Catalytic Conversion of Xylo-Oligomers to Furfural in Pulping Pre-Hydrolysis Liquor Using a Hydroxyl-Functionalized Covalent Organic Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Characterization of XOS
2.3. Preparation of Functionalized COF
2.4. Characterization of Functionalized COF
2.5. Catalytic Conversion of XOS into Furfural
3. Results and Discussion
3.1. Basic Properties of XOS
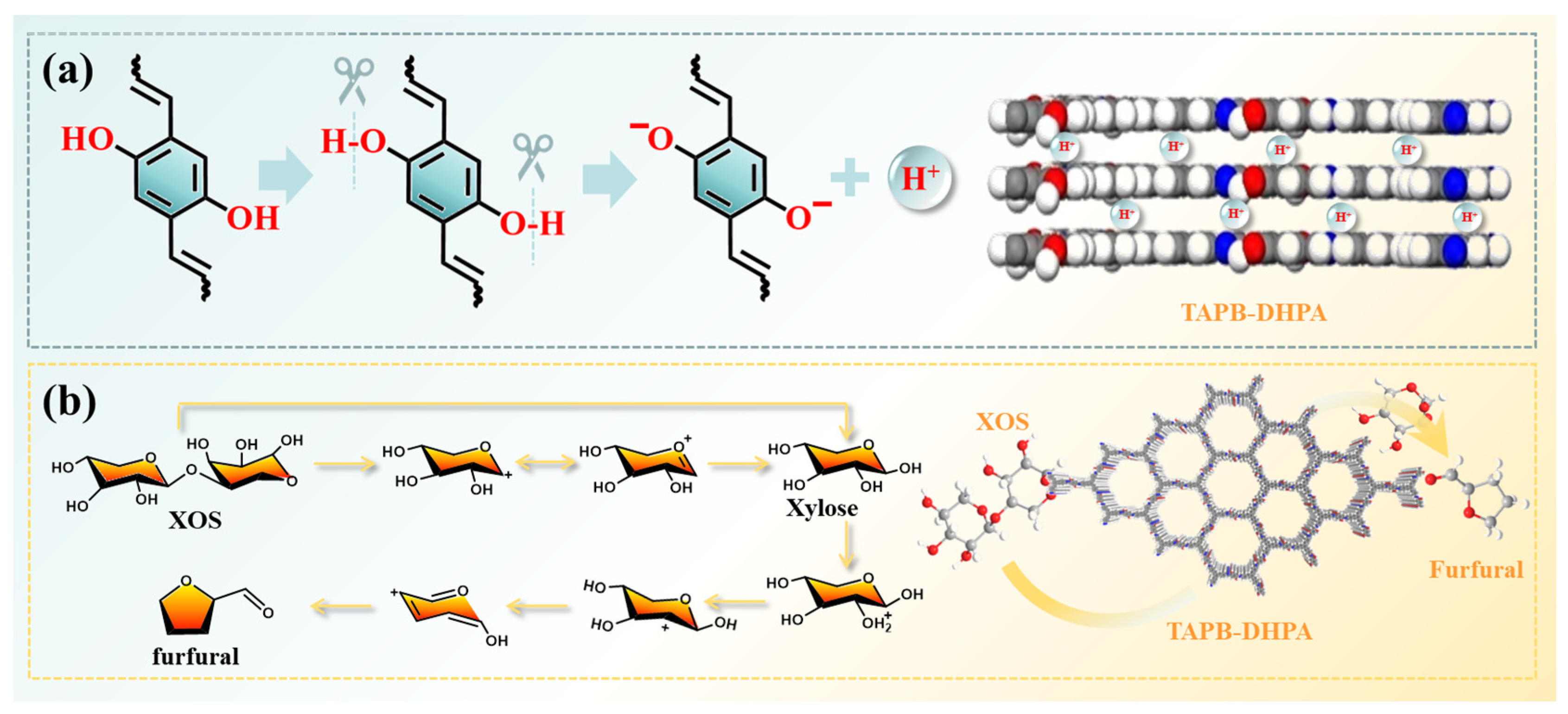
3.2. Structure and Properties of TAPB-DHPA
3.3. Catalytic Conversion of XOS to Furfural Using TAPB-DHPA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.Q.; Meng, Y.; Li, H.; Yang, S. Hierarchical Porous MIL-101(Cr) Solid Acid-Catalyzed Production of Value-Added Acetals from Biomass-Derived Furfural. Polymers 2021, 13, 3498. [Google Scholar] [CrossRef] [PubMed]
- Puke, M.; Godina, D.; Brazdausks, P. Catalyzed Hydrothermal Pretreatment of Oat Husks for Integrated Production of Furfural and Lignocellulosic Residue. Polymers 2024, 16, 707. [Google Scholar] [CrossRef]
- Cunha, A.E.P.; Simoes, R.M.S. Dissolving Kraft Pulp Production and Xylooligosaccharide Coproduction: Effect of Pre-Hydrolysis Conditions. Acs Omega 2023, 8, 13626. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Smit, A.T.; de Wild, P.J.; den Uil, H. Fractionation of wheat straw by prehydrolysis, organosolv delignification and enzymatic hydrolysis for production of sugars and lignin. Bioresour. Technol. 2012, 114, 389–398. [Google Scholar] [CrossRef]
- Lou, R.; Zhang, X. Evaluation of pretreatment effect on lignin extraction from wheat straw by deep eutectic solvent. Bioresour. Technol. 2022, 344, 126174. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shao, L.S.; Wu, Z.P.; Zhan, P.; Zhang, L. Design and Synthesis of Porous Organic Polymers: Promising Catalysts for Lignocellulose Conversion to 5-Hydroxymethylfurfural and Derivates. Polymers 2023, 15, 2630. [Google Scholar] [CrossRef]
- Chai, Y.D.; Pang, Y.L.; Lim, S.; Chong, W.C.; Lai, C.W.; Abdullah, A.Z. Recent Progress on Tailoring the Biomass-Derived Cellulose Hybrid Composite Photocatalysts. Polymers 2022, 14, 5244. [Google Scholar] [CrossRef]
- Gan, P.; Zhang, K.; Yang, G.H.; Li, J.Z.; Zhao, Y.; Chen, J.C. Catalytic Production and Upgrading of Furfural: A Platform Compound. Int. J. Mol. Sci. 2024, 25, 11992. [Google Scholar] [CrossRef]
- Deng, W.P.; Feng, Y.C.; Fu, J.; Guo, H.W.; Guo, Y.; Han, B.X.; Jiang, Z.C.; Kong, L.Z.; Li, C.Z.; Liu, H.C.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Ke, K.; Ji, H.R.; Shen, X.N.; Kong, F.O.; Li, B. Pressure Reduction Enhancing the Production of 5-Hydroxymethylfurfural from Glucose in Aqueous Phase Catalysis System. Polymers 2021, 13, 2096. [Google Scholar] [CrossRef]
- Peng, Y.W.; Hu, Z.G.; Gao, Y.J.; Yuan, D.Q.; Kang, Z.X.; Qian, Y.H.; Yan, N.; Zhao, D. Synthesis of a Sulfonated Two-Dimensional Covalent Organic Framework as an Efficient Solid Acid Catalyst for Biobased Chemical Conversion. ChemSusChem 2015, 8, 3208–3212. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.R.; Xu, B.H.; Pan, J.S.; Wu, Y.W.; Peng, X.M.; Wang, Y.F.; Zhang, S.J. Confinement of Bronsted acidic ionic liquids into covalent organic frameworks as a catalyst for dehydrative formation of isosorbide from sorbitol. Green Chem. 2019, 21, 4792–4799. [Google Scholar] [CrossRef]
- Gan, P.; Zhang, K.; Li, Z.H.; Zhang, C.X.; Yang, G.H.; Zhang, L.; Wang, B.B.; Chen, J.C. One-pot furfural production from sustainable biomass-derived sugars using a functionalized covalent organic framework as a heterogeneous catalyst. Green Chem. 2024, 26, 5155–5159. [Google Scholar] [CrossRef]
- Ju, Y.; Zhang, K.; Gan, P.; Guo, B.Z.; Xia, H.M.; Zhang, L.L.; Wang, B.B.; Zhang, L.; Chen, J.C. Functionalized covalent organic frameworks for catalytic conversion of biomass-derived xylan to furfural. Int. J. Biol. Macromol. 2025, 294, 139541. [Google Scholar] [CrossRef]
- Pang, J.X.; Zhang, Y.; Tong, X.Y.; Zhong, Y.G.; Kong, F.J.; Li, D.; Liu, X.F.; Qiao, Y.J. Recent Developments in Molecular Characterization, Bioactivity, and Application of Arabinoxylans from Different Sources. Polymers 2023, 15, 225. [Google Scholar] [CrossRef]
- Chen, Z.W.; Hu, T.Q.; Jang, H.F.; Grant, E. Modification of xylan in alkaline treated bleached hardwood kraft pulps as classified by attenuated total-internal-reflection (AIR) FTIR spectroscopy. Carbohydr. Polym. 2015, 127, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Åkerholm, M.; Hinterstoisser, B.; Salmén, L. Characterization of the crystalline structure of cellulose using static and dynamic FT-IR spectroscopy. Carbohydr. Res. 2004, 339, 569–578. [Google Scholar] [CrossRef]
- Chen, H.; Ferrari, C.; Angiuli, M.; Yao, J.; Raspi, C.; Bramanti, E. Qualitative and quantitative analysis of wood samples by Fourier transform infrared spectroscopy and multivariate analysis. Carbohydr. Polym. 2010, 82, 772–778. [Google Scholar] [CrossRef]
- Palaniappan, A.; Balasubramaniam, V.G.; Antony, U. Prebiotic potential of xylooligosaccharides derived from finger millet seed coat. Food Biotechnol. 2017, 31, 264–280. [Google Scholar] [CrossRef]
- Yang, D.; Zhong, L.X.; Yuan, T.Q.; Peng, X.W.; Sun, R.C. Studies on the structural characterization of lignin, hemicelluloses and cellulose fractionated by ionic liquid followed by alkaline extraction from bamboo. Ind. Crop. Prod. 2013, 43, 141–149. [Google Scholar] [CrossRef]
- Lu, F.F.; Wu, M.Q.; Lin, C.C.; Lin, X.C.; Xie, Z.H. Efficient and selective solid-phase microextraction of polychlorinated biphenyls by using a three-dimensional covalent organic framework as functional coating. J. Chromatogr. A 2022, 1681, 463419. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Yang, C.; Chen, F.S.; Zheng, G.F.; Han, Q. A Crystalline Partially Fluorinated Triazine Covalent Organic Framework for Efficient Photosynthesis of Hydrogen Peroxide. Angew. Chem. Int. Ed. 2022, 61, e202202328. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.J.; Wang, S.Q.; Wan, H.F.; Chen, L.L.; Wang, L.; Song, Y.H. Designing Fluorescent Covalent Organic Frameworks by Controlling Layer Spacing, Size of Aromatic Linker and Side Chains for Detection of Nitrofurazone. Adv. Opt. Mater. 2023, 11, 2202975. [Google Scholar] [CrossRef]
- Hu, H.; Tao, Y.L.; Wang, D.; Li, C.L.; Jiang, Q.C.; Shi, Y.X.; Wang, J.; Qin, J.P.; Zhou, S.J.; Kong, Y. Rational modification of hydroxy-functionalized covalent organic frameworks for enhanced photocatalytic hydrogen peroxide evolution. J. Colloid Interface Sci. 2023, 629, 750–762. [Google Scholar] [CrossRef]
- Kandambeth, S.; Venkatesh, V.; Shinde, D.B.; Kumari, S.; Halder, A.; Verma, S.; Banerjee, R. Self-templated chemically stable hollow spherical covalent organic framework. Nat. Commun. 2015, 6, 6786. [Google Scholar] [CrossRef]
- Shen, G.F.; Andrioletti, B.; Queneau, Y. Furfural and 5-(hydroxymethyl)furfural: Two pivotal intermediates for bio-based chemistry. Curr. Opin. Green Sustain. Chem. 2020, 26, 100384. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, K.F.; Xu, H.; Zhu, L.J.; Wang, S.R. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew. Sust. Energ. Rev. 2021, 139, 110706. [Google Scholar] [CrossRef]
- Lin, Q.X.; Zhan, Q.W.; Li, R.; Liao, S.W.; Ren, J.L.; Peng, F.; Li, L.B. Solvent effect on xylose-to-furfural reaction in biphasic systems: Combined experiments with theoretical calculations. Green Chem. 2021, 23, 8510–8518. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Chen, S.S.; Wang, L.; Hunt, A.J.; Sherwood, J.; Vigier, K.D.; Jerome, F.; Ok, Y.S.; Poon, C.S. Polar aprotic solvent-water mixture as the medium for catalytic production of hydroxymethylfurfural (HMF) from bread waste. Bioresour. Technol. 2017, 245, 456–462. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Conner, W.C.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423–1429. [Google Scholar] [CrossRef]
- Pang, S.H.; Medlin, J.W. Adsorption and Reaction of Furfural and Furfuryl Alcohol on Pd(111): Unique Reaction Pathways for Multifunctional Reagents. ACS Catal. 2011, 1, 1272–1283. [Google Scholar] [CrossRef]
- Xu, Z.P.; Li, W.Z.; Du, Z.J.; Wu, H.; Jameel, H.; Chang, H.M.; Ma, L.L. Conversion of corn stalk into furfural using a novel heterogeneous strong acid catalyst in γ-valerolactone. Bioresour. Technol. 2015, 198, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, H.T.; Li, X.D.; Ye, X.G.; Liu, L. When Furfural Meets Piancatelli Rearrangement. ACS Sustain. Chem. Eng. 2024, 12, 12378–12385. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, W.M.; Wang, X.G.; Zhang, B.; Liu, J.; Lu, Q. The formation mechanism of furfural in xylan pyrolysis: A machine learning study based on neural network potential. Fuel Process. Technol. 2023, 247, 107807. [Google Scholar] [CrossRef]
- Bhaumik, P.; Dhepe, P.L. From Lignocellulosic Biomass to Furfural: Insight into the Active Species of a Silica-Supported Tungsten Oxide Catalyst. Chemcatchem 2017, 9, 2709–2716. [Google Scholar] [CrossRef]
- Gai, X.T.; Ding, W.; He, J.; Guo, J.; Song, K. Furfural production from xylan using a Pueraria Residues carbon-based solid-acid catalyst. J. Sci. Food Agric. 2025, 105, 2002–2011. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Wu, Y.; Fan, J.; Wu, N.; Gao, L.; Li, W.; Xiao, G. Catalytic Conversion of Xylose and Xylan into Furfural Over Cr3+/P-SBA-15 Catalyst Derived from Spent Adsorbent. Ind. Eng. Chem. Res. 2019, 58, 13013–13020. [Google Scholar] [CrossRef]
- Wang, Y.; Delbecq, F.; Kwapinski, W.; Len, C. Application of sulfonated carbon-based catalyst for the furfural production from d-xylose and xylan in a microwave-assisted biphasic reaction. Mol. Catal. 2017, 438, 167–172. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.W.; Wang, X.T.; Lu, X.B.; Qi, X.H.; Yu, H.B. Recent progress in furfural production from hemicellulose and its derivatives: Conversion mechanism, catalytic system, solvent selection. Mol. Catal. 2021, 515, 111899. [Google Scholar] [CrossRef]
- Fan, X.Y.; Ren, M.N.; Zhou, C.S.; Kong, F.G.; Hua, C.H.; Fakayode, O.A.; Okonkwo, C.E.; Li, H.X.; Liang, J.K.; Wang, X. Total utilization of lignocellulosic biomass with xylooligosaccharides production priority: A review. Biomass Bioenergy 2024, 181, 107038. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Mamman, A.S.; Lee, J.M.; Kim, Y.C.; Hwang, I.T.; Park, N.J.; Hwang, Y.K.; Chang, J.S.; Hwang, J.S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefining 2008, 2, 438–454. [Google Scholar] [CrossRef]

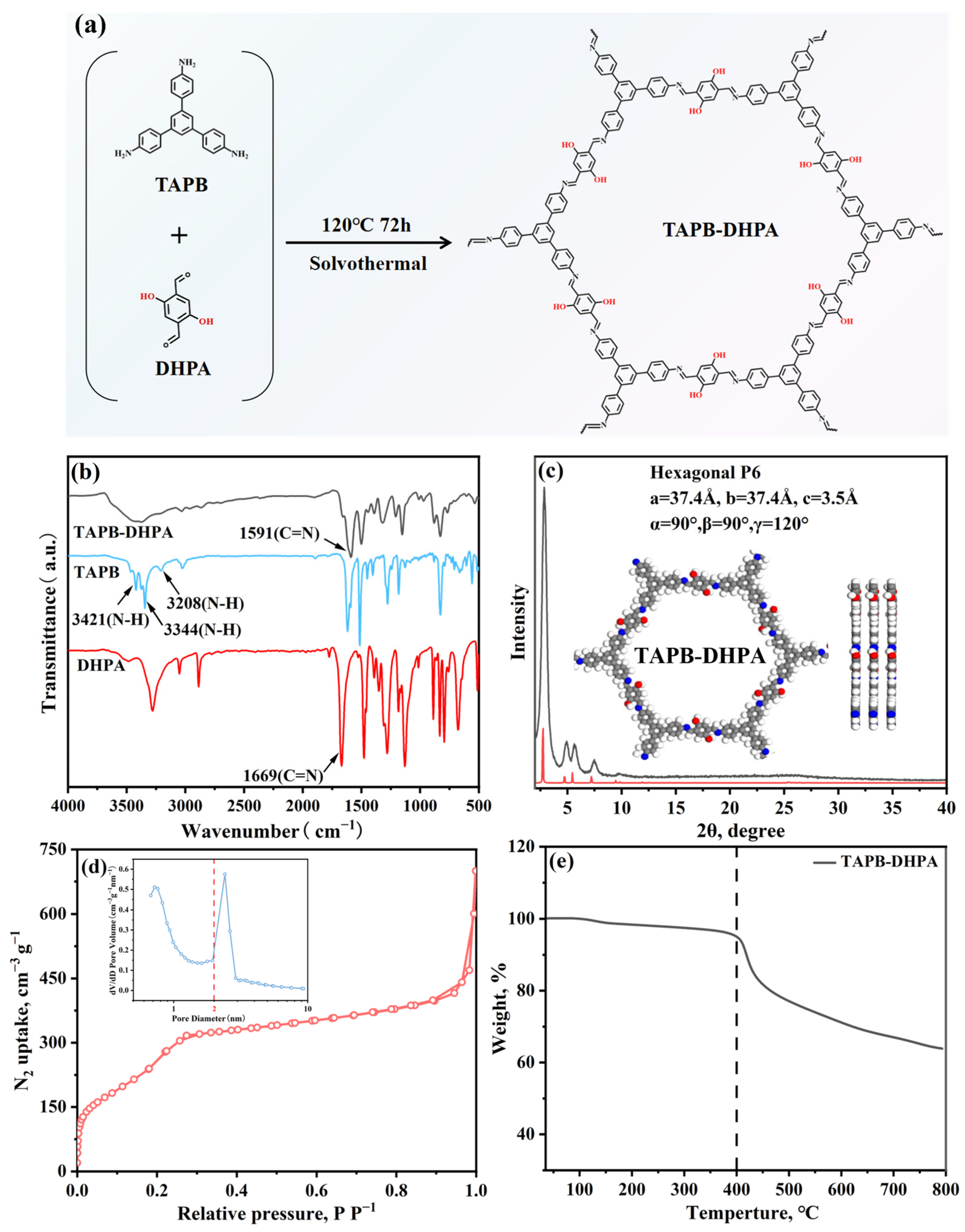
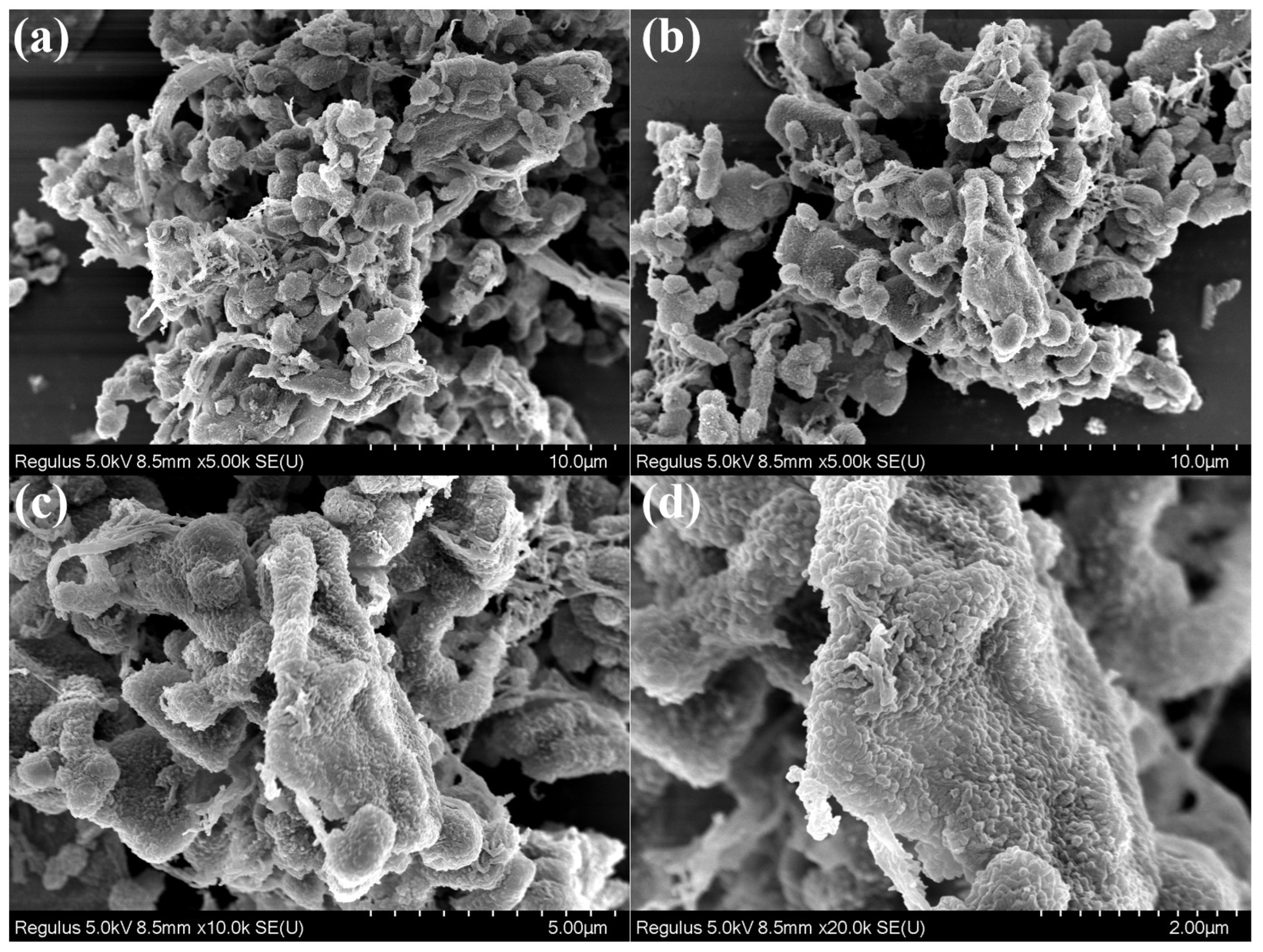
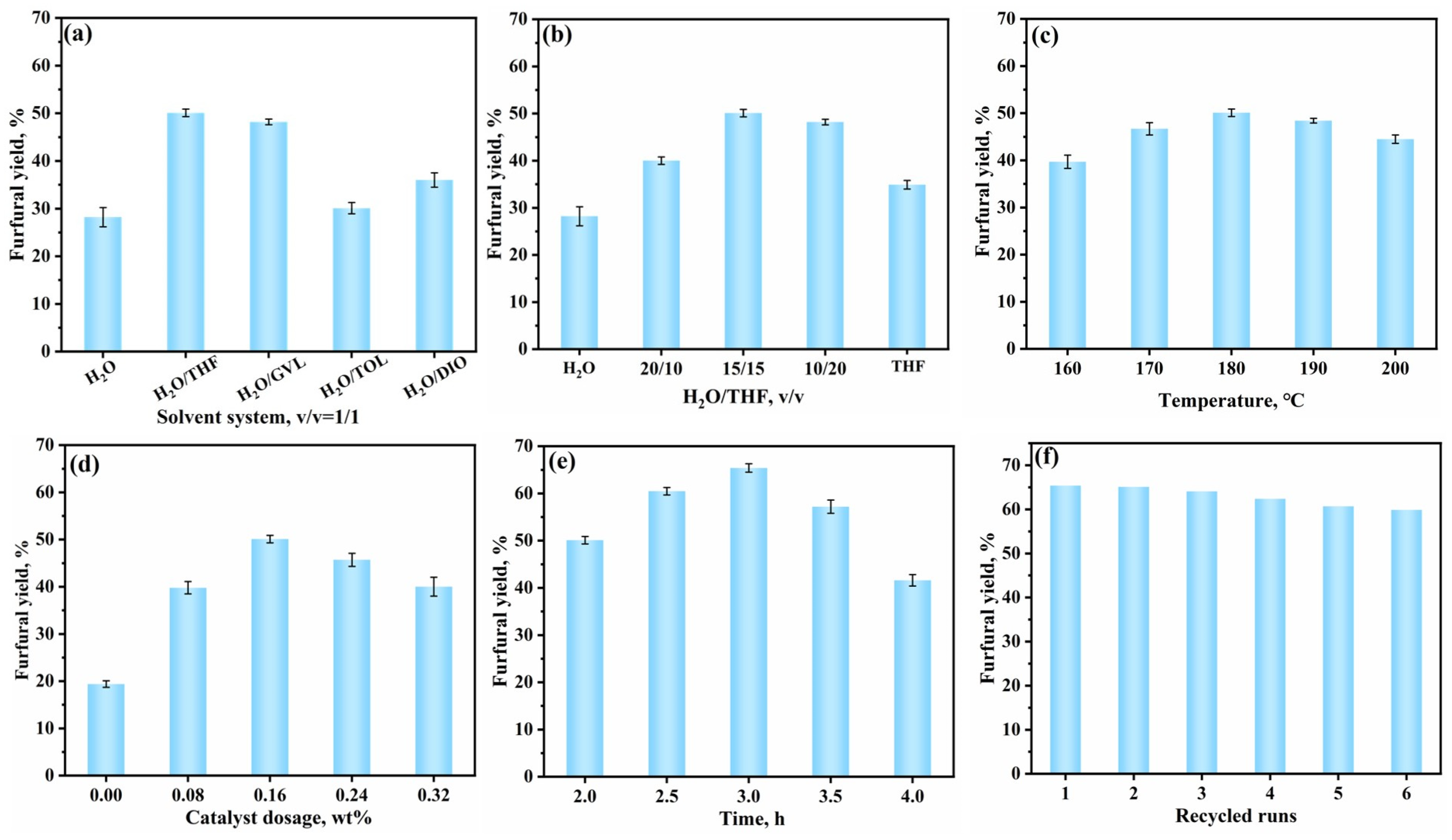
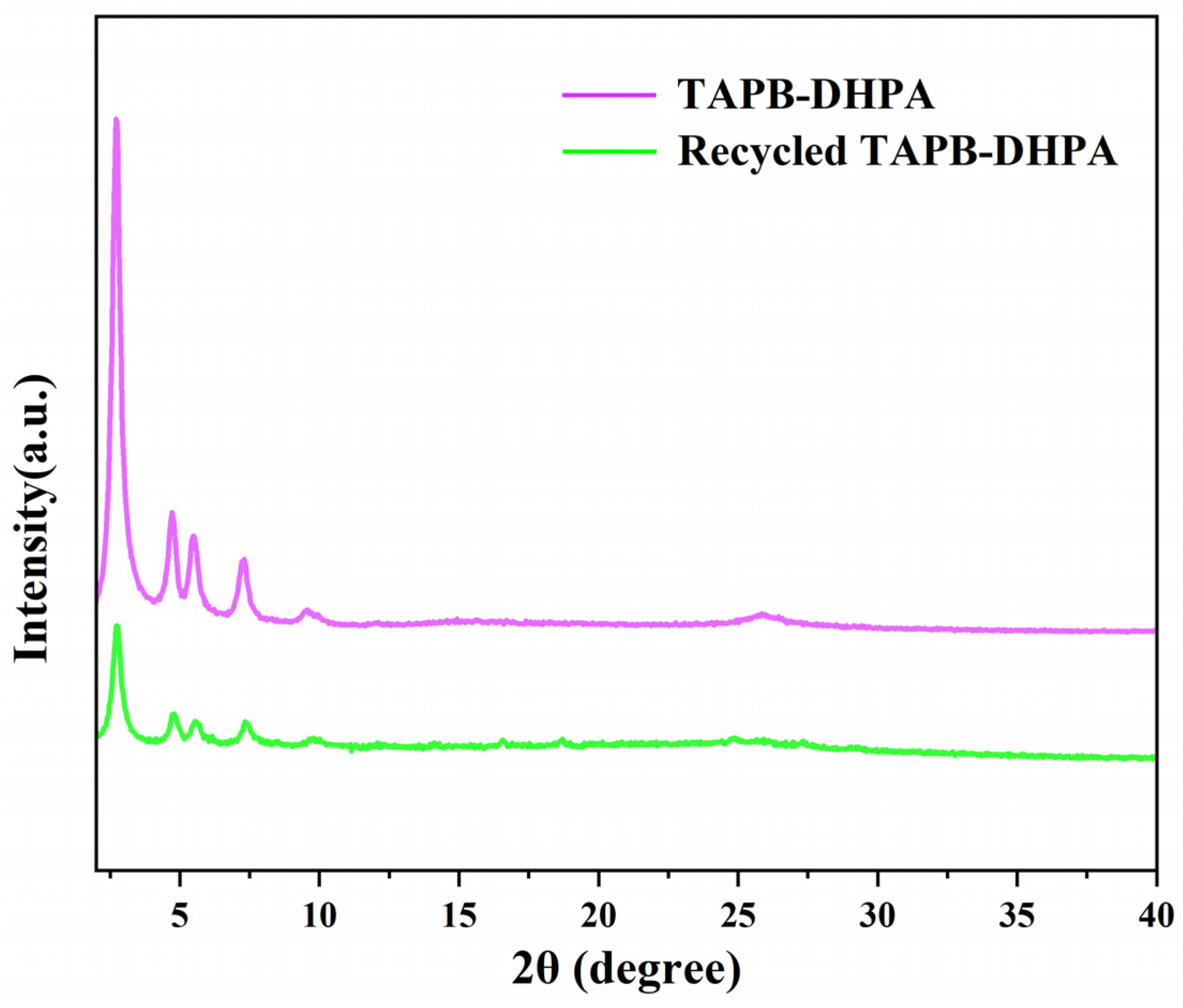
| Sample | Moisture Content (%) | Purity (%) | Mw (g/mol) | Mn (g/mol) | PDI |
|---|---|---|---|---|---|
| XOS | 0.44 | 96.7 | 306 | 286 | 1.07 |
| Synthesized COF | BET (m2/g) | Pore Sizes (nm) | TA (mmol/g) | T (°C) |
|---|---|---|---|---|
| TAPB-DHPA | 938 | 2.40 | 1.27 | 400 |
| Catalyst | Catalyst Dosage | Reaction | Yield | Ref. |
|---|---|---|---|---|
| TAPB-DHPA | 0.16 wt% | 180 °C, 180 min | 65.4% | This work |
| TP-DAB | 0.33 wt% | 160 °C, 180 min | 63.5% | [14] |
| TAPT-DHPA-COF | 0.33 wt% | 200 °C, 90 min | 72.2% | [13] |
| WO3/SiO2 | 10 wt% | 170 °C, 10 h | 71.0% | [35] |
| PR/C-SO3H-Fe | 0.71 wt% | 160 °C, 180 min | 79.0% | [36] |
| Cr3+/P-SBA-15 | 40 wt% | 180 °C, 120 min | 58.0% | [37] |
| SCh | 0.50 wt% | 190 °C, 80 min | 42.0% | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Xia, H.; Cheng, G.; Gan, P.; Ju, Y.; Guo, B.; Yang, J.; Qiao, C.; Lin, J.; Chen, J. Catalytic Conversion of Xylo-Oligomers to Furfural in Pulping Pre-Hydrolysis Liquor Using a Hydroxyl-Functionalized Covalent Organic Framework. Polymers 2025, 17, 1102. https://doi.org/10.3390/polym17081102
Zhang K, Xia H, Cheng G, Gan P, Ju Y, Guo B, Yang J, Qiao C, Lin J, Chen J. Catalytic Conversion of Xylo-Oligomers to Furfural in Pulping Pre-Hydrolysis Liquor Using a Hydroxyl-Functionalized Covalent Organic Framework. Polymers. 2025; 17(8):1102. https://doi.org/10.3390/polym17081102
Chicago/Turabian StyleZhang, Kai, Huanmei Xia, Guangyao Cheng, Peng Gan, Yuan Ju, Baozhen Guo, Jingli Yang, Chengcheng Qiao, Jixiang Lin, and Jiachuan Chen. 2025. "Catalytic Conversion of Xylo-Oligomers to Furfural in Pulping Pre-Hydrolysis Liquor Using a Hydroxyl-Functionalized Covalent Organic Framework" Polymers 17, no. 8: 1102. https://doi.org/10.3390/polym17081102
APA StyleZhang, K., Xia, H., Cheng, G., Gan, P., Ju, Y., Guo, B., Yang, J., Qiao, C., Lin, J., & Chen, J. (2025). Catalytic Conversion of Xylo-Oligomers to Furfural in Pulping Pre-Hydrolysis Liquor Using a Hydroxyl-Functionalized Covalent Organic Framework. Polymers, 17(8), 1102. https://doi.org/10.3390/polym17081102






