One-Step Synergistic SDS-H2O2 Process for High-Purity Chitin Extraction from Fly Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitin
2.3. Orthogonal Experimental Design
2.4. Control Experimental Design
2.5. Protein Content Measurement
2.6. Lipid Content Measurement
2.7. Ash and Moisture Measurement
2.8. Purity Measurement
2.9. Characterizations
2.10. Heavy Metal Adsorption Capacity
3. Results and Discussion
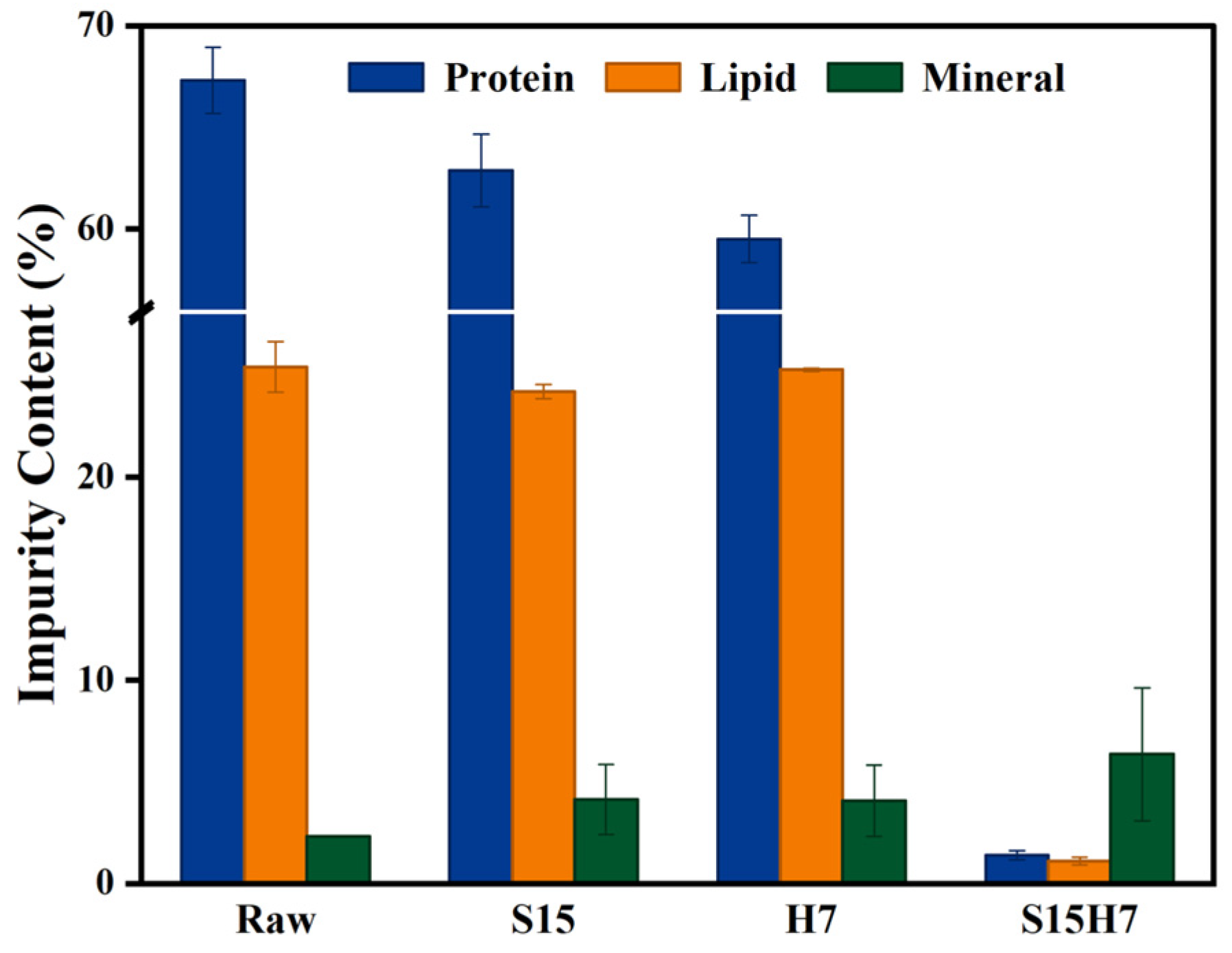
3.1. Orthogonal Experimental Results
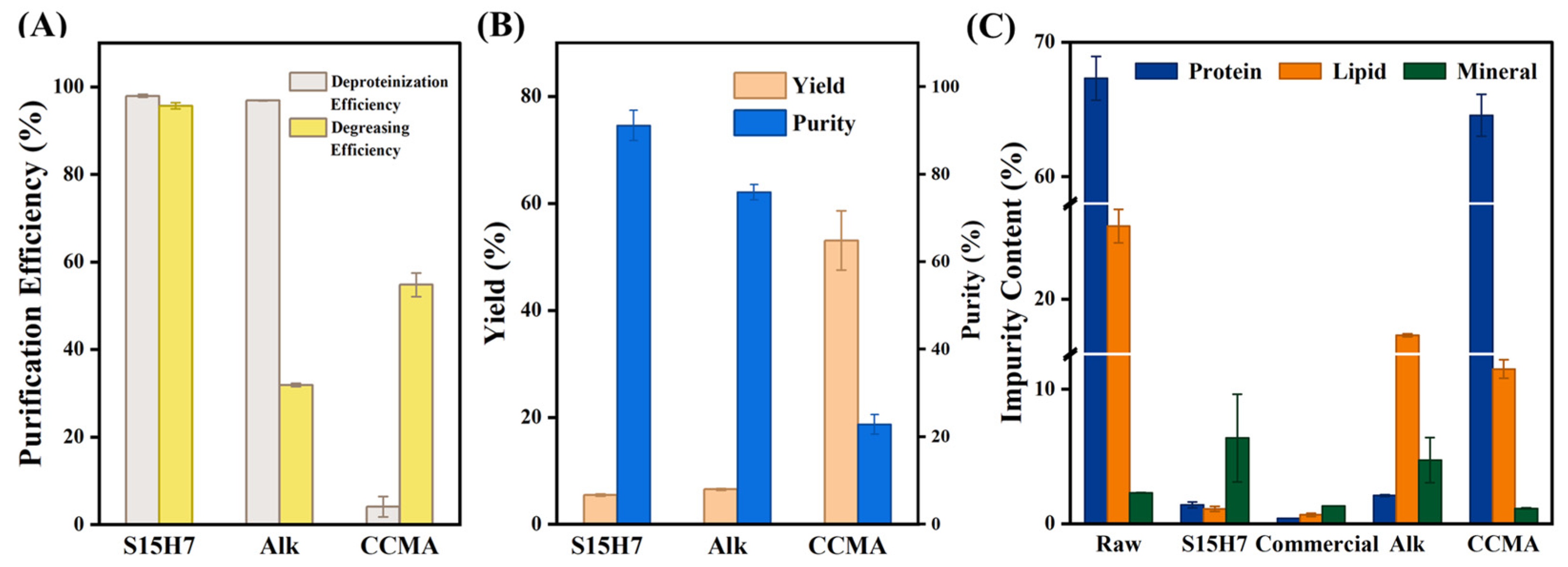
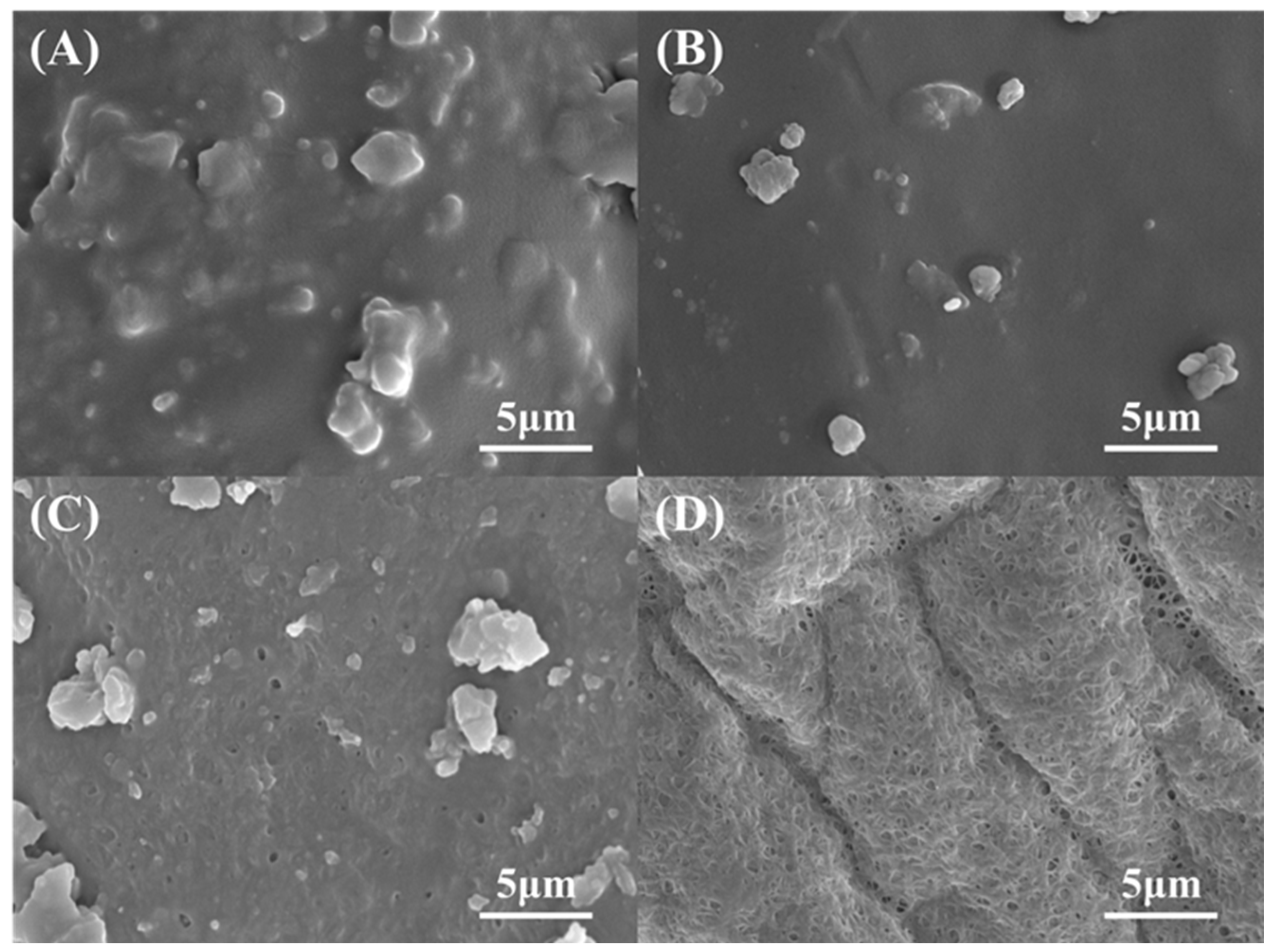
3.2. Purification Performance
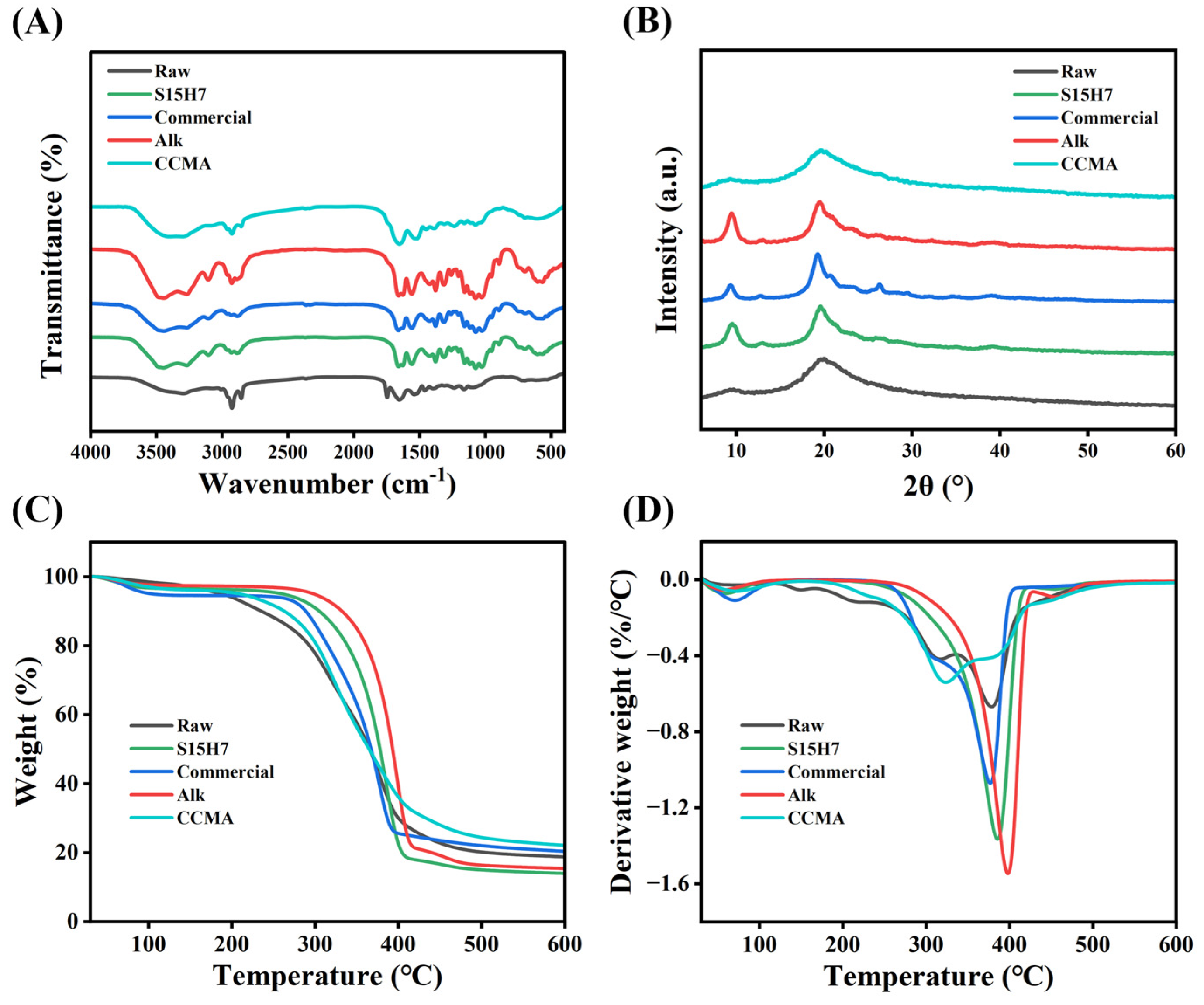
3.3. Purification Mechanism
3.4. Heavy Metal Adsorption Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asero, R.; Scala, E.; Villalta, D.; Pravettoni, V.; Arena, A.; Billeri, L.; Colombo, G.; Cortellini, G.; Cucinelli, F.; De Cristofaro, M.L.; et al. Shrimp Allergy: Analysis of Commercially Available Extracts for In Vivo Diagnosis. J. Investig. Allergol. Clin. Immunol. 2017, 27, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Mohan, K.; Muralisankar, T.; Jayakumar, R.; Rajeevgandhi, C. A study on structural comparisons of α-chitin extracted from marine crustacean shell waste. Carbohydr. Polym. Technol. Appl. 2021, 2, 100037. [Google Scholar] [CrossRef]
- Novikov, V.; Derkach, S.; Konovalova, I. Chitosan technology from crustacean shells of the northern seas. KnE Life Sci. 2020, 65–74. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Rahman, M.M.; Byanju, B.; Lamsal, B.P. Protein, lipid, and chitin fractions from insects: Method of extraction, functional properties, and potential applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 6415–6431. [Google Scholar] [CrossRef]
- Cauchie, H.-M. Chitin production by arthropods in the hydrosphere. Hydrobiologia 2002, 470, 63–95. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential extraction and characterisation of lipids, proteins, and chitin from black soldier fly (Hermetia illucens) larvae, prepupae, and pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Shin, C.-S.; Kim, D.-Y.; Shin, W.-S. Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Wu, S.-J.; Pan, S.-K.; Wang, H.-B.; Wu, J.-H. Preparation of chitooligosaccharides from cicada slough and their antibacterial activity. Int. J. Biol. Macromol. 2013, 62, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Malm, M.; Liceaga, A.M. Physicochemical Properties of Chitosan from Two Commonly Reared Edible Cricket Species, and Its Application as a Hypolipidemic and Antimicrobial Agent. Polysaccharides 2021, 2, 339–353. [Google Scholar] [CrossRef]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of conventional and sustainable lipid extraction methods for the production of oil and protein isolate from edible insect meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef]
- Sheng, Z.; Guo, A.; Wang, J.; Chen, X. Preparation, physicochemical properties and antimicrobial activity of chitosan from fly pupae. Heliyon 2022, 8, e11168. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Ji, R.; Schmitt, E.; Zibek, S. Chitosan production with larval exoskeletons derived from the insect protein production. J. Biotechnol. 2020, 310, 62–67. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Huet, G.; Hadad, C.; González-Domínguez, J.M.; Courty, M.; Jamali, A.; Cailleu, D.; van Nhien, A.N. IL versus DES: Impact on chitin pretreatment to afford high quality and highly functionalizable chitosan. Carbohydr. Polym. 2021, 269, 118332. [Google Scholar] [CrossRef]
- Li, R.; Hsueh, P.-H.; Ulfadillah, S.A.; Wang, S.-T.; Tsai, M.-L. Exploring the Sustainable Utilization of Deep Eutectic Solvents for Chitin Isolation from Diverse Sources. Polymers 2024, 16, 3187. [Google Scholar] [CrossRef] [PubMed]
- Jantzen da Silva Lucas, A.; Quadro Oreste, E.; Leão Gouveia Costa, H.; Martín López, H.; Dias Medeiros Saad, C.; Prentice, C. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, J.; Tong, J.; Yang, C.; Deng, H.; Du, Y.; Shi, X. Ultra-low protein residue of chitosan by one step H2O2 and sodium dodecyl sulfate treatment. Int. J. Biol. Macromol. 2022, 222, 2977–2986. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Yan, T.; Wang, X.; Huang, J.; Kuang, Z.; Ye, M.; Pan, M. Selectivity of deproteinization and demineralization using natural deep eutectic solvents for production of insect chitin (Hermetia illucens). Carbohydr. Polym. 2019, 225, 115255. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Kang, S.H.; Kim, S.A.; Jung, W.J. Extraction of chitin and chitosan from housefly, Musca domestica, pupa shells. Entomol. Res. 2016, 46, 324–328. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Barber, A.R.; Smith, P.; Luo, X.; Zhang, W. Application and optimization of the highly efficient and environmentally-friendly microwave-intensified lactic acid demineralization of deproteinized Rock lobster shells (Jasus edwardsii) for chitin production. Food Bioprod. Process. 2017, 102, 367–374. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H.M. Isolation and characterization of chitosan from different local insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, R.; Zhu, Y.; Yang, P.; Lin, Z.; Wang, Z.; Cong, W. Efficient extraction of chitin from crustacean waste via a novel ternary natural deep eutectic solvents. Carbohydr. Polym. 2022, 286, 119281. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; von Seggern, N.; Falabella, P.; Salvia, R.; Thomä, J.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Stegbauer, L. Purification of chitin from pupal exuviae of the black soldier fly. Waste Biomass Valorization 2022, 13, 1993–2008. [Google Scholar] [CrossRef]
- Yanase, K.; Hatta, I. Disruption of human stratum corneum lipid structure by sodium dodecyl sulphate. Int. J. Cosmet. Sci. 2018, 40, 44–49. [Google Scholar] [CrossRef]
- Shamim, G.; RANjAN, S.K.; PANdEy, D.M.; RAmANI, R. Biochemistry and biosynthesis of insect pigments. EJE 2014, 111, 149–164. [Google Scholar] [CrossRef]
- Zettler, J.A.; Adler, P.H.; McCreadie, J.W. Factors influencing larval color in the Simulium vittatum complex (Diptera: Simuliidae). Invertebr. Biol. 1998, 117, 245–252. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Mentes, A.; Asaroglu, M.; Sezen, G.; Tozak, K.O. Extraction and characterization of α-chitin and chitosan from six different aquatic invertebrates. Food Biophys. 2014, 9, 145–157. [Google Scholar] [CrossRef]
- Gomba, G.K.; Synytsya, A.; Švecová, P.; Coimbra, M.A.; Čopíková, J. Distinction of fungal polysaccharides by N/C ratio and mid infrared spectroscopy. Int. J. Biol. Macromol. 2015, 80, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sargin, I.; Tozak, K.Ö.; Baran, T.; Erdogan, S.; Sezen, G. Chitin extraction and characterization from Daphnia magna resting eggs. Int. J. Biol. Macromol. 2013, 61, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Ghadage, V.H.; Kulkarni, G.R.; Bhoraskar, S.V. Study of Fourier transform infrared spectra of cockroach nervous tissue and chitin. Proc. Opt. Interact. Tissue Cells XXIII 2012, 8221, 149–157. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Pavlikov, A.V.; Savchenko, N.F. Resistive Gas Sensors Based on Porous Sp-Containing Films Obtained by Dehydrohalogenation of PVDC and PVDC-PVC Copolymer. C 2023, 9, 82. [Google Scholar] [CrossRef]
- Deshpande, P.S.; Deshpande, T.D.; Kulkarni, R.D.; Mahulikar, P.P. Synthesis of sucrose–coconut fatty acids esters: Reaction kinetics and rheological analysis. Ind. Eng. Chem. Res. 2013, 52, 15024–15033. [Google Scholar] [CrossRef]
- Feng, F.; Liu, Y.; Hu, K. Influence of alkali-freezing treatment on the solid state structure of chitin. Carbohydr. Res. 2004, 339, 2321–2324. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.-M.; Wang, P.; Zhou, G.-E.; Xu, Z.-D. Solid state structure and properties of chitin/chitosan. Chem. J. Chin. Univ.-Chin. Ed. 1998, 19, 989–993. [Google Scholar] [CrossRef]
- Huang, W.-C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef] [PubMed]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of chitin extracted from black soldier fly in different life stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin adsorbents for toxic metals: A review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef]
- Forutan, R.; Ehsandoost, E.; Hadipour, S.; Mobaraki, Z.; Saleki, M.; Mohebbi, G. Kinetic and equilibrium studies on the adsorption of lead by the chitin of pink shrimp (Solenocera melantho). Entomol. Appl. Sci. Lett. 2016, 3, 20–26. [Google Scholar]
- Rhazi, M.; Desbrieres, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A.; El Meray, M. Influence of the nature of the metal ions on the complexation with chitosan.: Application to the treatment of liquid waste. Eur. Polym. J. 2002, 38, 1523–1530. [Google Scholar] [CrossRef]
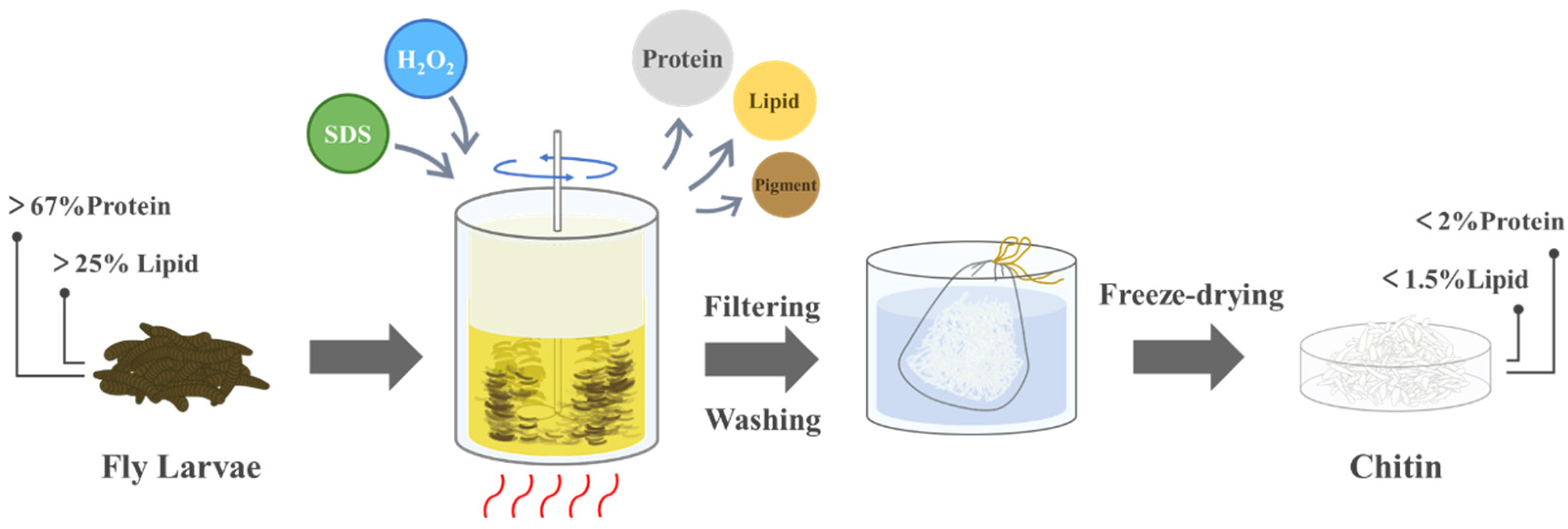

| Parameter | Level | |
|---|---|---|
| 1 | 2 | |
| SDS (wt%) | 9 | 15 |
| H2O2 (wt%) | 3 | 7 |
| Temperature (℃) | 70 | 80 |
| Experimental Group | SDS (wt%) | H2O2 (wt%) | Temperature (°C) |
|---|---|---|---|
| S9H3-70 | 9 | 3 | 70 |
| S9H3-80 | 9 | 3 | 80 |
| S9H7-70 | 9 | 7 | 70 |
| S9H7-80 | 9 | 7 | 80 |
| S15H3-70 | 15 | 3 | 70 |
| S15H3-80 | 15 | 3 | 80 |
| S15H7-70 | 15 | 7 | 70 |
| S15H7-80 | 15 | 7 | 80 |
| Experimental Group | Yield (%) | Protein (%) | Deproteinization Efficiency (%) | Purity of Chitin (%) |
|---|---|---|---|---|
| S9H3-70 | 12.47 ± 0.42 | 25.76 ± 2.59 | 61.73 ± 3.85 | 39.82 ± 1.32 |
| S9H7-70 | 5.84 ± 0.18 | 3.36 ± 1.11 | 95.00 ± 1.64 | 84.99 ± 2.69 |
| S15H3-70 | 11.07 ± 1.01 | 24.2 ± 1.88 | 64.05 ± 2.8 | 45.08 ± 4.35 |
| S15H7-70 | 6.16 ± 0.12 | 5.49 ± 1.32 | 91.85 ± 1.96 | 80.54 ± 1.65 |
| S9H3-80 | 6.05 ± 0.19 | 4.44 ± 1.07 | 93.41 ± 1.59 | 82.08 ± 2.58 |
| S9H7-80 | 5.55 ± 0.10 | 1.76 ± 0.52 | 97.38 ± 0.77 | 89.34 ± 1.66 |
| S15H3-80 | 6.38 ± 0.14 | 7.97 ± 0.73 | 88.16 ± 1.09 | 77.77 ± 1.67 |
| S15H7-80 | 5.45 ± 0.21 | 1.39 ± 0.22 | 97.93 ± 0.33 | 91.15 ± 3.48 |
| Metal Ion | Adsorption Rate (%) | ||
|---|---|---|---|
| S15H7-80 | Alk | Commercial | |
| Cd2+ | 35.05 ± 2.89 | 76.11 ± 1.28 | 43.03 ± 8.64 |
| Cu2+ | 46.02 ± 0.63 | 94.00 ± 0.07 | 81.15 ± 0.08 |
| Pb2+ | 99.91 ± 0.04 | 99.63 ± 0.10 | 96.04 ± 0.35 |
| Zn2+ | 52.47 ± 4.40 | 72.83 ± 1.15 | 34.64 ± 5.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Zhao, Z.; Hu, F.; Liu, M.; Shi, X. One-Step Synergistic SDS-H2O2 Process for High-Purity Chitin Extraction from Fly Larvae. Polymers 2025, 17, 994. https://doi.org/10.3390/polym17070994
Qiu Y, Zhao Z, Hu F, Liu M, Shi X. One-Step Synergistic SDS-H2O2 Process for High-Purity Chitin Extraction from Fly Larvae. Polymers. 2025; 17(7):994. https://doi.org/10.3390/polym17070994
Chicago/Turabian StyleQiu, Yuhuan, Zhongtao Zhao, Feng Hu, Mengyi Liu, and Xiaowen Shi. 2025. "One-Step Synergistic SDS-H2O2 Process for High-Purity Chitin Extraction from Fly Larvae" Polymers 17, no. 7: 994. https://doi.org/10.3390/polym17070994
APA StyleQiu, Y., Zhao, Z., Hu, F., Liu, M., & Shi, X. (2025). One-Step Synergistic SDS-H2O2 Process for High-Purity Chitin Extraction from Fly Larvae. Polymers, 17(7), 994. https://doi.org/10.3390/polym17070994






