Enhancing the Adhesion of Polyaniline on Steel Substrates Without a Binding Agent: Evaluated by ASTM D 3359 Tape Test and Sodium Chloride (NaCl) Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyaniline Synthesis
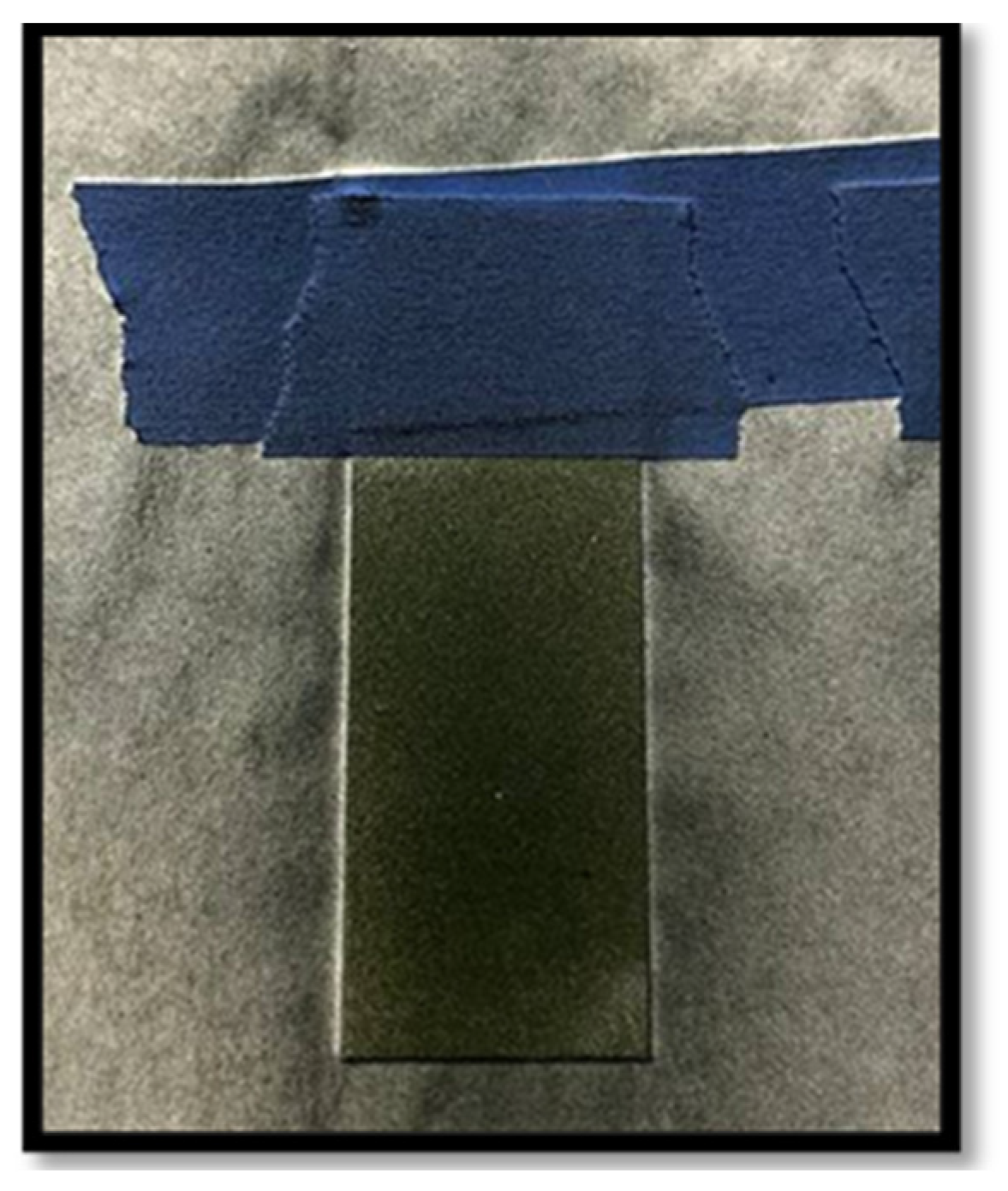
2.3. Pretreatment Surface on Substrate Approach
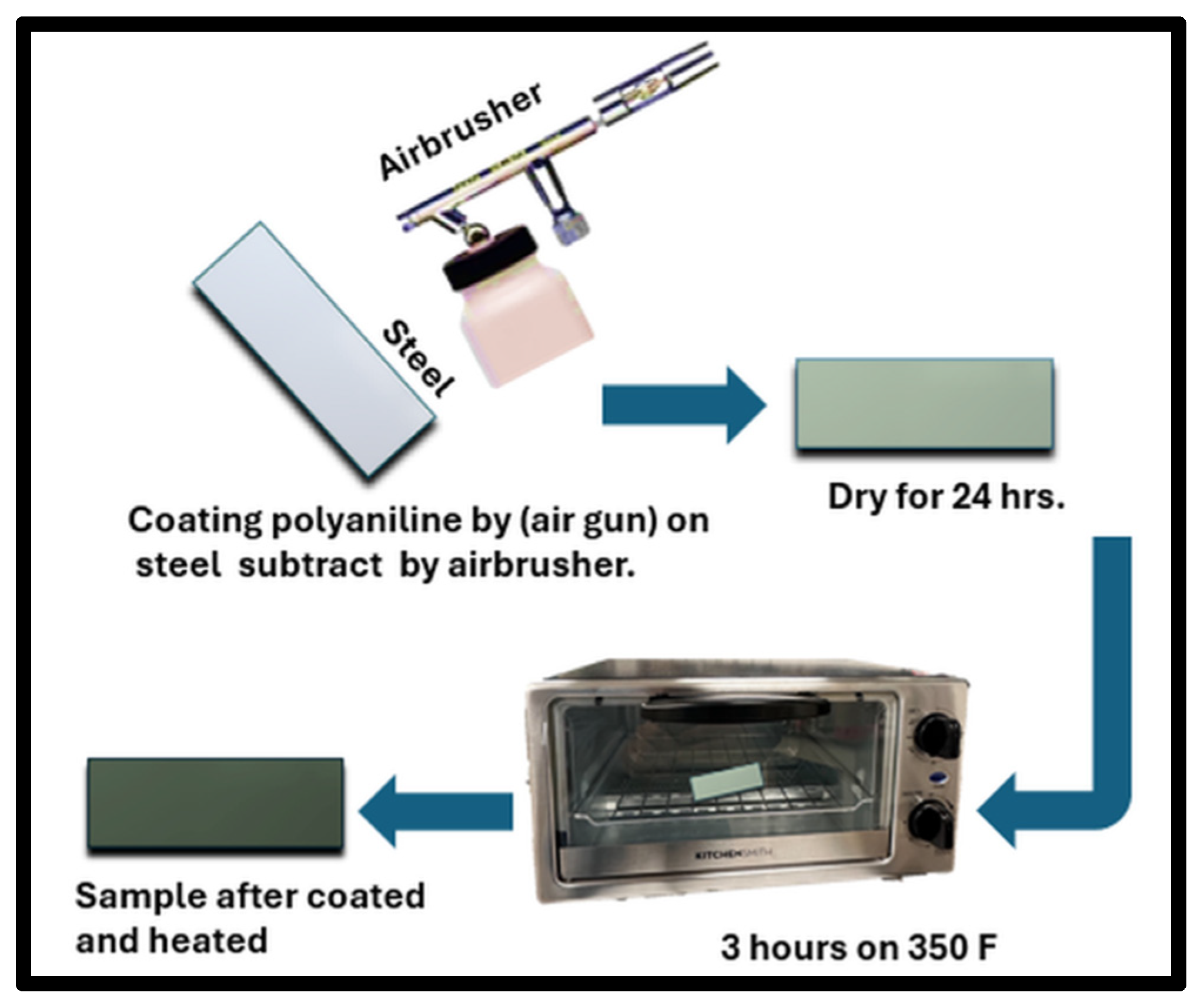
2.4. Coating Process (Air Spray)
2.5. Fabrication (Heat Treatment)
3. Testing
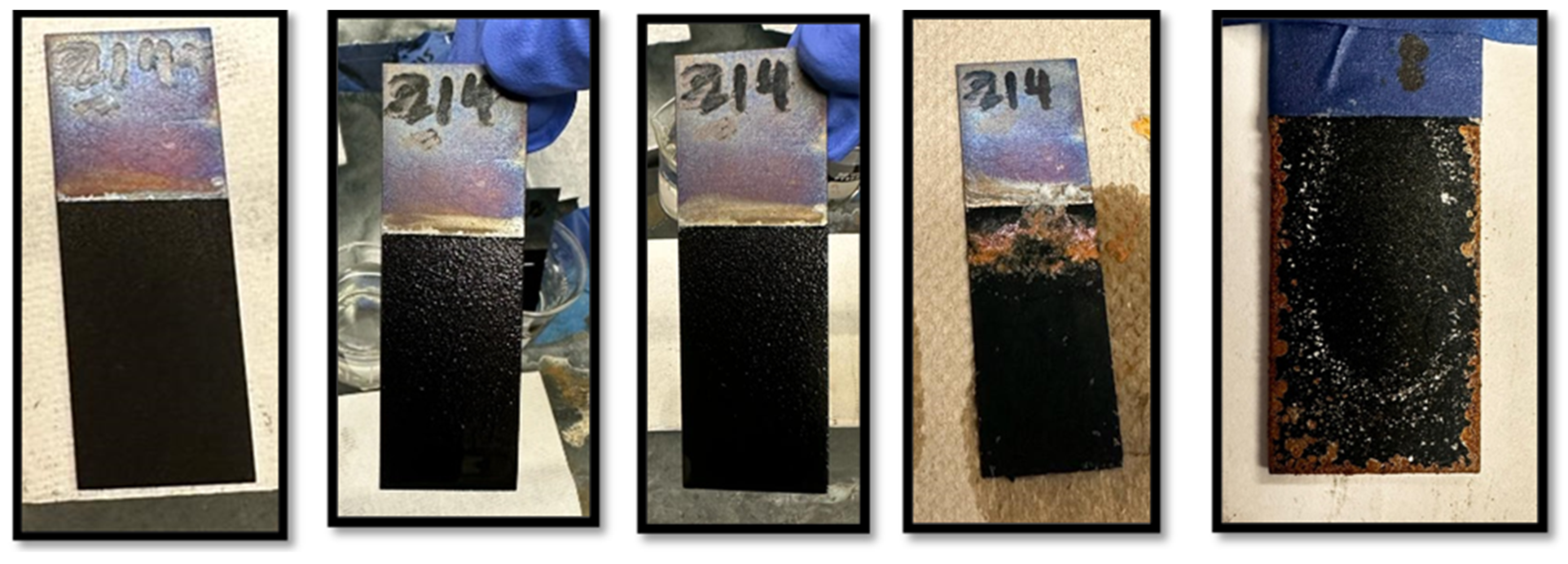
3.1. NaCl Adhesion Test of Polyaniline-Coated Steel Samples
3.2. Post-Cleaning Surface Comparison (Day 100)
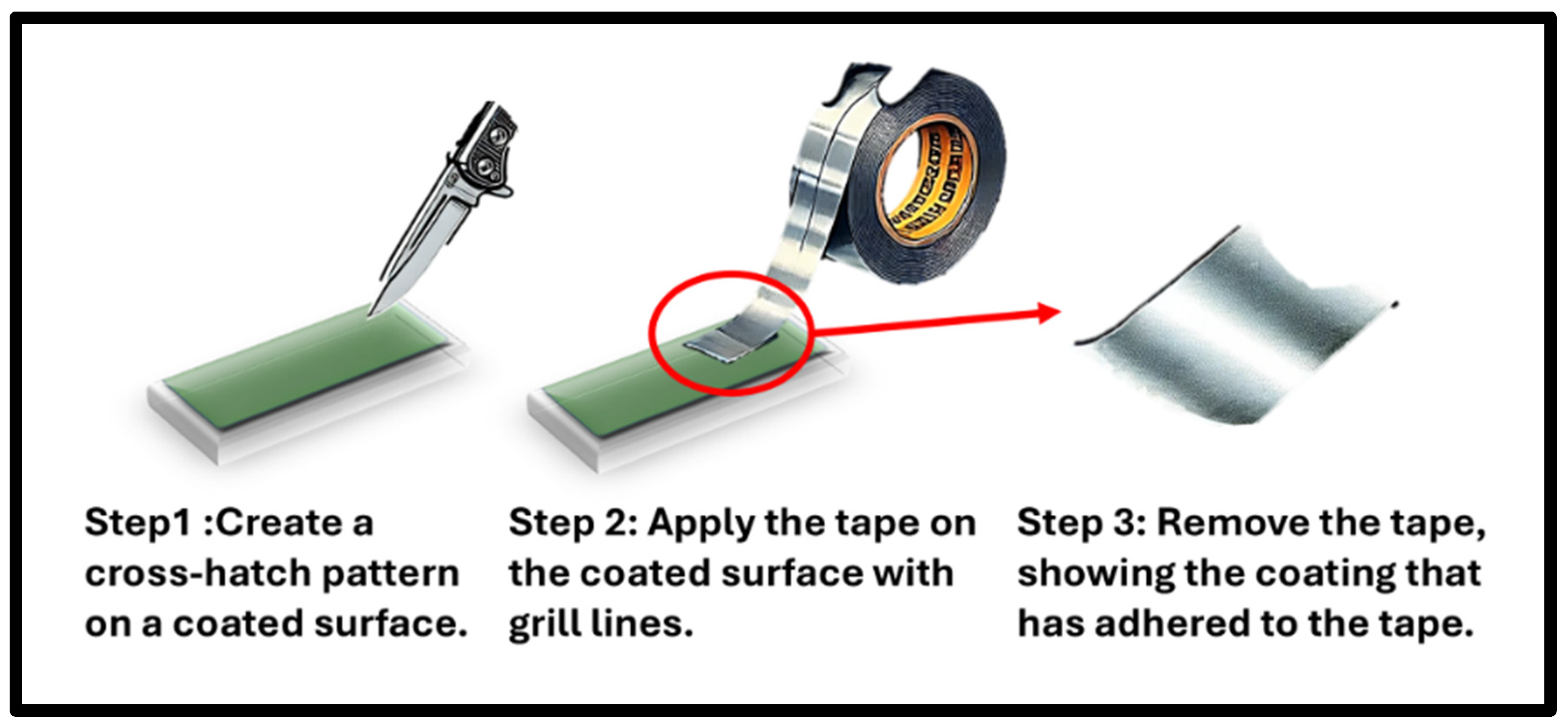
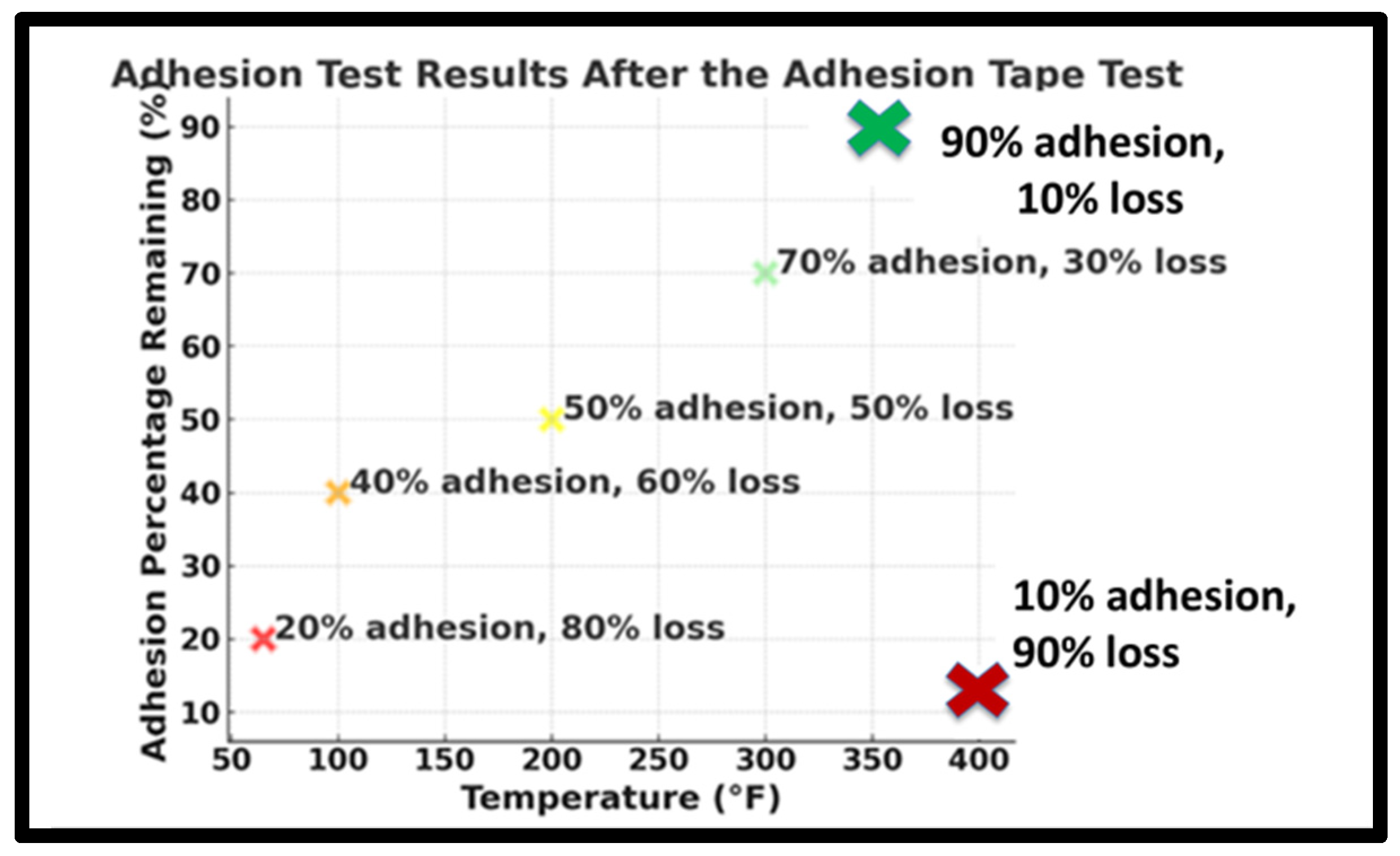
3.3. Adhesion Test by ASTM D 3359 Adhesive Tape
4. Results and Discussion
4.1. Post-Cleaning Surface Comparison (Day 100)
4.2. Adhesion Test Analysis Using ASTM D 3359 Tape
4.3. The Heat Treatment Temperature Analysis
4.4. Scanning Electron Microscope (SEM)
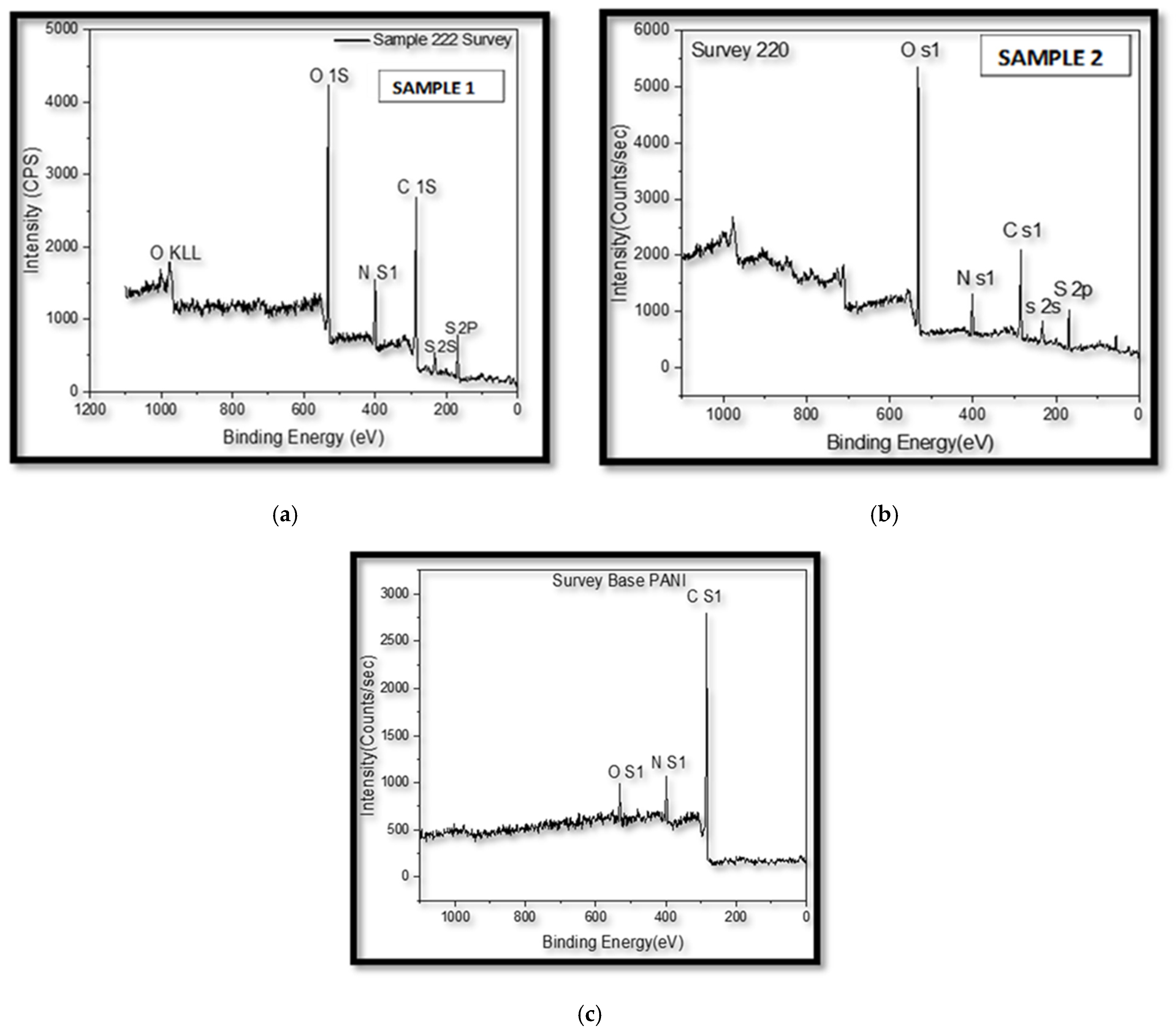
4.5. X-Ray Photoelectron Spectroscopy (XPS)
4.6. X-Ray Diffraction (XRD) Analysis
5. Correlation of Crystallinity and Surface Chemistry with Adhesion Performance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; Macdiarmid, A.G.; Chiang, C.K.; Heegert, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Macdiarmid, A.G.; Yang, L.S.; Huang, W.S.; Humphrey, B.D. Polyaniline: Electrochemistry and application to rechargeable batteries. Synth. Met. 1987, 18, 393–398. [Google Scholar] [CrossRef]
- Chiang, J.-C.; Macdiarmid, A.G. ‘Polyaniline’: Protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Wiley. Frontmatter. In Uhlig’s Corrosion Handbook; Wiley: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Costs and Preventive Strategies in the United States; Report No. FHWA-RD-01-156; U.S. Federal Highway Administration: Washington, DC, USA, 2002.
- Zhang, Y.; Shao, Y.; Liu, X.; Shi, C.; Wang, Y.; Meng, G.; Zeng, X.; Yang, Y. A study on corrosion protection of different polyaniline coatings for mild steel. Prog. Org. Coat. 2017, 111, 240–247. [Google Scholar] [CrossRef]
- Munger, C.G.; Vincent, L.D. Corrosion Prevention by Protective Coatings, 3rd ed.; NACE International: Houston, TX, USA, 2014. [Google Scholar]
- Shah, S.S.; Oladepo, S.; Ehsan, M.A.; Iali, W.; Alenaizan, A.; Siddiqui, M.N.; Oyama, M.; Al-Betar, A.-R.; Aziz, M.A. Recent Progress in Polyaniline and its Composites for Supercapacitors. Chem. Rec. 2024, 24, e202300105. [Google Scholar] [CrossRef] [PubMed]
- Arshak’, A.; Gill, E.I.; Arshak, K.; Korostynska, O.; Cunniffe, C. Drop-Coated Polyaniline Composite Conductimetric pH Sensors. In Proceedings of the 2007 30th International Spring Seminar on Electronics Technology (ISSE), Cluj-Napoca, Romania, 9–13 May 2007. [Google Scholar]
- Pour-Ali, S.; Dehghanian, C.; Kosari, A. In situ synthesis of polyaniline-camphorsulfonate particles in an epoxy matrix for corrosion protection of mild steel in NaCl solution. Corros. Sci. 2014, 85, 204–214. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Sonawane, N.; Siju, C.R. Epoxy powder coatings containing polyaniline for enhanced corrosion protection. Prog. Org. Coat. 2009, 64, 383–386. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Yao, H.; Di, Y.; Shu, M.; Li, J.; Zhang, B.; Cao, G.; Guan, S. Positive effect of the addition of polyaniline on the anticorrosive property of polyethersulfone two-layer composite coating. J. Appl. Polym. Sci. 2021, 138, 50758. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.H.; Li, J.; Lu, J.L.; Wang, F.S. Polyaniline for corrosion prevention of mild steel coupled with copper. Electrochim. Acta 2007, 52, 5392–5399. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Smirnov, A.A.; Silkin, S.A.; Parfenyuk, V.I.; Belkin, P.N. Influence of mechanical alloying on the microstructure and properties of coatings. Surf. Coat. Technol. 2016, 307, 1291–1296. [Google Scholar] [CrossRef]
- Belkin, P.N.; Kusmanov, S.A.; Dyakov, I.G.; Komissarova, M.R.; Parfenyuk, V.I. Effects of plasma electrolytic polishing on surface properties of titanium alloys after chemical-thermal treatment. Surf. Coat. Technol. 2016, 307, 1303–1309. [Google Scholar] [CrossRef]
- Choudhary, H.K.; Pawar, S.P.; Kumar, R.; V, A.A.; Bose, S.; Sahoo, B. Supporting Information Mechanistic Insight into the Critical Concentration of Barium Hexaferrite and the Conductive Polymeric Phase with Respect to Synergistically Electromagnetic Interference (EMI) Shielding. ChemistrySelect 2017, 2, 830–841. [Google Scholar] [CrossRef]
- Bagherzadeh, M.R.; Mahdavi, F.; Ghasemi, M.; Shariatpanahi, H.; Faridi, H.R. Using nanoemeraldine salt-polyaniline for preparation of a new anticorrosive water-based epoxy coating. Prog. Org. Coat. 2010, 68, 319–322. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Preparation of polyaniline-TiO2 composite and its comparative corrosion protection performance with polyaniline. Synth. Met. 2007, 157, 205–213. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, X.N.; Wu, C.-Y.; Chen, M.-T.; Hsieh, H.-H.; Tran, H.D.; Huang, S.-C.; Kaner, R.B. Processable colloidal dispersions of polyaniline-based copolymers for transparent electrodes. Polym. Chem. 2013, 4, 4814–4820. [Google Scholar] [CrossRef]
- Mcknight, M.E.; Seiler, J.F.; Nguyen, T.; Rossiter, W.J. Measuring Peel Adhesion of Coatings A. J. Prot. Coat. Linings 1995, 12, 82. [Google Scholar]
- Considine, T.A.; Braswell, T.E.; Labukas, J.P. Characterization of Tape Adhesion to Chemical Agent Resistant Coatings; US Army Research Laboratory: Adelphi, MD, USA, 2015.
- Magdaleno-López, C.; de Jesús Pérez-Bueno, J. Quantitative evaluation for the ASTM D4541-17/D7234 and ASTM D3359 adhesion norms with digital optical microscopy for surface modifications with flame and APPJ. Int. J. Adhes. Adhes. 2020, 98, 102551. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Cui, X.; Wang, H. The use of Photoshop software to estimate the adhesion and rust-resistant properties of coating film. Surf. Interface Anal. 2011, 43, 913–917. [Google Scholar] [CrossRef]
- ASTM D 3359; Standard Test Methods for Measuring Adhesion by Tape Test 1. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Smith, W.F.; Hashemi, J.; Presuel-Moreno, F. Foundations of Materials Science and Engineering; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Lee, D.; Char, K. Thermal Degradation Behavior of Polyaniline in Polyaniline/Na+-Montmorillonite Nanocomposites. Available online: www.elsevier.com/locate/polydegstab (accessed on 15 October 2023).
- Silva, A.L.C.; Ugucioni, J.C.; Correa, S.; Ardisson, J.D.; Macedo, W.A.A.; Silva, J.P.; Cotta, A.A.C.; Brito, A.D.B. Synthesis and characterization of nanocomposites consisting of polyaniline, chitosan and tin dioxide. Mater. Chem. Phys. 2018, 216, 402–412. [Google Scholar] [CrossRef]
- Vadiraj, K.T.; Belagali, S.L. Characterization of Polyaniline for Optical and Electrical Properties. IOSR J. Appl. Chem. 2015, 8, 53–56. [Google Scholar] [CrossRef]











| Sample Number | Before ASTM Test | After ASTM Test | Tape Result |
|---|---|---|---|
Sample 1:
|  |  |  |
Sample 2:
|  |  |  |
| Classification of Adhesion Test Results | ||
|---|---|---|
| Classification | Percent Area Removed | Surface of Cross-Cut Area from Which Flaking Has Occurred for Six Parallel Cuts and Adhesion Range by Percent |
| 5B | None (0%) |  |
| 4B | Less than 5% |  |
| 3B | 5–15% | 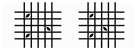 |
| 2B | 15–35% | 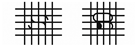 |
| 1B | 35–65% |  |
| 0B | Greater than 65% |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldwais, S.; Al-Muntaser, A.A.; Chen, C.; Robles, J.; Pal, A.; Abiade, J.T. Enhancing the Adhesion of Polyaniline on Steel Substrates Without a Binding Agent: Evaluated by ASTM D 3359 Tape Test and Sodium Chloride (NaCl) Exposure. Polymers 2025, 17, 1082. https://doi.org/10.3390/polym17081082
Aldwais S, Al-Muntaser AA, Chen C, Robles J, Pal A, Abiade JT. Enhancing the Adhesion of Polyaniline on Steel Substrates Without a Binding Agent: Evaluated by ASTM D 3359 Tape Test and Sodium Chloride (NaCl) Exposure. Polymers. 2025; 17(8):1082. https://doi.org/10.3390/polym17081082
Chicago/Turabian StyleAldwais, Saleh, Ali A. Al-Muntaser, Chen Chen, Jaqueline Robles, Anish Pal, and Jeremiah T. Abiade. 2025. "Enhancing the Adhesion of Polyaniline on Steel Substrates Without a Binding Agent: Evaluated by ASTM D 3359 Tape Test and Sodium Chloride (NaCl) Exposure" Polymers 17, no. 8: 1082. https://doi.org/10.3390/polym17081082
APA StyleAldwais, S., Al-Muntaser, A. A., Chen, C., Robles, J., Pal, A., & Abiade, J. T. (2025). Enhancing the Adhesion of Polyaniline on Steel Substrates Without a Binding Agent: Evaluated by ASTM D 3359 Tape Test and Sodium Chloride (NaCl) Exposure. Polymers, 17(8), 1082. https://doi.org/10.3390/polym17081082









