The Effect of Processing Methods and Nucleating Agents on the Wear Resistance and Crystallinity Behavior of Nylon 11
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Processing Methods
2.3. The Nucleating Methods
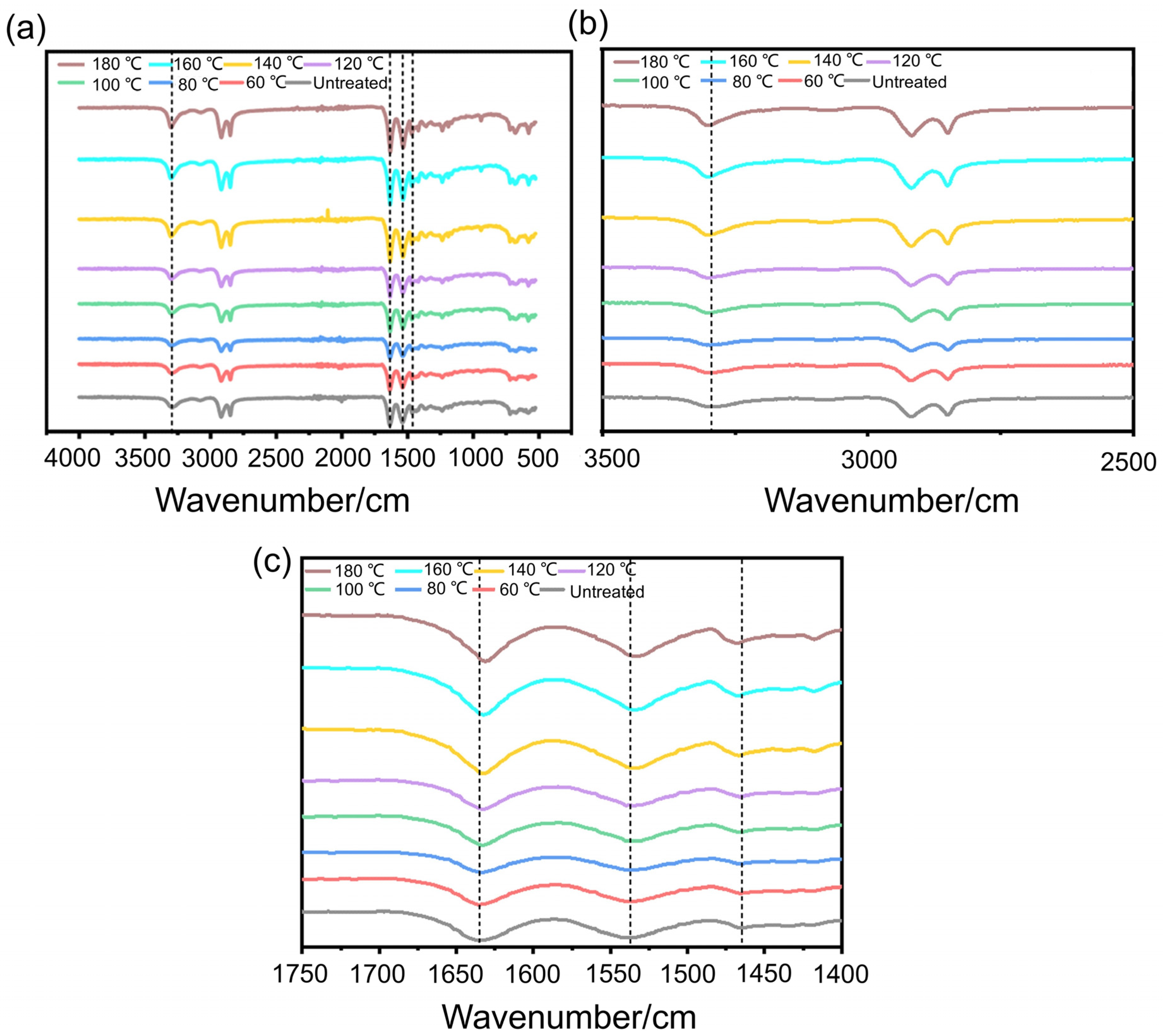
2.4. Characterization and Measurements
3. Results and Discussion
3.1. The Effect of the Pre-Treatment Temperature on the Crystallinity and Wear Resistance
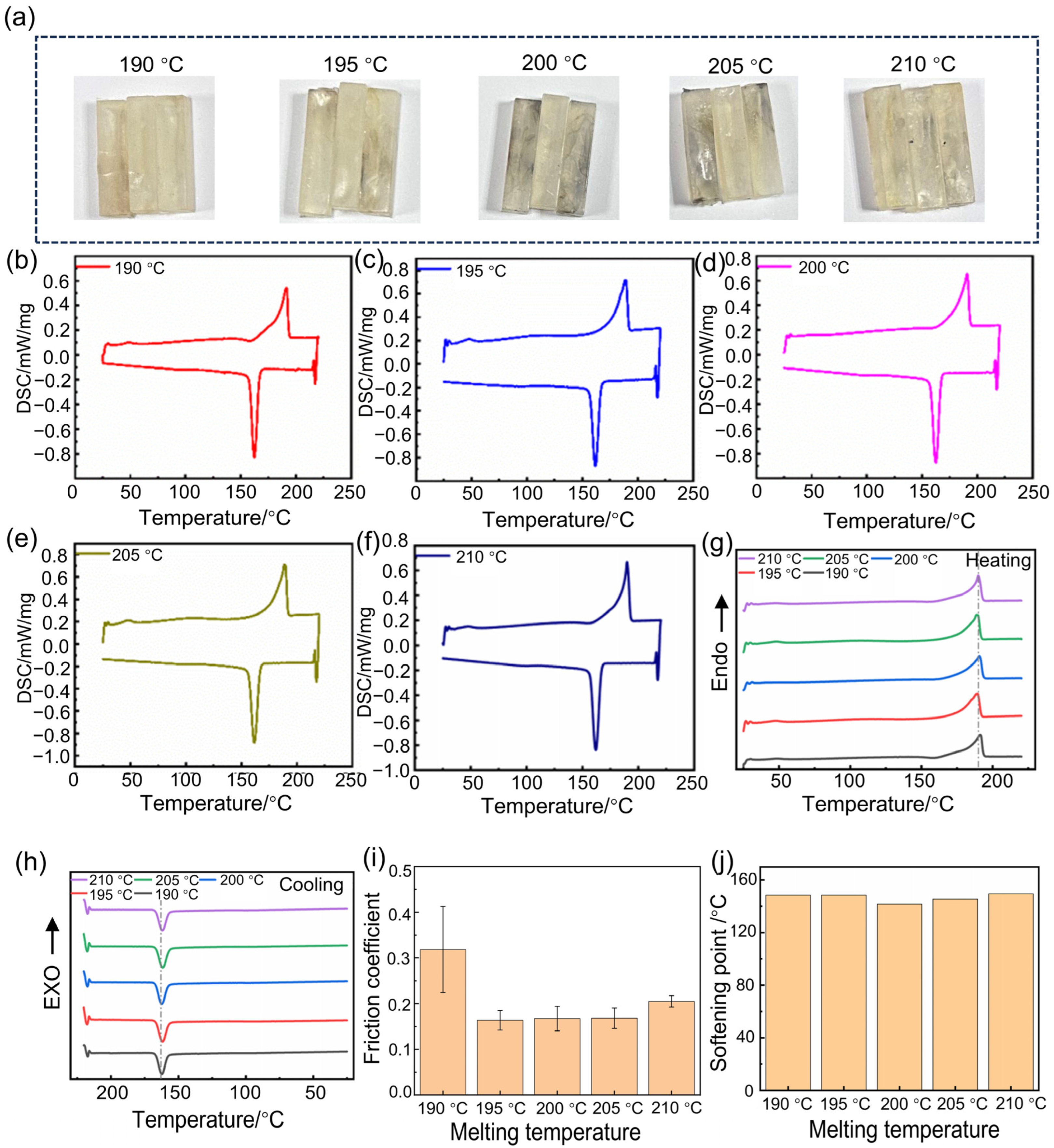
3.2. The Effect of the Different Melting Temperatures on the Crystallinity and Wear Resistance
3.3. The Effect of the Different Heating Programs on the Crystallinity and Wear Resistance
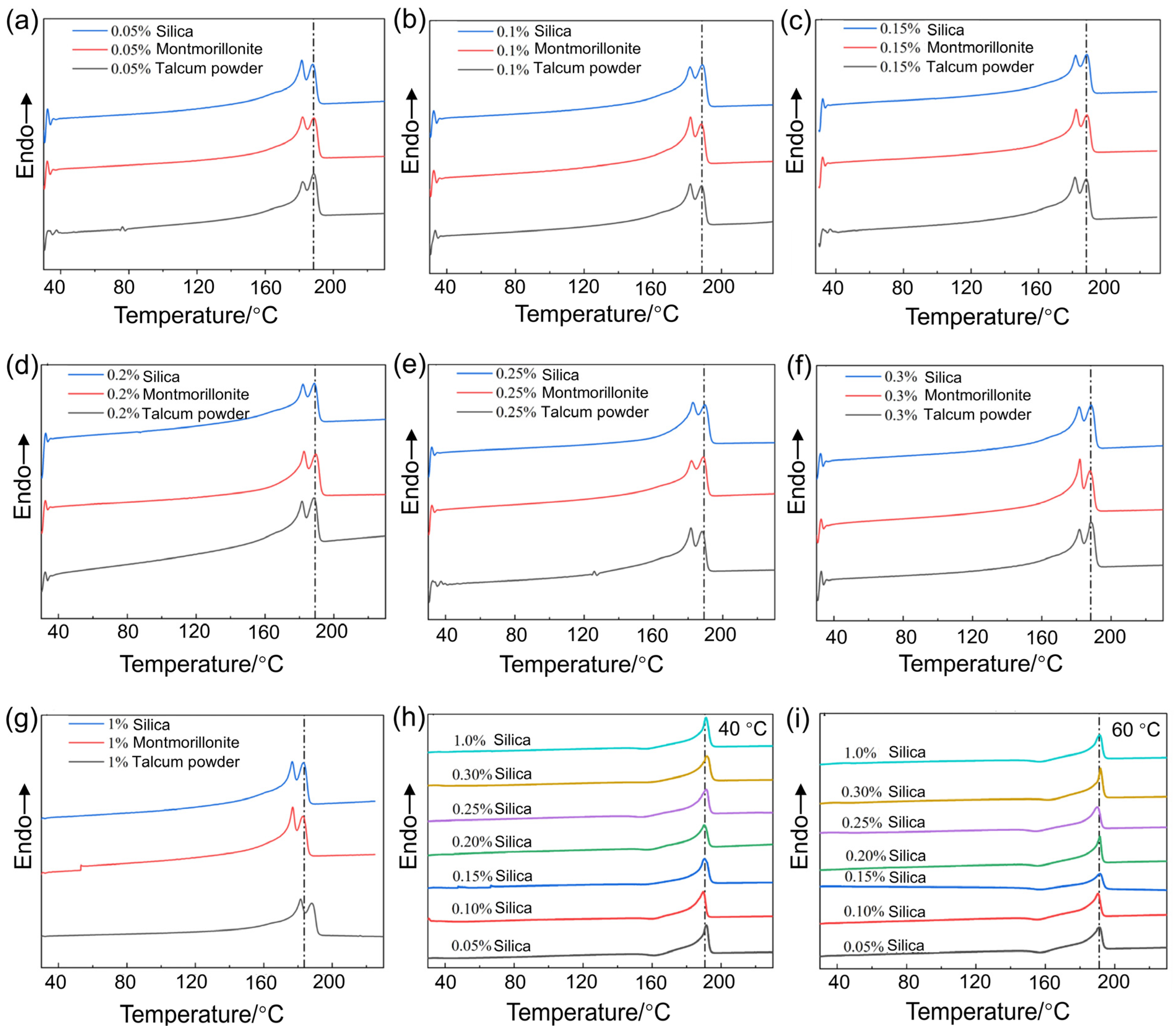
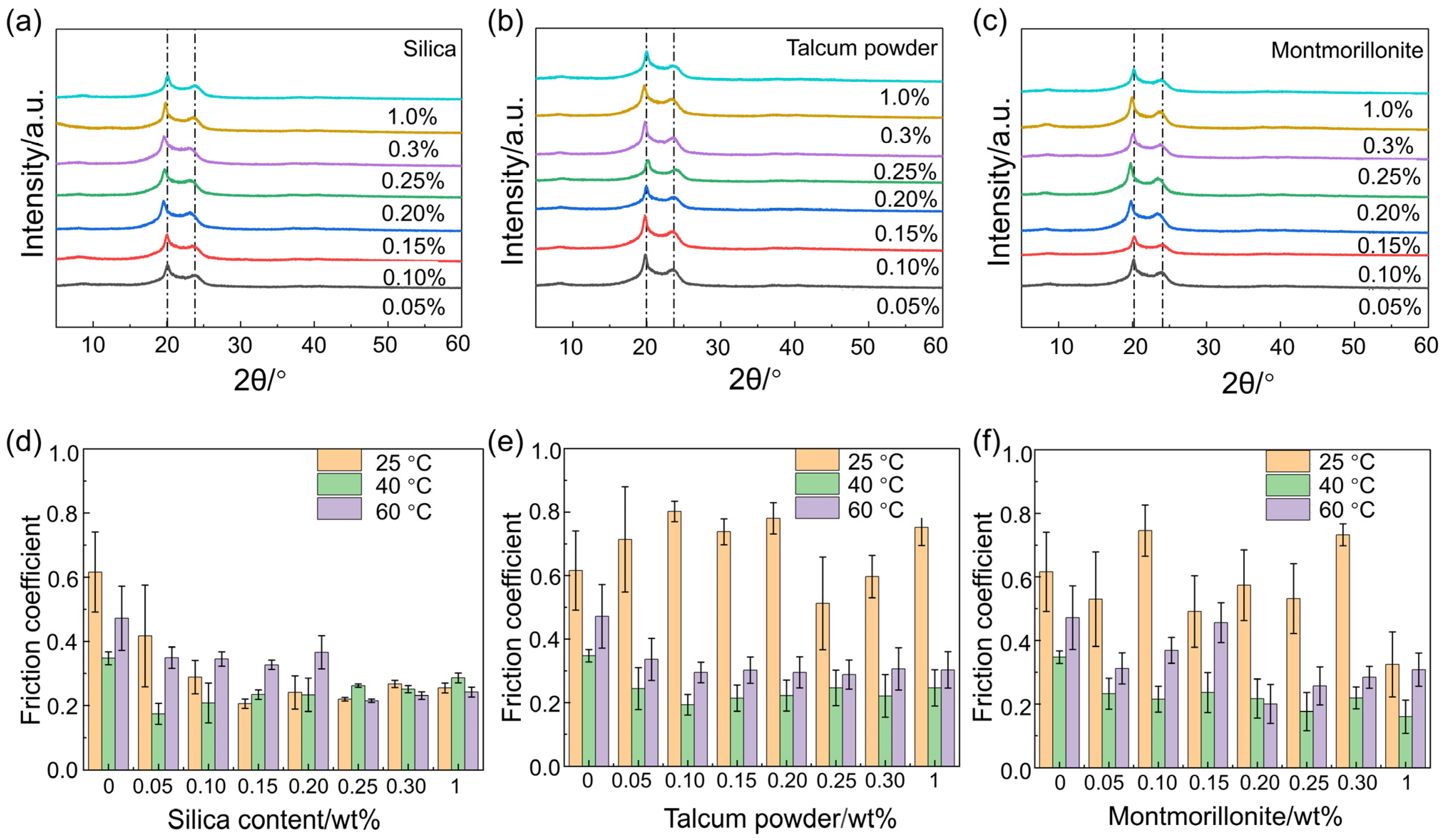
3.4. The Effect of the Varying Types and Concentrations of Nucleating Agents on the Crystallinity and Wear Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohyama, K.; Kaneko, F.; Fujiwara, H.; Nishimura, S. The study of the crystallinity effect on the structural change of Nylon 11 exposed to high-pressure hydrogen gas. Int. J. Hydrogen Energy 2024, 78, 460–469. [Google Scholar] [CrossRef]
- Jain, M.; Mutlu, Z.; Mao, J.H.; Zhou, J.R.; Zhou, P.A.; Wu, C.; Cao, Y.; Cakmak, M. Effect of uniaxial stretching on the structural and dielectric properties of melt cast Nylon 11 films. Polym. Eng. Sci. 2023, 63, 387–401. [Google Scholar] [CrossRef]
- Al-Shawabkeh, A. Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer. e-Polymers 2022, 22, 858–869. [Google Scholar] [CrossRef]
- De Jager, B.; Moxham, T.; Besnard, C.; Salvati, R.; Chen, J.W.; Dolbnya, I.P.; Korsunsky, A.M. Synchrotron X-ray scattering analysis of Nylon-12 crystallisation variation depending on 3D printing conditions. Polymers 2020, 12, 1169. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Shuttleworth, P.S. Investigation of the crystallization kinetics and melting behaviour of polymer blend nanocomposites Based on Poly(L-Lactic Acid), Nylon 11 and TMDCs WS2. Polymers 2022, 14, 2692. [Google Scholar] [CrossRef]
- Tseng, C.H.; Tsai, P.S. The isothermal and nonisothermal crystallization kinetics and morphology of solvent-precipitated Nylon 66. Polymers 2022, 14, 442. [Google Scholar] [CrossRef]
- Chen, K.J.; Koh, Z.H.; Le, K.Q.; Teo, H.W.B.; Zheng, H.; Zeng, J.; Zhou, K.; Du, H.J. Effects of build positions on the thermal history, crystallization, and mechanical properties or polyamide 12 parts printed by Multi Jet Fusion. Virtual Phys. Prototyp. 2022, 17, 631–648. [Google Scholar] [CrossRef]
- Yao, H.M.; Li, W.; Zeng, Z.; Wang, T.; Zhu, J.R.; Lin, Z.Y. Non-isothermal crystallization kinetics of poly (phthalazinone ether sulfone)/MC Nylon 6 in-situ composites. Iran. Polym. J. 2022, 31, 869–882. [Google Scholar] [CrossRef]
- He, X.F.; Guo, F.Q.; Tang, K.H.; Ge, T.J. Crystallization kinetics of modified nanocellulose/monomer casting Nylon composites. Polymers 2023, 15, 719. [Google Scholar] [CrossRef]
- Yin, X.T.; Zhao, X.W.; Ye, L. In situ-induced epiphytic crystallization of MC PA6 by self-assembled nucleator and reinforcing effect. Polym. Int. 2023, 72, 629–639. [Google Scholar] [CrossRef]
- Shi, K.; Liu, G.S.; Sun, H.; Yang, B.; Weng, Y.X. Effect of biomass as nucleating agents on crystallization behavior of polylactic acid. Polymers 2022, 14, 4305. [Google Scholar] [CrossRef]
- Zhao, X.P.; Yu, J.J.; Liang, X.Y.; Huang, Z.P.; Li, J.C.; Peng, S.X. Crystallization behaviors regulations and mechanical performances enhancement approaches of polylactic acid (PLA) biodegradable materials modified by organic nucleating agents. Int. J. Biol. Macromol. 2023, 233, 123581. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.H.; Sun, M.L.; Meng, X.; Cheng, R.H. Crystallization improvement of PLA by the talc with “grafting from” method of polymerization of lactide. J. Polym. Res. 2024, 31, 14. [Google Scholar] [CrossRef]
- Deng, R.; Ren, L.L.; Su, D.D.; Yang, J.K.; Jin, J.B.; Lei, T.; Zhang, K.Z.; Qin, S.H. Nylon 12 composite powder prepared by thermally induced phase separation for selective laser sintering. J. Mater. Res. 2024, 39, 2482–2492. [Google Scholar] [CrossRef]
- Fu, Y.; Huo, C.M.; Liu, S.Y.; Li, K.Q.; Meng, Y.Z. Non-isothermal crystallization Kinetics of montmorillonite/polyamide 610 nanocomposites. Nanomaterials 2023, 13, 1814. [Google Scholar] [CrossRef]
- Peng, C.; Yang, H.; Tang, W.F. Study on the flammability, crystal behaviors and mechanical performance of polyamide 11 composites by intercalated layered double hydroxides. Int. J. Mol. Sci. 2022, 23, 12818. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Pan, B.L.; Xie, M.X.; Gao, J.Y.; Zhao, G.M.; Niu, Y.M.; Wang, H.G. Synergistic effects of graphene oxide and paraffin wax on the tribological properties of monomer casting Nylon-6 composites. Tribol. Int. 2021, 154, 106726. [Google Scholar] [CrossRef]
- Randhawa, K.S.; Patel, A.D. A review on tribo-mechanical properties of micro- and nanoparticulate-filled Nylon composites. J. Polym. Eng. 2021, 41, 339–355. [Google Scholar] [CrossRef]
- Lin, J.E.; Mai, Z.Y.; Zhang, P.; Lin, C.X.; Cheng, Y.Q.; Zhang, Z.R. Superior mechanical and tribological properties of short-cut carbon fiber reinforced monomer casting Nylon composites after heat treatment. Mater. Lett. 2023, 338, 134005. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.M.; Wu, H.; Guo, S.Y.; Qiu, J.H. Constructing highly aligned crystalline structure to enhance sliding wear performance of bulk polyamide 6. Polymer 2021, 237, 124353. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Yousefi, E. Fabrication of a dye removal system through electrospun of TiO2/Nylon-6 nanocomposite on three-dimensional spacer fabrics. Polym. Bull. 2022, 79, 2953–2967. [Google Scholar] [CrossRef]
- Yu, S.R.; Xia, Z.Y.; Kiratitanavit, W.; Kulkarni, S.; Sharma, R.; Lu, D.N.; Morgan, A.B.; Kumar, J.; Mosurkal, R.; Nagarajan, R. Durable Flame Retardant Nylon-6,6 by Microwave-Assisted Surface Grafting of Polyacrylamide. ACS Appl. Polym. Mater. 2024, 6, 8876–8884. [Google Scholar] [CrossRef]
- Hou, C.M.; Zhang, Y.; Liao, Q.Q.; Chen, Q.R. Ultra-cheap and ultra-stable adsorption materials based on Nylon and removal performance of copper ion. Sep. Purif. Technol. 2024, 336, 126346. [Google Scholar] [CrossRef]
- Rashmi, B.J.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Toughening of poly (lactic acid) without sacrificing stiffness and strength by melt-blending with polyamide 11 and selective localization of halloysite nanotubes. Express Polym. Lett. 2015, 9, 721–735. [Google Scholar] [CrossRef]
- Yu, M.H.; Qi, L.L.; Cheng, L.L.; Min, W.; Mei, Z.H.; Gao, R.Z.; Sun, Z.Y. The effect of cooling rates on thermal, crystallization, mechanical and barrier properties of rotational molding polyamide 11 as the liner material for high-capacity high-pressure vessels. Molecules 2023, 28, 2425. [Google Scholar] [CrossRef]

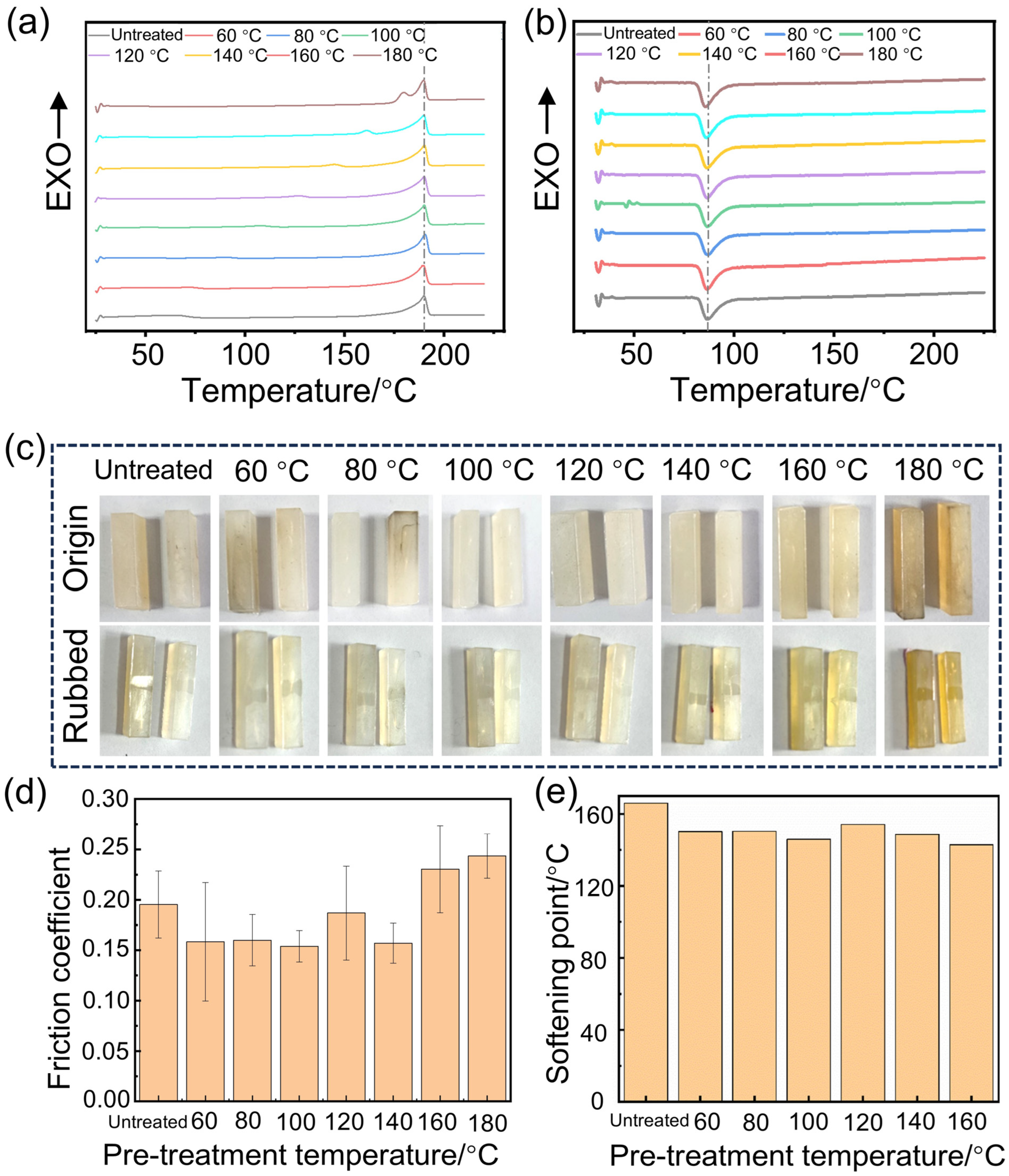

| Pre-Treatment Temperature/°C | Tm/°C | Hm/J·g−1 | Xc/% |
|---|---|---|---|
| Untreated | 189.90 | 58.39 | 30.89 |
| 60 | 189.46 | 57.87 | 30.61 |
| 80 | 190.36 | 51.61 | 27.31 |
| 100 | 190.00 | 64.83 | 34.30 |
| 120 | 189.97 | 70.61 | 37.36 |
| 140 | 190.03 | 69.89 | 36.98 |
| 160 | 189.73 | 67.07 | 35.49 |
| 180 | 189.73 | 52.71 | 27.89 |
| Melting Temperature/°C | Tm/°C | Hm/J·g−1 | Xc/% |
|---|---|---|---|
| 190 | 191.30 | 58.86 | 31.14 |
| 195 | 189.36 | 65.52 | 34.67 |
| 200 | 190.62 | 58.58 | 31.00 |
| 205 | 188.74 | 63.74 | 33.72 |
| 210 | 189.91 | 60.65 | 32.09 |
| Sample | Tm/°C | Hm/J·g−1 | Xc/% |
|---|---|---|---|
| Program 1 | 190.10 | 56.46 | 29.87 |
| Program 2 | 189.60 | 64.44 | 34.10 |
| Program 3 | 189.80 | 59.90 | 31.69 |
| Content/wt% | Montmorillonite | Talcum Powder | Silica | |||
|---|---|---|---|---|---|---|
| ΔHm/J·g−1 | Xc/% | ΔHm/J·g−1 | Xc/% | ΔHm/J·g−1 | Xc/% | |
| 0 | ~ | ~ | ~ | ~ | 66.95 | 35.42 |
| 0.05 | 68.96 | 36.51 | 81.99 | 43.40 | 80.89 | 42.82 |
| 0.1 | 83.65 | 44.34 | 86.04 | 45.76 | 87.55 | 46.48 |
| 0.15 | 84.19 | 44.61 | 79.71 | 42.17 | 78.55 | 41.56 |
| 0.2 | 85.75 | 45.46 | 84.19 | 44.54 | 79.86 | 42.25 |
| 0.25 | 85.75 | 45.48 | 77.61 | 41.06 | 79.92 | 42.29 |
| 0.3 | 77.33 | 41.04 | 87.43 | 46.40 | 82.31 | 43.68 |
| 1 | 65.88 | 35.21 | 71.01 | 37.95 | 69.91 | 37.63 |
| Silica Content/wt% | ΔHm/J·g−1 | Xc/% |
|---|---|---|
| 0.05% | 50.18 | 26.55 |
| 0.10% | 51.44 | 27.22 |
| 0.15% | 53.06 | 28.07 |
| 0.20% | 48.17 | 25.49 |
| 0.25% | 53.11 | 28.10 |
| 0.30% | 57.76 | 30.56 |
| 1.0% | 55.71 | 29.48 |
| Silica Content/wt% | ΔHm/J·g−1 | Xc/% |
|---|---|---|
| 0.05% | 54.64 | 28.91 |
| 0.10% | 54.92 | 29.06 |
| 0.15% | 39.95 | 21.14 |
| 0.20% | 53.08 | 28.08 |
| 0.25% | 52.00 | 27.51 |
| 0.30% | 51.64 | 27.32 |
| 1.0% | 54.42 | 28.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, H.; Sun, D.; Li, Y.; Yu, G.; Liu, S.; Huo, P.; Zhang, D.; Kong, X. The Effect of Processing Methods and Nucleating Agents on the Wear Resistance and Crystallinity Behavior of Nylon 11. Polymers 2025, 17, 1073. https://doi.org/10.3390/polym17081073
Lyu H, Sun D, Li Y, Yu G, Liu S, Huo P, Zhang D, Kong X. The Effect of Processing Methods and Nucleating Agents on the Wear Resistance and Crystallinity Behavior of Nylon 11. Polymers. 2025; 17(8):1073. https://doi.org/10.3390/polym17081073
Chicago/Turabian StyleLyu, Hu, Dongzhou Sun, Yue Li, Guoliang Yu, Shudi Liu, Pengfei Huo, Dawei Zhang, and Xianzhi Kong. 2025. "The Effect of Processing Methods and Nucleating Agents on the Wear Resistance and Crystallinity Behavior of Nylon 11" Polymers 17, no. 8: 1073. https://doi.org/10.3390/polym17081073
APA StyleLyu, H., Sun, D., Li, Y., Yu, G., Liu, S., Huo, P., Zhang, D., & Kong, X. (2025). The Effect of Processing Methods and Nucleating Agents on the Wear Resistance and Crystallinity Behavior of Nylon 11. Polymers, 17(8), 1073. https://doi.org/10.3390/polym17081073







