Enhanced Xylan/PVA Composite Films via Nano-ZnO Reinforcement for Sustainable Food Packaging
Abstract
1. Introduction
2. Materials and Research Procedures
2.1. Materials
2.2. Preparation of Nano-ZnO-Modified Xylan/PVA Composite Film
2.3. Analytical Methods
2.3.1. FTIR Analysis
2.3.2. SEM Analysis
2.3.3. Mechanical Properties Testing
2.3.4. Thermal Stability Testing
2.3.5. Solubility and Swelling Degree
2.3.6. Water Vapor Permeability Testing
2.3.7. Oxygen Barrier Properties
2.3.8. Contact Angle
2.3.9. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of Hemicellulose-Based Composite Films Incorporating Nano-ZnO
3.2. Thermal Stability
3.3. Structural and Morphological Analysis
3.4. Surface Morphology Analysis
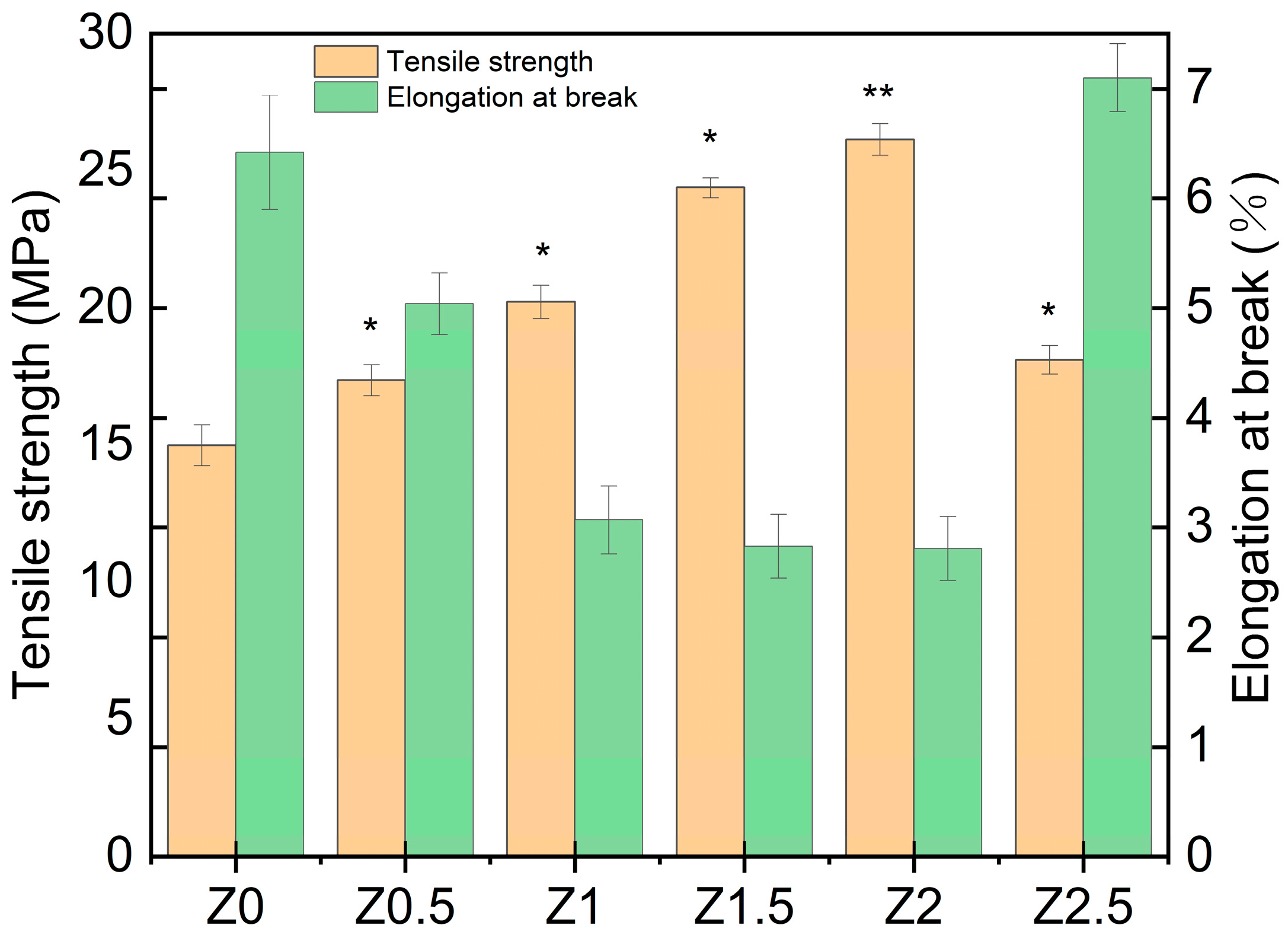
3.5. Mechanical Properties
3.6. Oxygen Permeability
3.7. Water Vapor Permeability
3.8. Surface Wettability
3.9. Swelling Degree and Solubility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, J.; Han, B. Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and Disadvantages of Bioplastics Production from Starch and Lignocellulosic Components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Macedo, J.V.C.; Martins, J.R.; Abe, M.M.; Branciforti, M.C.; Brienzo, M. Hemicellulose Application for the Production of Bioplastics and Biomaterials. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Brienzo, M., Ed.; Springer Nature: Singapore, 2022; pp. 231–273. ISBN 978-981-16-3682-0. [Google Scholar]
- Ebringerová, A.; Heinze, T. Xylan and Xylan Derivatives—Biopolymers with Valuable Properties, 1. Naturally Occurring Xylans Structures, Isolation Procedures and Properties. Macromol. Rapid Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial Packaging Efficiency of ZnO-SiO2 Nanocomposites Infused into PVA/CS Film for Enhancing the Shelf Life of Food Products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Chen, M.; Wei, Y.; Liu, C. Functional Packaging Films Originating from Hemicelluloses Laurate by Direct Transesterification in Ionic Liquid. Carbohydr. Polym. 2020, 229, 115336. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Zhang, M. Hemicellulose and Unlocking Potential for Sustainable Applications in Biomedical, Packaging, and Material Sciences: A Narrative Review. Int. J. Biol. Macromol. 2024, 280, 135657. [Google Scholar] [CrossRef]
- Hussain, S.A.; Yadav, M.P.; Sharma, B.K.; Qi, P.X.; Jin, T.Z. Biodegradable Food Packaging Films Using a Combination of Hemicellulose and Cellulose Derivatives. Polymers 2024, 16, 3171. [Google Scholar] [CrossRef]
- Le, T.-A.; Huynh, T.-P. Hemicellulose-Based Sensors: When Sustainability Meets Complexity. ACS Sens. 2024, 9, 4975–5001. [Google Scholar] [CrossRef] [PubMed]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef]
- Ren, J.L.; Sun, R.C.; Liu, C.F.; Chao, Z.Y.; Luo, W. Two-Step Preparation and Thermal Characterization of Cationic 2-Hydroxypropyltrimethylammonium Chloride Hemicellulose Polymers from Sugarcane Bagasse. Polym. Degrad. Stab. 2006, 91, 2579–2587. [Google Scholar] [CrossRef]
- Peng, X.-W.; Ren, J.-L.; Zhong, L.-X.; Cao, X.-F.; Sun, R.-C. Microwave-Induced Synthesis of Carboxymethyl Hemicelluloses and Their Rheological Properties. J. Agric. Food Chem. 2011, 59, 570–576. [Google Scholar] [CrossRef]
- Escalante, A.; Gonçalves, A.; Bodin, A.; Stepan, A.; Sandström, C.; Toriz, G.; Gatenholm, P. Flexible Oxygen Barrier Films from Spruce Xylan. Carbohydr. Polym. 2012, 87, 2381–2387. [Google Scholar] [CrossRef]
- Guan, Y.; Qi, X.-M.; Chen, G.-G.; Peng, F.; Sun, R.-C. Facile Approach to Prepare Drug-Loading Film from Hemicelluloses and Chitosan. Carbohydr. Polym. 2016, 153, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of Polyvinyl Alcohol/Xylan Composite Films with Citric Acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Belmokaddem, F.-Z.; Pinel, C.; Huber, P.; Petit-Conil, M.; Da Silva Perez, D. Green Synthesis of Xylan Hemicellulose Esters. Carbohydr. Res. 2011, 346, 2896–2904. [Google Scholar] [CrossRef]
- Carson, J.F.; Maclay, W.D. Esters of Lima Bean Pod and Corn Cob Hemicelluloses. J. Am. Chem. Soc. 1948, 70, 293–295. [Google Scholar] [CrossRef]
- Shibuya, N.; Iwasaki, T. Structural Features of Rice Bran Hemicellulose. Phytochemistry 1985, 24, 285–289. [Google Scholar] [CrossRef]
- Church, J.A. A Redox-Initiated Xylan–Poly(Sodium Acrylate) Graft Copolymer. J. Polym. Sci. Part A-1 Polym. Chem. 1967, 5, 3183–3192. [Google Scholar] [CrossRef]
- Li, N.; Sun, D.; Su, Z.; Hao, X.; Li, M.; Ren, J.; Peng, F. Rapid Fabrication of Xylan-Based Hydrogel by Graft Polymerization via a Dynamic Lignin-Fe3+ Plant Catechol System. Carbohydr. Polym. 2021, 269, 118306. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased Plastics and Bionanocomposites: Current Status and Future Opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Petzold, K.; Günther, W.; Kötteritzsch, M.; Heinze, T. Synthesis and Characterization of Methyl Xylan. Carbohydr. Polym. 2008, 74, 327–332. [Google Scholar] [CrossRef]
- Daus, S.; Petzold-Welcke, K.; Kötteritzsch, M.; Baumgaertel, A.; Schubert, U.S.; Heinze, T. Homogeneous Sulfation of Xylan from Different Sources. Macromol. Mater. Eng. 2011, 296, 551–561. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, W.; Xiao, N.; Chen, M.; Liu, C. Construction of Functional Composite Films Originating from Hemicellulose Reinforced with Poly(Vinyl Alcohol) and Nano-ZnO. Cellulose 2020, 27, 1341–1355. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, W.; Wang, B.; Kang, X.; Liu, P.; Cui, B.; Abd El-Aty, A.M. Preparation and Evaluation of Starch-Based Extrusion-Blown Nanocomposite Films Incorporated with Nano-ZnO and Nano-SiO2. Int. J. Biol. Macromol. 2021, 183, 1371–1378. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; López, D.; López, J.; Kenny, J.M. 12—An Overview of Nanoparticles Role in the Improvement of Barrier Properties of Bioplastics for Food Packaging Applications. In Food Packaging; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 391–424. ISBN 978-0-12-804302-8. [Google Scholar]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of Nano-ZnO and Nano-SiO2 Particles on Properties of PVA/Xylan Composite Films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of Nanomaterial Aging and Transformations through the Life Cycle of Nano-Enhanced Products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Teófilo, R.F.; Coimbra, J.S.d.R.; Vitor, D.M.; Batista, R.A.; Ferreira, S.O.; de Andrade, N.J.; Medeiros, E.A.A. Physical–Mechanical and Antimicrobial Properties of Nanocomposite Films with Pediocin and ZnO Nanoparticles. Carbohydr. Polym. 2013, 94, 199–208. [Google Scholar] [CrossRef]
- Liu, G.; Shi, K.; Sun, H.; Yang, B.; Weng, Y. Nano-SiO2-Modified Xylan-PVOH-Based Composite Films: Mechanical and Barrier Properties Investigation. BioResources 2023, 18, 4195–4211. [Google Scholar] [CrossRef]
- Dejene, B.K. Advancing Natural Fiber-Reinforced Composites Through Incorporating ZnO Nanofillers in the Polymeric Matrix: A Review. J. Nat. Fibers 2024, 21, 2356015. [Google Scholar] [CrossRef]
- TiO2-KH550 Nanoparticle-Reinforced PVA/Xylan Composite Films with Multifunctional Properties. Available online: https://www.mdpi.com/1996-1944/11/9/1589 (accessed on 2 March 2025).
- Kherfi, A.; Madani, A.; Chalal, D.; Benidir, S. Improvement of the Protective Properties of the Nanocomposite Polypyrrole-Zinc Oxide Coating on Mild Steel by Adding the Dispersant Sodium Hexametaphosphate. Synth. Met. 2021, 277, 116795. [Google Scholar] [CrossRef]
- GB/T 1040.2-2006; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. Standards Press of China: Beijing, China, 2006.
- Kavoosi, G.; Dadfar, S.M.M.; Dadfar, S.M.A.; Ahmadi, F.; Niakosari, M. Investigation of Gelatin/Multi-Walled Carbon Nanotube Nanocomposite Films as Packaging Materials. Food Sci. Nutr. 2014, 2, 65–73. [Google Scholar] [CrossRef]
- GB/T 1037-2021; Plastics—Film and Sheeting—Determination of Water Vapor Transmission Rate. Standards Press of China: Beijing, China, 2021.
- Sztuka, K.; Kołodziejska, I. The Influence of Hydrophobic Substances on Water Vapor Permeability of Fish Gelatin Films Modified with Transglutaminase or 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide (EDC). Food Hydrocoll. 2009, 23, 1062–1064. [Google Scholar] [CrossRef]
- Gao, C.; Ren, J.; Wang, S.; Sun, R.; Zhao, L. Preparation of Polyvinyl Alcohol/Xylan Blending Films with 1,2,3,4-Butane Tetracarboxylic Acid as a New Plasticizer. J. Nanomater. 2014, 2014, 764031. [Google Scholar] [CrossRef]
- Ren, J.; Wang, S.; Gao, C.; Chen, X.; Li, W.; Peng, F. TiO2-Containing PVA/Xylan Composite Films with Enhanced Mechanical Properties, High Hydrophobicity and UV Shielding Performance. Cellulose 2015, 22, 593–602. [Google Scholar] [CrossRef]
- Park, S.; Choi, G.R.; Kim, J.H.; Lee, J.C.; Kim, S.-J. Dispersion Effect of Sodium Hexametaphosphate on the Photocatalytic Efficiency of a Solution-Combusted ZnO Nanopowder. J. Korean Phys. Soc. 2012, 61, 1400–1403. [Google Scholar] [CrossRef]
- Saadatmand, S.; Edlund, U.; Albertsson, A.-C.; Danielsson, S.; Dahlman, O.; Karlström, K. Turning Hardwood Dissolving Pulp Polysaccharide Residual Material into Barrier Packaging. Biomacromolecules 2013, 14, 2929–2936. [Google Scholar] [CrossRef]
- Svagan, A.J.; Åkesson, A.; Cárdenas, M.; Bulut, S.; Knudsen, J.C.; Risbo, J.; Plackett, D. Transparent Films Based on PLA and Montmorillonite with Tunable Oxygen Barrier Properties. Biomacromolecules 2012, 13, 397–405. [Google Scholar] [CrossRef]
- Hao, Y.; Cheng, L.; Song, X.; Gao, Q. Functional Properties and Characterization of Maize Starch Films Blended with Chitosan. J. Thermoplast. Compos. Mater. 2023, 36, 4977–4996. [Google Scholar] [CrossRef]




| Samples | Tmax (°C) | 600 °C Carbon Residual Rate (%) | ||
|---|---|---|---|---|
| Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | ||
| Z0 | 105.35 | 308.33 | 420.34 | 15.20 |
| Z0.5 | 107.93 | 309.56 | 427.02 | 19.12 |
| Z1 | 109.32 | 312.32 | 423.35 | 18.65 |
| Z1.5 | 109.91 | 309.021 | 424.45 | 21.51 |
| Z2 | 115.72 | 310.59 | 430.71 | 20.55 |
| Z2.5 | 118.00 | 309.58 | 433.78 | 20.34 |
| Samples | Oxygen Permeability [(cm3·μm)/(m2·d·kPa)] |
|---|---|
| Z0 | 1.83 ± 0.09 |
| Z0.5 | 1.00 ± 0.03 * |
| Z1 | 0.80 ± 0.07 * |
| Z1.5 | 0.67 ± 0.05 * |
| Z2 | 0.50 ± 0.06 * |
| Z2.5 | 0.75 ± 0.07 * |
| Samples | WVP (10−6·g·h−1∙m−1∙Pa−1) |
|---|---|
| Z0 | 1.88 ± 0.05 |
| Z0.5 | 1.80 ± 0.20 |
| Z1 | 1.63 ± 0.14 |
| Z1.5 | 1.60 ± 0.11 |
| Z2 | 1.53 ± 0.17 * |
| Z2.5 | 1.87 ± 0.13 |
| Samples | Solubility (%) | Swelling Degree (%) |
|---|---|---|
| Z0 | 63.94 ± 2.07 | 219.74 ± 5.98 |
| Z0.5 | 58.61 ± 4.09 | 213.52 ± 14.69 |
| Z1 | 53.89 ± 2.86 | 211.03 ± 2.77 |
| Z1.5 | 53.29 ± 5.79 | 207.46 ± 12.94 |
| Z2 | 47.43 ± 5.59 * | 156.25 ± 10.17 * |
| Z2.5 | 53.74 ± 2.58 * | 158.90 ± 6.27 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Sun, H.; Yu, C.; Weng, Y. Enhanced Xylan/PVA Composite Films via Nano-ZnO Reinforcement for Sustainable Food Packaging. Polymers 2025, 17, 1080. https://doi.org/10.3390/polym17081080
Yao L, Sun H, Yu C, Weng Y. Enhanced Xylan/PVA Composite Films via Nano-ZnO Reinforcement for Sustainable Food Packaging. Polymers. 2025; 17(8):1080. https://doi.org/10.3390/polym17081080
Chicago/Turabian StyleYao, Lin, Hui Sun, Chang Yu, and Yunxuan Weng. 2025. "Enhanced Xylan/PVA Composite Films via Nano-ZnO Reinforcement for Sustainable Food Packaging" Polymers 17, no. 8: 1080. https://doi.org/10.3390/polym17081080
APA StyleYao, L., Sun, H., Yu, C., & Weng, Y. (2025). Enhanced Xylan/PVA Composite Films via Nano-ZnO Reinforcement for Sustainable Food Packaging. Polymers, 17(8), 1080. https://doi.org/10.3390/polym17081080







