Preparation and Flame-Retardant Properties of DMMP/Nano-Silica/WPU Composite Materials
Abstract
1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Modification of Nano Silicon Dioxide
2.3. Preparation of DMMP Nano Silicon Dioxide/WPU Composite Materials
2.4. Measurement and Characterisation
2.4.1. Physical Properties of PU Composite Materials
2.4.2. Structural Characterisation
2.4.3. Mechanical Properties
2.4.4. Thermal Analysis
2.4.5. Flammability Testing
2.4.6. Analysis of Residual Carbon
3. Results and Discussion
3.1. Modification of Nano Silicon Dioxide
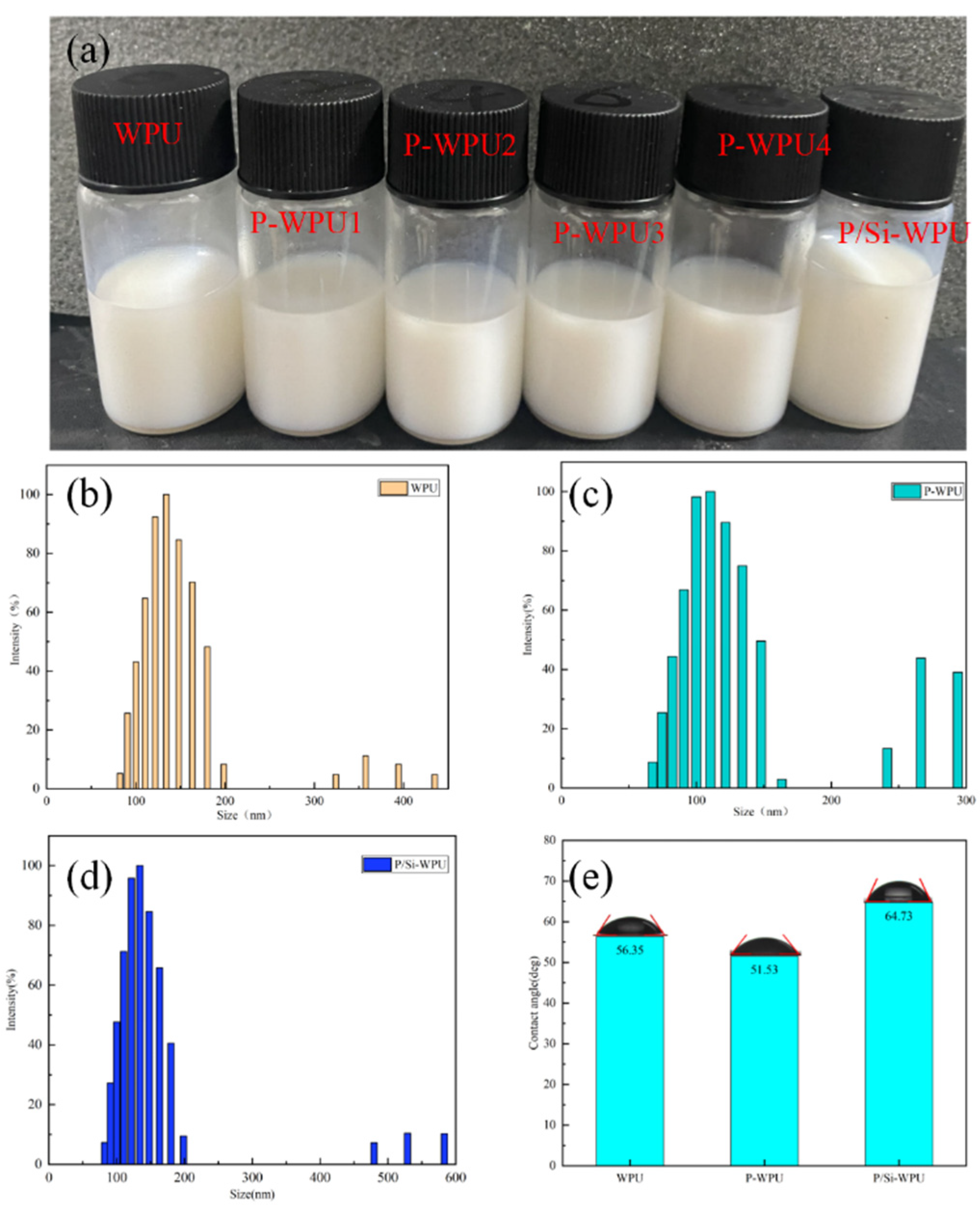
3.2. Stability and Hydrophobicity of WPU Lotion and Its Composites
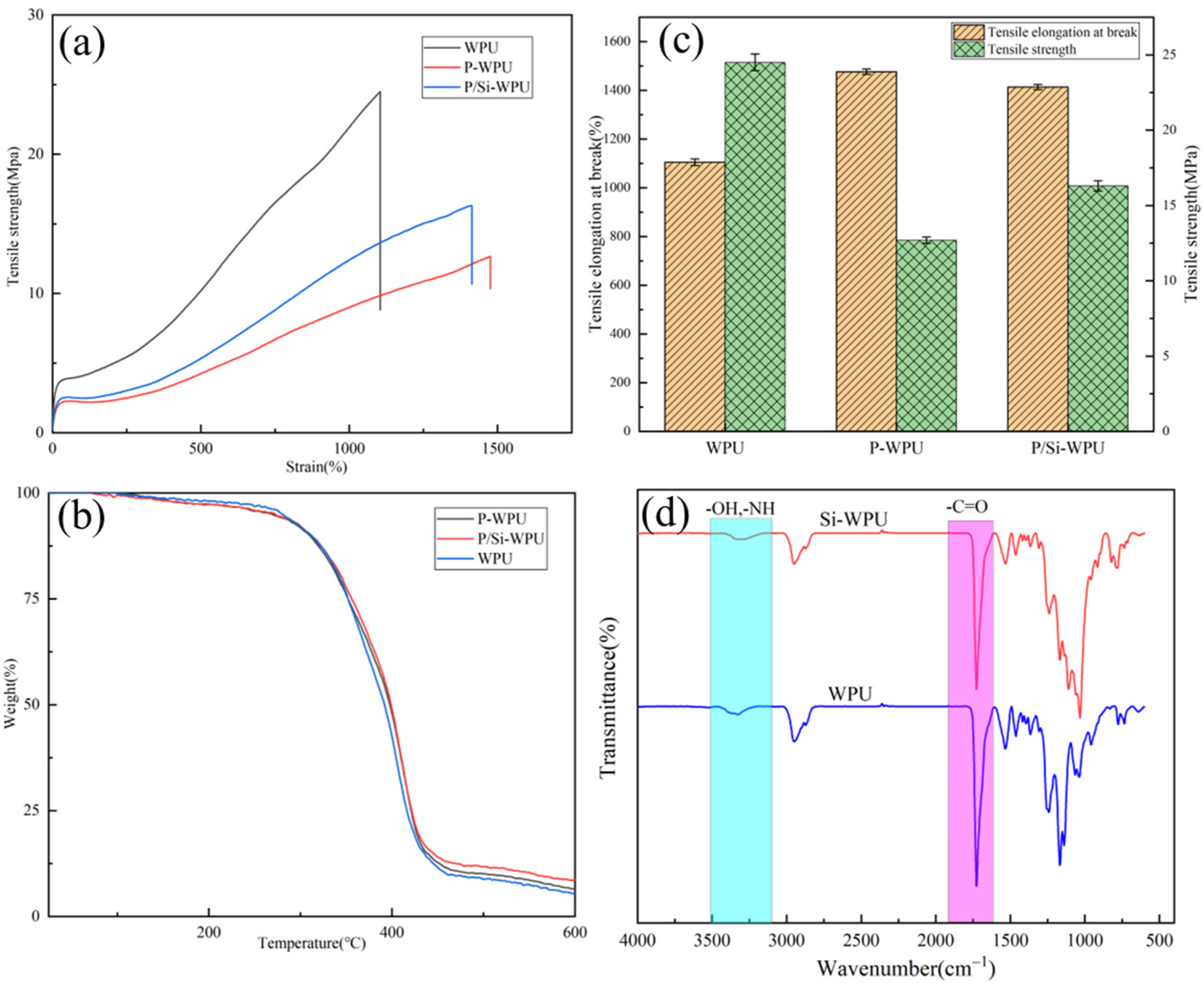
3.3. Mechanical Properties and Thermal Analysis of WPU and Its Composite Materials
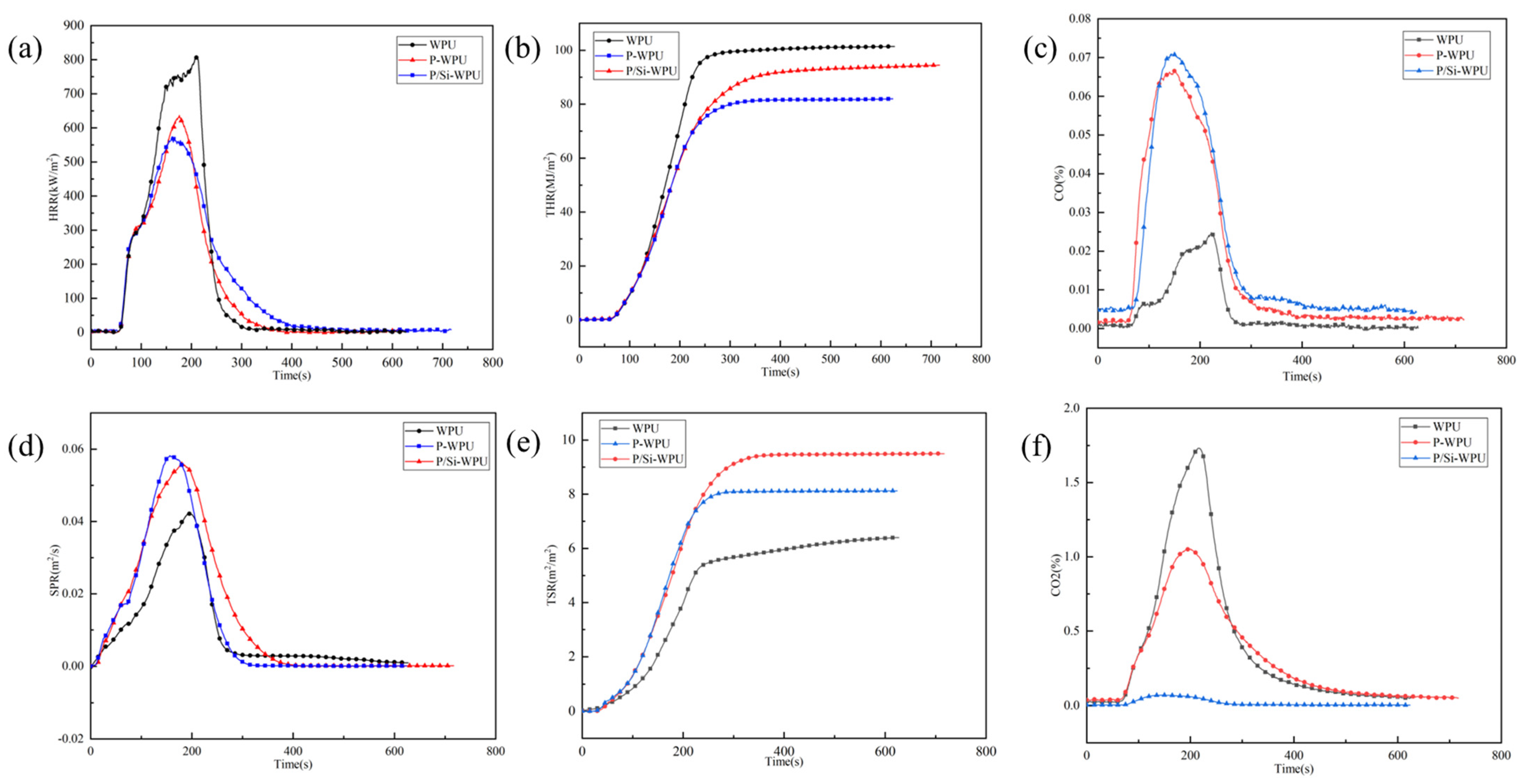
3.4. Flame Retardant Performance Analysis
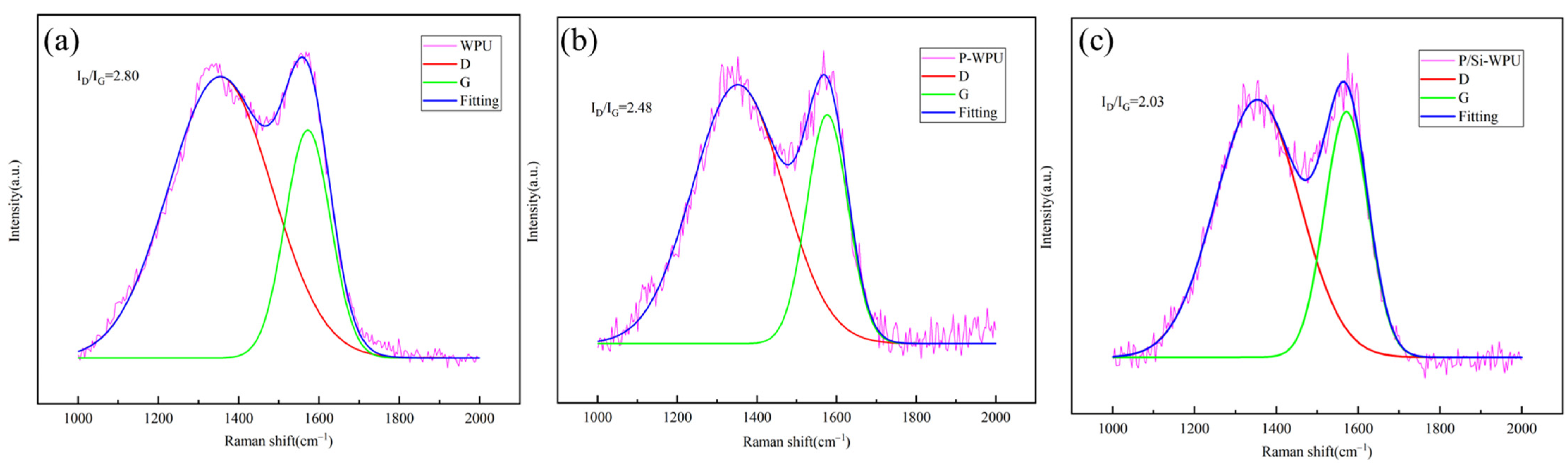
3.5. Residual Carbon Analysis
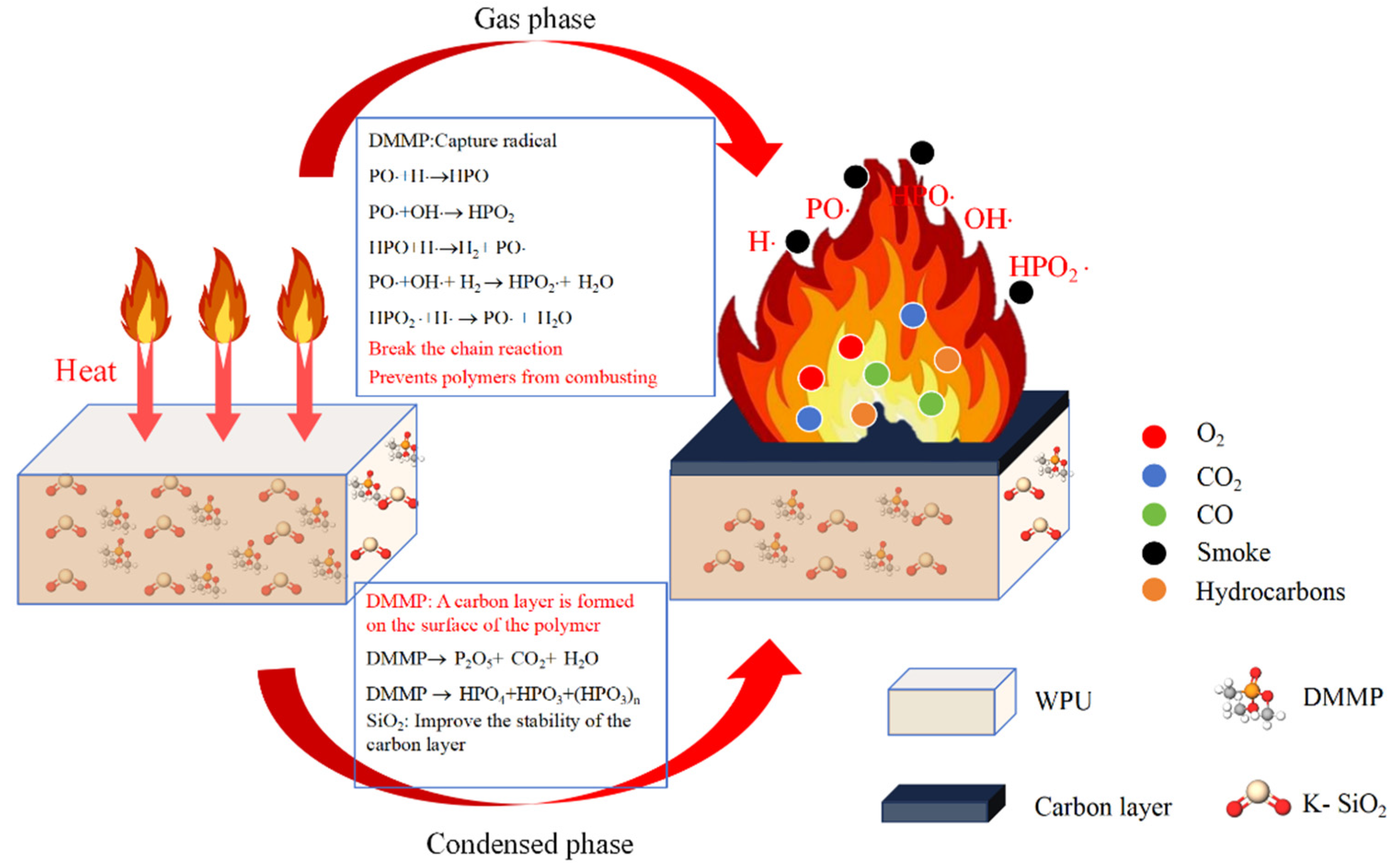
3.6. Flame-Retardant Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dave, D.; Mestry, S.; Mhaske, S.T. Development of flame-retardant waterborne polyurethane dispersions (WPUDs) from sulfonated phosphorus-based reactive water-dispersible agents. J. Coat. Technol. Res. 2021, 18, 1037–1049. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Wang, H.; He, M.; Ding, L.; Zhao, W. Simultaneously improving the fracture toughness and flame retardancy of soybean oil-based waterborne polyurethane coatings by phosphorus-nitrogen chain extender. Ind. Crops Prod. 2021, 163, 113328. [Google Scholar] [CrossRef]
- Pérez-Limiñana, M.A.; Arán-Aís, F.; Torró-Palau, A.M.; Orgilés-Barceló, A.C.; Martín-Martínez, J.M. Characterization of waterborne polyurethane adhesives containing different amounts of ionic groups. Int. J. Adhes. Adhes. 2005, 25, 507–517. [Google Scholar] [CrossRef]
- Shi, M.; Wang, X.; Yang, J. Development of lignin-based waterborne polyurethane materials for flame retardant leather application. Polym. Bull. 2022, 80, 5553–5571. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, C.; Liu, Z.; Liu, T.; Dou, X.; Chen, Y.; Ou, R.; Wang, Q. Flexible decorative wood veneer with high strength, wearability and moisture penetrability enabled by infiltrating castor oil-based waterborne polyurethanes. Compos. Part B Eng. 2022, 230, 109502. [Google Scholar]
- Oussama, A.; Laila, S.; Aicha, B.; Sanaa, M.; Zakia, E.; Abbès, B.; Gmouh, S. Mechanical and aging performances of Palm/Wool and Palm/Polyester nonwovens coated by waterborne polyurethane for automotive interiors. Ind. Crops Prod. 2021, 170, 113681. [Google Scholar]
- Wang, S.; Du, X.; Fu, X.; Du, Z.; Wang, H.; Cheng, X. Highly effective flame-retarded polyester diol with synergistic effects for waterborne polyurethane application. J. Appl. Polym. Sci. 2020, 137, 13. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Zhao, J.; Shi, H. New pivot for investigating flame-retarding mechanism: Quantitative analysis of zinc phosphate doped aliphatic waterborne polyurethane-based intumescent coatings for flame-retarding plywood. Polym. Adv. Technol. 2021, 32, 153–164. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Huang, Z.; Cao, Y.; Li, L. Continuous flame-retardant actions of two phosphate esters with expandable graphite in rigid polyurethane foams. Polym. Degrad. Stab. 2016, 130, 97–102. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Zhang, L.; Zhang, J.; Xiong, W.; Wu, Y.; Song, B.; Mai, Y. Enhanced Flame Retardancy and Mechanical Properties of Intumescent Flame-Retardant Polypropylene with Triazine Derivative-Modified Nano-SiO2. Polym. Sci. Ser. B Chem. 2020, 62, 306–318. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Yang, H.; Yang, H.; Lang, J.; Zhang, Q. Synergistic Flame-Retardant Mechanism of Dicyclohexenyl Aluminum Hypophosphite and Nano-Silica. Polymers 2019, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, W.; Song, B.; Zhu, X.; Wang, L.; Sha, R.; Li, T.; Pei, X.; Wang, L.; Qian, X.; et al. Synthesis of P, N and Si-containing waterborne polyurethane with excellent flame retardant, alkali resistance and flexibility via one-step synthetic approach. Prog. Org. Coat. 2023, 174, 107286. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, H.; Wu, B.; Fu, X.; Hu, K.; Zhou, C.; Lei, J. Preparation and characterization of waterborne polyurethane based on diphenylmethane diisocyanate-50. Adv. Polym. Technol. 2018, 37, 596–605. [Google Scholar] [CrossRef]
- Safronava, N.; Lyon, R.E.; Crowley, S.; Stoliarov, S.I. Effect of Moisture on Ignition Time of Polymers. Fire Technol. 2015, 51, 1093–1112. [Google Scholar] [CrossRef]
- Xu, P.; Luo, Y.; Zhang, P. Interfacial architecting of organic–inorganic hybrid toward mechanically reinforced, fire-resistant and smoke-suppressed polyurethane composites. J. Colloid Interface Sci. 2022, 621, 385–397. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Du, X.; Wang, H.; Cheng, X.; Du, Z. Synthesis of a novel flame retardant based on DOPO derivatives and its application in waterborne polyurethane. RSC Adv. 2019, 9, 7411–7419. [Google Scholar] [CrossRef]
- Yin, S.; Ren, X.; Zheng, R.; Li, Y.; Zhao, J.; Xie, D.; Mei, Y. Improving fire safety and mechanical properties of waterborne polyurethane by montmorillonite-passivated black phosphorus. Chem. Eng. J. 2023, 464, 142683. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. Hazard. Mater. 2018, 346, 140–151. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, R.K.K.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. NANO⋅MICRO Small 2019, 15, 13. [Google Scholar] [CrossRef]
- Zhu, S.; Gong, W.; Luo, J.; Meng, X.; Xin, Z.; Wu, J.; Jiang, Z. Flame Retardancy and Mechanism of Novel Phosphorus-Silicon Flame Retardant Based on Polysilsesquioxane. Polymers 2019, 11, 1304. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Yuan, Y.; Mu, X.; Pan, Y.; Song, L.; Hu, Y. An insight into gas phase flame retardantmechanisms of AHP versus AlPi in PBT:Online pyrolysis vacuum ultravioletphotoionization time-of-flight massspectrometry. Combust. Flame 2019, 209, 467–477. [Google Scholar] [CrossRef]



| Characteristic | WPU | P-WPU | P/Si-WPU |
|---|---|---|---|
| Colour | milky white | milky white | milky white |
| Zeta potential (mV) | −66.52 | −65.62 | −61.10 |
| Effective particle size (nm) | 128.78 | 129.47 | 141.86 |
| Stability | ≥6 months | ≥6 months | ≥6 months |
| Contact angle | 56.35 | 51.53 | 64.73 |
| Physical Characteristics | WPU | P-WPU | P/Si-WPU |
|---|---|---|---|
| Tensile strength (Mpa) | 24.5 ± 0.55 | 12.7 ± 0.21 | 16.3 ± 0.34 |
| Tensile elongation at break (%) | 1104.8 ± 13.2 | 1476.1 ± 11.3 | 1413.1 ± 10.6 |
| Shore hardness (HA) | 93.0 ± 1.1 | 72.0 ± 2.3 | 87.0 ± 1.8 |
| WPU | P-WPU | P/Si-WPU | |
|---|---|---|---|
| T5% (°C) | 279.8 | 268.9 | 272.8 |
| T10% (°C) | 311.9 | 310.2 | 312.1 |
| T50% (°C) | 391.9 | 397.6 | 405.1 |
| R600 (%) | 5.5 | 6.4 | 8.7 |
| Thermal diffusivity coefficient (mm2/s) | 0.209 | 0.099 | 0.087 |
| Thermal conductivity (W/(m·K)) | 0.385 | 0.230 | 0.206 |
| Sample | LOL(%) | UL-94 Test | ||
|---|---|---|---|---|
| UL-94 Rating | Dripping | Ignite the Cotton | ||
| WPU | 18.1 ± 0.2 | No rating | Yes | Yes |
| P-WPU | 27.6 ± 0.1 | V-0 | Yes | No |
| P-WPU1 | 21.6 ± 0.3 | No rating | Yes | Yes |
| P-WPU2 | 23.0 ± 0.1 | No rating | Yes | Yes |
| P-WPU3 | 25.2 ± 0.2 | V-1 | Yes | No |
| P/Si-WPU | 28.3 ± 0.3 | V-0 | Yes | No |
| Samples | TTI (s) | THR (MJ/m2) | PHRR (kW/m2) | TSR (m2/m2) | FGI |
|---|---|---|---|---|---|
| WPU | 53.0 | 101.39 | 809.37 | 6.40 | 3.87 |
| P-WPU | 45.0 | 81.98 | 636.55 | 8.12 | 3.61 |
| P/Si-WPU | 48.0 | 94.47 | 574.21 | 9.50 | 3.54 |
| Sample | C-C | C-O | C=O |
|---|---|---|---|
| WPU | 61.89% | 31.43% | 6.68% |
| P/Si-WPU | 61.36% | 27.06% | 11.58% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Huang, X.; Mo, Y.; Ye, M.; Hu, Q.; Chen, Q.; Wang, Y.; Hu, C. Preparation and Flame-Retardant Properties of DMMP/Nano-Silica/WPU Composite Materials. Polymers 2025, 17, 1052. https://doi.org/10.3390/polym17081052
Wu W, Huang X, Mo Y, Ye M, Hu Q, Chen Q, Wang Y, Hu C. Preparation and Flame-Retardant Properties of DMMP/Nano-Silica/WPU Composite Materials. Polymers. 2025; 17(8):1052. https://doi.org/10.3390/polym17081052
Chicago/Turabian StyleWu, Wanchao, Xiaoyue Huang, Ya Mo, Miaojia Ye, Qian Hu, Quankai Chen, Yiwen Wang, and Chuanqun Hu. 2025. "Preparation and Flame-Retardant Properties of DMMP/Nano-Silica/WPU Composite Materials" Polymers 17, no. 8: 1052. https://doi.org/10.3390/polym17081052
APA StyleWu, W., Huang, X., Mo, Y., Ye, M., Hu, Q., Chen, Q., Wang, Y., & Hu, C. (2025). Preparation and Flame-Retardant Properties of DMMP/Nano-Silica/WPU Composite Materials. Polymers, 17(8), 1052. https://doi.org/10.3390/polym17081052






