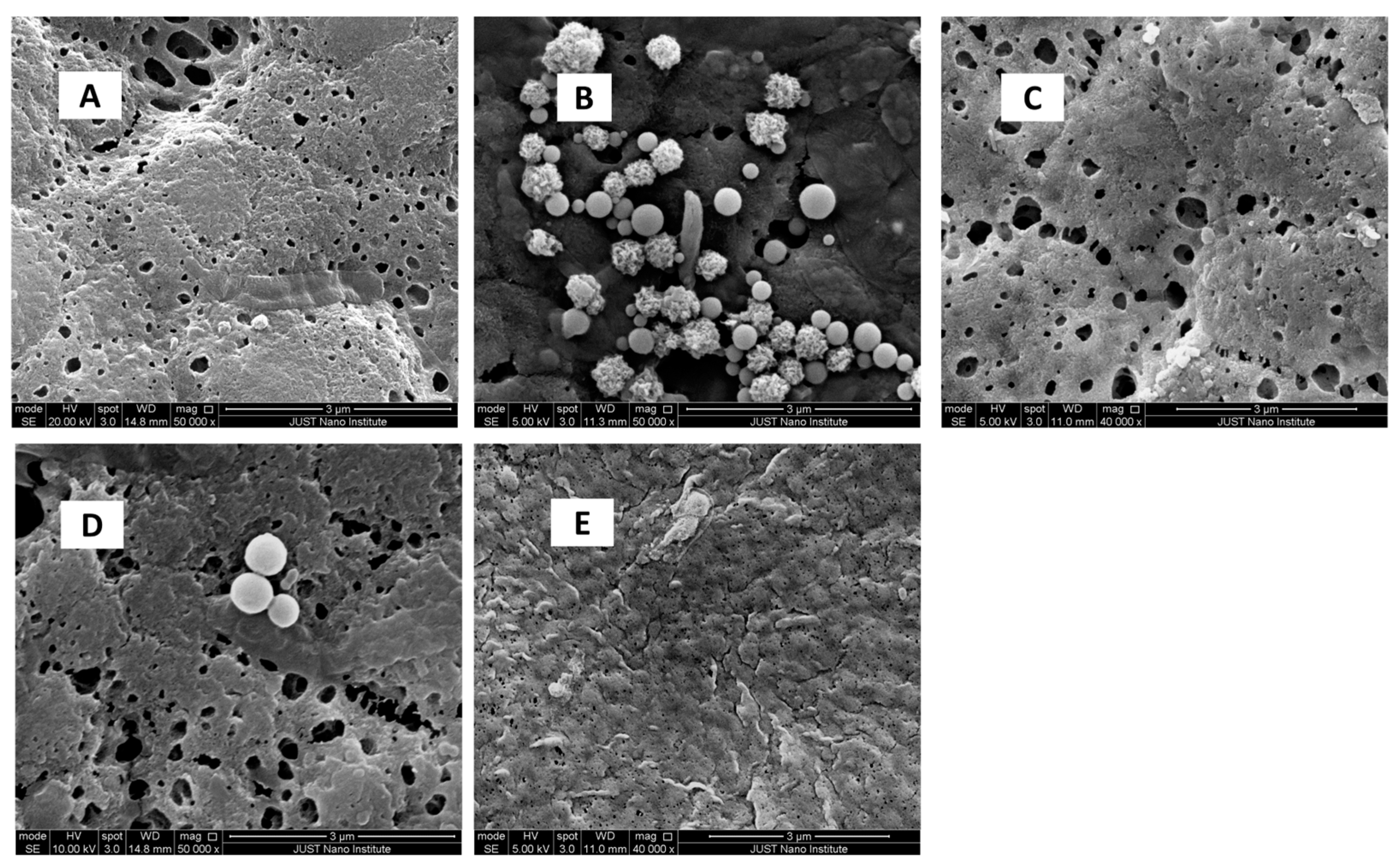
SEM
Porosity
Contact Angle and Surface Roughness
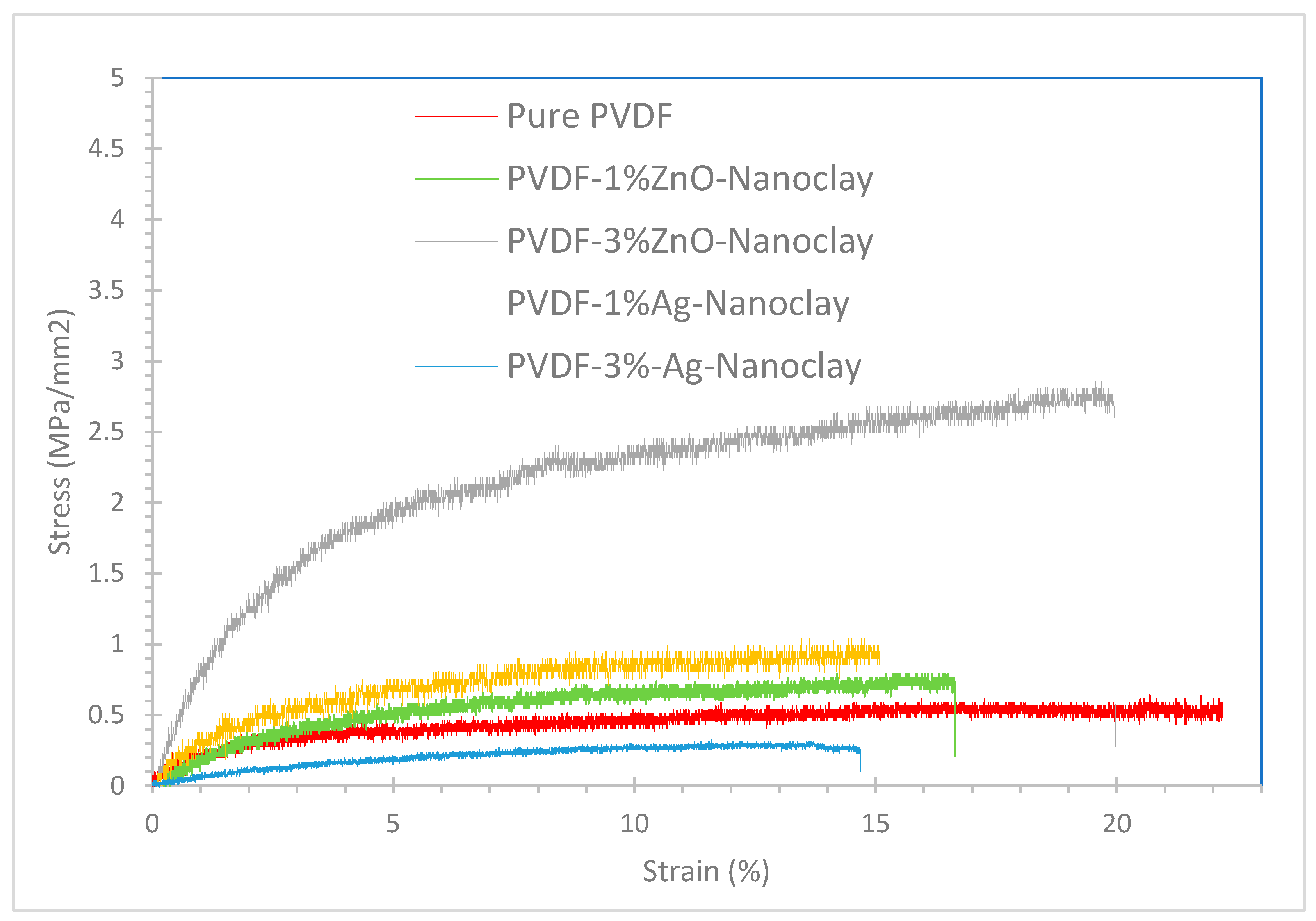
3.2.2. Mechanical Properties
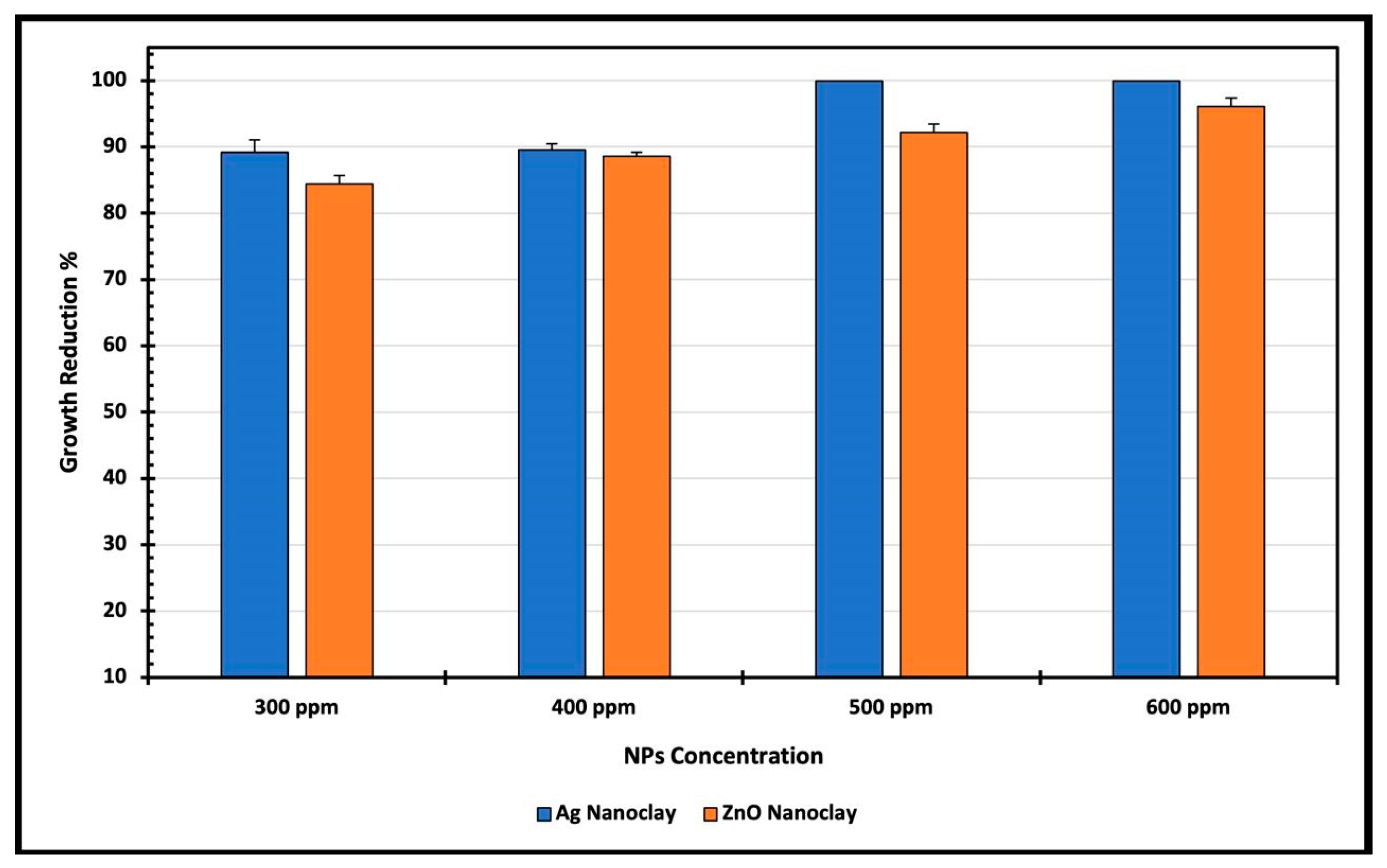
3.2.3. Antibacterial Properties
3.2.4. Barrier Properties
4. Structure–Properties–Performance Relationship
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017: Special Focus on Inequalities; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P. Nanotechnology for a Safe and Sustainable Water Supply: Enabling Integrated Water Treatment and Reuse. Accounts Chem. Res. 2012, 46, 834–843. [Google Scholar] [CrossRef]
- Khalaf, A.; Abu-Dalo, D.; AlShamaileh, E. Synthesis, Characterization, and Application of Fe2O3 Nanophotocatalyst for the Treatment of Various Pollutants in Aqueous Phase: A Systematic Review. Sci. World J. 2024, 2024, 8644322. [Google Scholar] [CrossRef]
- Elkacmi, R.; Bennajah, M. Advanced oxidation technologies for the treatment and detoxification of olive mill wastewater: A general review. J. Water Reuse Desalination 2019, 9, 463–505. [Google Scholar] [CrossRef]
- Dwairi, I.M. Evaluation of Jordanian Phillipsite Tuff in Removal of Ammonia from Wastewater Experimental Study. Al-Balqa J. 1991, 1, 53–66. [Google Scholar]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Water Treatment by Membrane Filtration Techniques. In Environmental Water; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–154. [Google Scholar] [CrossRef]
- Cheng, Y.; Xia, C.; Garalleh, H.A.; Garaleh, M.; Chi, N.T.L.; Brindhadevi, K. A review on optimistic development of polymeric nanocomposite membrane on environmental remediation. Chemosphere 2023, 315, 137706. [Google Scholar] [CrossRef]
- Ji, J.; Liu, F.; Hashim, N.A.; Abed, M.R.M.; Li, K. Poly(vinylidene fluoride) (PVDF) membranes for fluid separation. React. Funct. Polym. 2015, 86, 134–153. [Google Scholar] [CrossRef]
- Lai, C.Y.; Groth, A.; Gray, S.; Duke, M. Nanocomposites for Improved Physical Durability of Porous PVDF Membranes. Membranes 2014, 4, 55–78. [Google Scholar] [CrossRef]
- Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Mohamed, M.A.; Rahman, M.A.; Othman, M.H.D.; Lau, W.J.; Yusof, N. Preparation and performance of PVDF-based nanocomposite membrane consisting of TiO2 nanofibers for organic pollutant decomposition in wastewater under UV irradiation. Desalination 2016, 391, 89–97. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Effects of nano sized zinc oxide on the performance of PVDF microfiltration membranes. Desalination 2012, 302, 71–79. [Google Scholar] [CrossRef]
- Habba, Y.G.; Capochichi-Gnambodoe, M.; Serairi, L.; Leprince-Wang, Y. Enhanced photocatalytic activity of ZnO nanostructure for water purification. Phys. Status Solidi (b) 2016, 253, 1480–1484. [Google Scholar] [CrossRef]
- Taha, A.K.T.; Amir; Korkmaz, A.D.; Al-Messiere, M.A.; Baykal, A.; Karakuş, S.; Kilislioglu, A. Development of Novel Nano-ZnO Enhanced Polymeric Membranes for Water Purification. J. Inorg. Organomet. Polym. Mater. 2018, 29, 979–988. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Al Omari, R.; Younes, M.; Algburi, S. Cross-linked chitosan-adipic/zirconia (ZrO2) nanocomposite for removal of eosin y dye: Insight into physicochemical properties, adsorption modelling, isotherms, and kinetics. Int. J. Environ. Anal. Chem. 2024, 1–18. [Google Scholar] [CrossRef]
- Al-Maliki, R.M.; Alsalhy, Q.F.; Al-Jubouri, S.; Salih, I.K.; AbdulRazak, A.A.; Shehab, M.A.; Németh, Z.; Hernadi, K. Classification of Nanomaterials and the Effect of Graphene Oxide (GO) and Recently Developed Nanoparticles on the Ultrafiltration Membrane and Their Applications: A Review. Membranes 2022, 12, 1043. [Google Scholar] [CrossRef]
- Ma, C.; Hu, J.; Sun, W.; Ma, Z.; Yang, W.; Wang, L.; Ran, Z.; Zhao, B.; Zhang, Z.; Zhang, H. Graphene oxide-polyethylene glycol incorporated PVDF nanocomposite ultrafiltration membrane with enhanced hydrophilicity, permeability, and antifouling performance. Chemosphere 2020, 253, 126649. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, X.; Kaneda, M.; Zhang, W.; Bernstein, R.; Ma, J.; Elimelech, M. Graphene Oxide Functionalized Membranes: The Importance of Nanosheet Surface Exposure for Biofouling Resistance. Environ. Sci. Technol. 2019, 54, 517–526. [Google Scholar] [CrossRef]
- Alnairat, N.; Dalo, M.A.; Abu-Zurayk, R.; Mallouh, S.A.; Odeh, F.; Al Bawab, A. Green Synthesis of Silver Nanoparticles as an Effective Antibiofouling Material for Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane. Polymers 2021, 13, 3683. [Google Scholar] [CrossRef]
- Mecha, A.C.; Chollom, M.N.; Babatunde, B.F.; Tetteh, E.K.; Rathilal, S. Versatile Silver-Nanoparticle-Impregnated Membranes for Water Treatment: A Review. Membranes 2023, 13, 432. [Google Scholar] [CrossRef]
- Lai, C.Y.; Groth, A.; Gray, S.; Duke, M. Enhanced abrasion resistant PVDF/nanoclay hollow fibre composite membranes for water treatment. J. Membr. Sci. 2014, 449, 146–157. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Gao, S.; Cui, J.; Qu, Q.; Wang, Y.; Huang, C.; Fu, G.-D. Self-Healing and Superwettable Nanofibrous Membranes with Excellent Stability toward Multifunctional Applications in Water Purification. ACS Appl. Mater. Interfaces 2020, 12, 23644–23654. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Alnairat, N.; Bozeya, A.; Khalaf, A.; Abu-Dalo, D. Enhanced properties of PVDF membranes using green Ag-nanoclay composite nanoarchitectonics. Mater. Res. Express 2024, 11, 045007. [Google Scholar] [CrossRef]
- Al-Khaial, M.Q.; Chan, S.Y.; Abu-Zurayk, R.A.; Alnairat, N. Biosynthesis and Characterization of Zinc Oxide Nanoparticles (ZnO-NPs) Utilizing Banana Peel Extract. Inorganics 2024, 12, 121. [Google Scholar] [CrossRef]
- Mahdy, S.A.; Raheed, Q.J.; Kalaichelvan, P. Antimicrobial activity of zero-valent iron nanoparticles. Int. J. Mod. Eng. Res. 2012, 2, 578–581. [Google Scholar]
- ISO 22196; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Standards Organization: Geneva, Switzerland, 2011.
- Huang, L.; Zhao, S.; Wang, Z.; Wu, J.; Wang, J.; Wang, S. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General Aspects: A Chemical Reduction Approach to the Synthesis of Nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef] [PubMed]
- Alsoud, A.; Daradkeh, S.I.; Al-Bashaish, S.R.; Shaheen, A.A.; Jaber, A.M.D.; Abuamr, A.M.; Mousa, M.S.; Holcman, V. Electrical Characterization of Epoxy Nanocomposite under High DC Voltage. Polymers 2024, 16, 963. [Google Scholar] [CrossRef]
- Zhao, Q.; Fu, L.; Jiang, D.; Ouyang, J.; Hu, Y.; Yang, H.; Xi, Y. Nanoclay-modulated oxygen vacancies of metal oxide. Commun. Chem. 2019, 2, 11. [Google Scholar] [CrossRef]
- Ye, J.; Li, X.; Hong, J.; Chen, J.; Fan, Q. Photocatalytic degradation of phenol over ZnO nanosheets immobilized on montmorillonite. Mater. Sci. Semicond. Process. 2015, 39, 17–22. [Google Scholar] [CrossRef]
- Awwad, A.; Abdeen, A. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef]
- Vanaja, M.; Gurusamy, A. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Assayed, G.A.I.; Shaheen, A.A.; Alsoud, A.; Al-Bashaish, S.R.; Mousa, M.S.; Knápek, A.; Sobola, D. Structural and electrical characterization of cadmium phosphate glasses doped with different concentration of sodium chloride. Phys. Scr. 2024, 99, 125018. [Google Scholar] [CrossRef]
- Naqvi, S.M.A.; Soleimani, H.; Yahya, N.; Irshad, K. Structural and optical properties of chromium doped zinc oxide nanoparticles synthesized by sol-gel method. AIP Conf. Proc. 2014, 1621, 530–537. [Google Scholar] [CrossRef]
- Ashraf, R.; Riaz, S.; Kayani, Z.N.; Naseem, S. Effect of Calcination on Properties of ZnO Nanoparticles. Mater. Today Proc. 2015, 2 Pt B, 5468–5472. [Google Scholar] [CrossRef]
- Ahmad, T.; Pandey, V.; Husain, M.S.; Adiba; Munjal, S. Structural and spectroscopic analysis of pure phase hexagonal wurtzite ZnO nanoparticles synthesized by sol-gel. Mater. Today Proc. 2022, 49, 1694–1697. [Google Scholar] [CrossRef]
- Inoue, M.; Hirasawa, I. The relationship between crystal morphology and XRD peak intensity on CaSO4 2H2O. J. Cryst. Growth 2013, 380, 169–175. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Hu, J.; Liu, L. Theory of polymer diffusion in polymer-nanoparticle mixtures: Effect of nanoparticle concentration and polymer length. Soft Matter 2021, 17, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Prihandana, G.; Sriani, T.; Muthi’ah, A.; Machmudah, A.; Mahardika, M.; Miki, N. Study Effect of nAg Particle Size on the Properties and Antibacterial Characteristics of Polysulfone Membranes. Nanomaterials 2022, 12, 388. [Google Scholar] [CrossRef]
- Roshani, R.; Ardeshiri, F.; Peyravi, M.; Jahanshahi, M. Highly permeable PVDF membrane with PS/ZnO nanocomposite incorporated for distillation process. RSC Adv. 2018, 8, 23499–23515. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Shen, J.; Ruan, H.; Wu, L.; Gao, C. Preparation and characterization of PES–SiO2 organic–inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]
- Li, J.-B.; Zhu, J.-W.; Zheng, M.-S. Morphologies and properties of poly(phthalazinone ether sulfone ketone) matrix ultrafiltration membranes with entrapped TiO2 nanoparticles. J. Appl. Polym. Sci. 2007, 103, 3623–3629. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Zhang, W.; Zhao, X.; Zhang, L.; Gao, Y. Rheological mechanism of polymer nanocomposites filled with spherical nanoparticles: Insight from molecular dynamics simulation. Polymer 2021, 231, 124129. [Google Scholar] [CrossRef]
- Ardeshiri, F.; Salehi, S.; Peyravi, M.; Jahanshahi, M.; Amiri, A.; Rad, A. PVDF membrane assisted by modified hydrophobic ZnO nanoparticle for membrane distillation. Asia-Pacific J. Chem. Eng. 2018, 13, e2196. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Feng, C.; Matsuura, T.; Kapantaidakis, G.C.; Wessling, M.; Koops, G.H. Characterization of polyethersulfone-polyimide hollow fiber membranes by atomic force microscopy and contact angle goniometery. J. Membr. Sci. 2003, 226, 63–73. [Google Scholar] [CrossRef]
- Mierzwa, J.C.; Arieta, V.; Verlage, M.; Carvalho, J.; Vecitis, C.D. Effect of clay nanoparticles on the structure and performance of polyethersulfone ultrafiltration membranes. Desalination 2013, 314, 147–158. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Liu, W.; Fu, R.; Tu, S.; Zhao, Y.; Dong, L.; Yan, B.; Gu, Y. High Performance Piezoelectric Nanogenerators Based on Electrospun ZnO Nanorods/Poly(vinylidene fluoride) Composite Membranes. J. Phys. Chem. C 2019, 123, 11378–11387. [Google Scholar] [CrossRef]
- Hwang, H.-Y.; Kim, D.-J.; Kim, H.-J.; Hong, Y.-T.; Nam, S.-Y. Effect of nanoclay on properties of porous PVdF membranes. Trans. Nonferrous Met. Soc. China 2011, 21, s141–s147. [Google Scholar] [CrossRef]
- He, Y.; Hong, J.M. Effect of Nano-Sized ZnO Particle Addition on PVDF Ultrafiltration Membrane Performance. Adv. Mater. Res. 2011, 311, 1818–1821. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Magdah, A.G.; Helmy, E.A.M.; Mabrouk, A.S. Recent advances in green synthesis of silver nanoparticles and their applications: About future directions. A review. BioNanoScience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Parvataneni, R. Biogenic synthesis and characterization of silver nanoparticles using aqueous leaf extract of Scoparia dulcis L. and assessment of their antimicrobial property. Drug Chem. Toxicol. 2020, 43, 307–321. [Google Scholar] [CrossRef]
- Ganash, M.; Ghany, T.A.; Omar, A. Morphological and biomolecules dynamics of phytopathogenic fungi under stress of silver nanoparticles. BioNanoScience 2018, 8, 566–573. [Google Scholar] [CrossRef]
- Girase, B.; Depan, D.; Shah, J.S.; Xu, W.; Misra, R.D.K. Silver–clay nanohybrid structure for effective and diffusion-controlled antimicrobial activity. Mater. Sci. Eng. C 2011, 31, 1759–1766. [Google Scholar] [CrossRef]
- Roy, A.; Butola, B.; Joshi, M. Synthesis, characterization and antibacterial properties of novel nano-silver loaded acid activated montmorillonite. Appl. Clay Sci. 2017, 146, 278–285. [Google Scholar] [CrossRef]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Atmaca, S.; Gül, K.; Cicek, R. The effect of zinc on microbial growth. Turk. J. Med. Sci. 1998, 28, 595–598. [Google Scholar]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Morrison, K.D.; Underwood, J.C.; Metge, D.W.; Eberl, D.D.; Williams, L.B. Mineralogical variables that control the antibacterial effectiveness of a natural clay deposit. Environ. Geochem. Health 2014, 36, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Bajaj, G.; Mukherji, S.; Mukherji, S. Arginine-assisted immobilization of silver nanoparticles on ZnO nanorods: An enhanced and reusable antibacterial substrate without human cell cytotoxicity. Nanoscale 2015, 7, 7415–7429. [Google Scholar] [CrossRef]
- Jaber, A.M.; Zahra, J.A.; El-Abadelah, M.M.; Al-Mahadeen, M.M.; Sabri, S.S.; Kasabri, V.; Haddadin, R.N. Evaluation of Spirooxindole-3,3’-pyrrolines-incorporating Isoquinoline Motif as Antitumor, Anti-Inflammatory, Antibacterial, Antifungal, and Antioxidant Agents. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2024, 23, 261–272. [Google Scholar] [CrossRef]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.; Dong, S.; Kang, Y.; Zhou, Y.; Wang, A. Hydrothermal Fabrication of Spindle-Shaped ZnO/Palygorskite Nanocomposites Using Nonionic Surfactant for Enhancement of Antibacterial Activity. Nanomaterials 2019, 9, 1453. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Wang, W.; Gao, B.; Wang, Z. Palygorskite/silver nanoparticles incorporated polyamide thin film nanocomposite membranes with enhanced water permeating, antifouling and antimicrobial performance. Chemosphere 2019, 236, 124396. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Al Omari, R.; Younes, M.; Algburi, S. Chitosan polymer/functionalised rambutan peel using carboxylic acid: Process optimisation using Box-Behnken Design for brilliant green dye removal. Int. J. Environ. Anal. Chem. 2024, 1–21. [Google Scholar] [CrossRef]
- Gabriel, J.S.; Gonzaga, V.A.M.; Poli, A.L.; Schmitt, C.C. Photochemical synthesis of silver nanoparticles on chitosans/montmorillonite nanocomposite films and antibacterial activity. Carbohydr. Polym. 2017, 171, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Al Hunaiti, A.; Ghazzy, A.; Aqel, H.; Abu Thiab, T.; Saeed, R.; Taha, M.; Hwaitat, E.; Zihlif, M.Z.; Imraish, A. New Chitosan-Based Trimetallic Cu0.5Zn0.5Fe2O4 Nanoparticles: Preparation, Characterization, and Anti-cancer Activity Original Research Article distributed under the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International (CC BY-NC-ND 4.0) license. Pharm. Pract. 2024, 22, 3016. [Google Scholar] [CrossRef]
- Tian, G.; Huang, Z.; Wang, H.; Cui, C.; Zhang, Y. Polycaprolactone nanofiber membrane modified with halloysite and ZnO for anti-bacterial and air filtration. Appl. Clay Sci. 2022, 223, 106512. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dinh, T.T.L.; Ahn, Y.-H. Enhanced antifouling performance of PVDF ultrafiltration membrane by blending zinc oxide with support of graphene oxide nanoparticle. Chemosphere 2020, 241, 125068. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Shao, X.-S.; Zhou, Q.; Li, M.-Z.; Zhang, Q.-Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Abdul-Majeed, M.A. Preparation and Characterization of AgNp/PVDF Cmposite Ultrafiltration Membrane. J. Eng. 2018, 24, 50. [Google Scholar] [CrossRef]
- Kamaludin, R.; Majid, L.A.; Othman, M.H.D.; Mansur, S.; Kadir, S.H.S.A.; Wong, K.Y.; Khongnakorn, W.; Puteh, M.H. Polyvinylidene Difluoride (PVDF) Hollow Fiber Membrane Incorporated with Antibacterial and Anti-Fouling by Zinc Oxide for Water and Wastewater Treatment. Membranes 2022, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wu, Z.; Liu, N. Effect of nano-ZnO with different particle size on the performance of PVDF composite membrane. Plast. Rubber Compos. 2017, 46, 1–7. [Google Scholar] [CrossRef]
| Sample | Average Pore Diameter (nm) |
|---|---|
| Pure PVDF | 295.2 |
| PVDF-1%Ag-Nanoclay | 314.5 |
| PVDF-3%Ag-Nanoclay | 310.2 |
| PVDF-1%ZnO-Nanoclay | 373.5 |
| PVDF-3%ZnO-Nanoclay | 242.3 |
| Sample | Average Porosity (%) |
|---|---|
| PVDF | 77.8 ± 1.7 |
| PVDF-1%Ag-Nanoclay | 79.4 ± 2.3 |
| PVDF-3%Ag-Nanoclay | 82.4 ± 0.4 |
| PVDF-1%ZnO-Nanoclay | 80.9 ± 1.3 |
| PVDF-3%ZnO-Nanoclay | 79.6 ± 2.5 |
| Sample | Contact Angle (°) | Average Roughness (nm) |
|---|---|---|
| PVDF | 53⁰ | 14.4 |
| PVDF-1%Ag-Nanoclay | 55⁰ | 29.3 |
| PVDF-3%Ag-Nanoclay | 66⁰ | 24.2 |
| PVDF-1%ZnO-Nanoclay | 63⁰ | 13.4 |
| PVDF-3%ZnO-Nanoclay | 79⁰ | 11.7 |
| Sample | Sterilization Against E. coli |
|---|---|
| PVDF-1%Ag-Nanoclay | 99.98% |
| PVDF-3%Ag-Nanoclay | 99.98% |
| PVDF-1%ZnO-Nanoclay | 61.54% |
| PVDF-3%ZnO-Nanoclay | 99.92% |
| Sample | Water Flux Compared to Pure PVDF Membrane (%) |
|---|---|
| PVDF-1%Ag-Nanoclay | −7.8 |
| PVDF-3%Ag-Nanoclay | +48.2 |
| PVDF-1%ZnO-Nanoclay | +16.1 |
| PVDF-3%ZnO-Nanoclay | −12.0 |
| Parameter | Nanocomposite | Structure | Properties | Performance |
|---|---|---|---|---|
| Pore Size | PVDF-Ag-Nanoclay | Small pores (310.2–314.5 nm), surface roughness (29.3 nm, 24.2 nm roughness) | Increased hydrophobicity (Contact angle increased by 130 compared to pure PVDF) | Enhanced antibacterial activity due to higher nanoparticle exposure on surface (99.98% sterilization) |
| PVDF-ZnO-Nanoclay | Larger pores at 1 wt% (373.5 nm), smaller pores at 3 wt% (242.3 nm) | Increased crystallinity, lower surface roughness at 3wt% (11.7 nm roughness) | Reduced water flux at 3 wt% (−12%), antibacterial activity due to ROS generation (99.92% sterilization) | |
| Porosity | PVDF-Ag-Nanoclay | Higher porosity at 3 wt% (82.4%), increased voids | Higher porosity facilitates water diffusion | Higher water flux (+48%), but reduced mechanical strength at higher loading (−44%) |
| PVDF-ZnO-Nanoclay | Lower porosity at 3 wt%, (79.6) reduced voids | Lower porosity limits water flow | Reduced water flux at 3 wt% (−12%), enhanced mechanical strength at 3 wt% (+500%) | |
| Nanoparticle Distribution | PVDF-Ag-Nanoclay | Nanoparticles are mostly on the surface | Higher nanoparticle loading leads to agglomeration at 3 wt% | Enhanced mechanical strength at 1 wt% (+108%), reduced at 3 wt% (−44%) due to agglomeration |
| PVDF-ZnO-Nanoclay | Well-dispersed nanoparticles within the polymer matrix | Better interaction with PVDF, increased viscosity at higher loading | Enhanced mechanical strength at both loadings (+77%, +500%), better distribution of nanoparticles | |
| Surface Roughness | PVDF-Ag-Nanoclay | Increased roughness (29.3 nm, 24.2 nm), nanoparticles on the surface | Increased hydrophobicity due to rough surface (Contact angle increased by 13⁰ compared to pure PVDF) | Higher antibacterial performance at lower concentrations (99.98% sterilization) |
| PVDF-ZnO-Nanoclay | More uniform surface, nanoparticles embedded within matrix | Reduced surface roughness due to better dispersion (11.7 nm) | Moderate antibacterial efficiency, mainly due to ROS and Zn2+ ion release | |
| Hydrophilicity | PVDF-Ag-Nanoclay | More hydrophobic | Increased contact angle and surface roughness (29.3 nm roughness at 1wt%) | Decreased water flux due to increased hydrophobicity (−7% at 1wt%) |
| PVDF-ZnO-Nanoclay | Less hydrophilic | Hydrophilicity influenced by nanoparticle distribution | Lower water flux at 3wt% (−12%), enhanced antibacterial due to ROS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Zurayk, R.; Alnairat, N.; Waleed, H.; Al-Khaial, M.Q.; Khalaf, A.; Bozeya, A.; Abu-Dalo, D.; Al-Yousef, S.; Afaneh, R. Polyvinylidene Fluoride (PVDF) and Nanoclay Composites’ Mixed-Matrix Membranes: Exploring Structure, Properties, and Performance Relationships. Polymers 2025, 17, 1120. https://doi.org/10.3390/polym17081120
Abu-Zurayk R, Alnairat N, Waleed H, Al-Khaial MQ, Khalaf A, Bozeya A, Abu-Dalo D, Al-Yousef S, Afaneh R. Polyvinylidene Fluoride (PVDF) and Nanoclay Composites’ Mixed-Matrix Membranes: Exploring Structure, Properties, and Performance Relationships. Polymers. 2025; 17(8):1120. https://doi.org/10.3390/polym17081120
Chicago/Turabian StyleAbu-Zurayk, Rund, Nour Alnairat, Haneen Waleed, Mohammed Q. Al-Khaial, Aya Khalaf, Ayat Bozeya, Duaa Abu-Dalo, Sojoud Al-Yousef, and Razan Afaneh. 2025. "Polyvinylidene Fluoride (PVDF) and Nanoclay Composites’ Mixed-Matrix Membranes: Exploring Structure, Properties, and Performance Relationships" Polymers 17, no. 8: 1120. https://doi.org/10.3390/polym17081120
APA StyleAbu-Zurayk, R., Alnairat, N., Waleed, H., Al-Khaial, M. Q., Khalaf, A., Bozeya, A., Abu-Dalo, D., Al-Yousef, S., & Afaneh, R. (2025). Polyvinylidene Fluoride (PVDF) and Nanoclay Composites’ Mixed-Matrix Membranes: Exploring Structure, Properties, and Performance Relationships. Polymers, 17(8), 1120. https://doi.org/10.3390/polym17081120







