1. Introduction
Hair is one of the most fundamental and essential physiological features of mammals, serving to protect the skin, regulate temperature, and provide sensory functions. It plays a crucial role in these processes. The basic structure of the hair fiber is distributed in concentric layers and is divided into the cuticle, cortex, and medulla, which function mechanically as a whole. The cuticle primarily comprises β-keratin and inelastic proteins, forming a multilayered squamous structure made up of dead, flattened, overlapping cells. The outer surface of these squamous cells is coated with a lipoprotein layer approximately 10–14 nm thick, known as the epidermis [
1]. Beneath the epidermis lies the outer cortex, which contains the majority of cystine residues. The innermost layer, the medulla, is located at the cortical interface, exhibiting variability in thickness and structure while having a low thionin content. The cells in the stratum corneum are interconnected by the cell membrane complex (CMC), which primarily consists of a lipid layer surrounding a central protein layer. The cortex constitutes the majority of the hair fiber mass (86–90%) and is primarily composed of keratin and structural lipids. It plays a crucial role in determining the hair’s appearance, strength, elasticity, and texture. Most of the hair pigment is concentrated in the cortex, particularly within melanosomes, where pigment granules contain melanin, the key determinant of hair color [
2]. The medulla is a loosely vacuolated cellular structure located near the center of the hair fiber, becoming more prominent as the fiber diameter increases. It is rich in lipids, and its primary protein component is hyaluronan, which has relatively weak disulfide bonds [
3]. Beyond natural aging [
4], hair damage arises from hormonal imbalances, microbial overgrowth, environmental stressors (e.g., UV exposure or pollution), and cosmetic treatments (e.g., dyeing or bleaching). These factors degrade cuticle integrity, increase alkalinity, and cause keratin loss, often leading to hair breakage or loss [
5,
6,
7,
8]. Unfortunately, hair lacks the ability to self-repair. Given that hair issues profoundly impact individuals’ physical, psychological, and social well-being, hair repair and enhancement have become topics of significant interest [
9]. As a result, the cosmetic industry is actively developing new haircare products aimed at improving hair health and appearance.
Natural materials (such as plants, animals, and microorganisms) are rich in protein resources and exhibit various biological activities (such as antihypertensive and antioxidant effects), while also possessing excellent biocompatibility, safety, and no side effects. Compared to chemical synthesis, peptide extraction from natural sources is more environmentally friendly and sustainable, aligning with the trends of green biotechnology development. In recent years, there has been a noticeable trend in the cosmetic industry toward incorporating marine-sourced ingredients into formulations due to their ability to meet diverse consumer demands and their broad range of bioactive properties, including antioxidant, moisturizing, and UV-resistant effects [
10]. Marine-derived collagen hydrolysates are proteins and their hydrolysates are extracted from marine organisms, known for their excellent moisturizing properties and restorative effects [
11]. Chen [
12] investigated the efficacy of gelatin hydrolysate extracted from the skin of
Gadus macrocephalus in reducing UV radiation-induced inflammation and collagen degradation. Gelatin and its hydrolysates from salmon skin have been shown to prevent photo-oxidation [
13], while low doses of collagen hydrolysates enhance hydration and reduce transepidermal water loss. Additionally, hydrolyzed collagen derived from the fish bones of
Gadus macrocephalus contains high levels of proline and hydroxyproline, which are essential for the stabilization of collagen’s triple-helix structure and the maintenance of protein integrity [
14]. Meanwhile, the antioxidant peptide APTBP, obtained from tuna skeleton proteins, has been found to significantly inhibit lipid peroxidation in the linoleic acid emulsion system [
15]. The hydrolysate of
Thunnus thynnus head protein, A21, exhibits high antioxidant activity and remarkable functional properties [
16]. Similarly,
Sardinella aurita head protein undergoes enzymatic hydrolysis by proteases to yield antioxidant peptides, which serve as natural antioxidants to enhance the antioxidant properties of functional foods and prevent oxidative reactions during food processing [
17]. Research has shown that marine-derived collagen peptides exhibit great potential in the development of modern haircare products. Natural collagen extracted from marine fish-processing by-products using acidic hydration technology, combined with ingredients such as hyaluronic acid and plant oils, has been developed into haircare products. The results demonstrate that it can effectively repair the hair cuticle [
18]. Collagen extracted from salmon skin through hydrolysis and ultrafiltration also effectively repairs the hair cuticle and enhances shine [
19]. Proteins and peptides contribute to hair repair primarily through two mechanisms: surface film formation and internal penetration. Surface film formation involves large proteins adsorbing to the hair surface via electrostatic interactions, creating a protective barrier. Internal penetration relies on small peptides diffusing into the cortex to stabilize keratin networks via disulfide bond rearrangement. Large-molecular-weight proteins and peptides predominantly adhere to the surface of damaged hair via electrostatic adsorption or chemical cross-linking, forming a protective protein film that shields the hair from adverse external conditions [
20]. In contrast, small-molecular-weight proteins and peptides possess superior permeability, allowing them to penetrate the lipid layer on the hair fiber’s surface and reach the interior of the hair strand. By facilitating the rearrangement of internal chemical bonds, these peptides improve hair texture and overall structural integrity [
21]. Thus, we selected three fish protein peptides,
Takifugu obscurus skin peptides (TSPs),
Pneumatophorus japonicus bone peptides (PBPs), and
Pneumatophorus japonicus head peptides (PHPs), with good biological activities to verify their combined (TSP–PBP–PHP) repair potential.
Currently available peptide delivery systems include emulsions, micelles, and peptide-based nanocarriers. Micelles possess an amphiphilic structure with both hydrophilic and hydrophobic domains, allowing them to effectively encapsulate and transport small bioactive molecules, thereby facilitating deeper penetration into the hair shaft [
22]. However, micellar systems often require high concentrations of surfactants [
23,
24], which may lead to cuticle damage or scalp irritation when used excessively. Peptide-based nanocarriers, owing to their nanoscale dimensions, exhibit enhanced penetration capabilities and can form a sustained-release system on the hair [
25] or skin surface [
26], thereby providing prolonged repair effects. However, these technologies typically involve complex fabrication processes and high production costs, limiting their large-scale application in commercial haircare products. In contrast, emulsions offer a more cost-effective and scalable natural repair solution, making them suitable for the development of commercial haircare formulations [
27]. Emulsions are widely utilized in hair and skincare products [
28,
29], as they can be optimized through emulsifiers and thickeners to improve permeability and adhesion, ensuring more effective delivery of active ingredients to damaged hair [
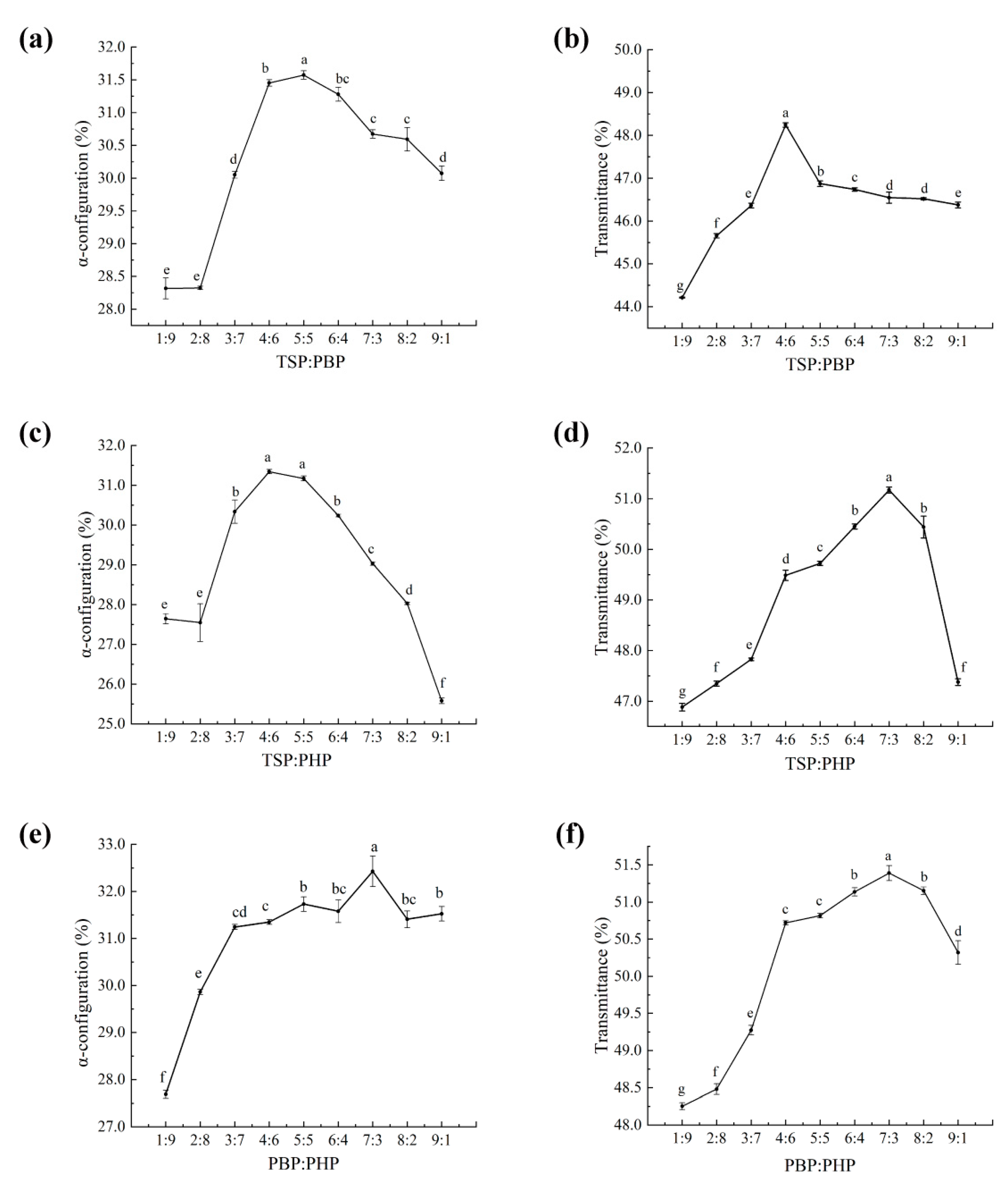
30]. However, conventional emulsions primarily remain on the hair surface, making deep repair more challenging. The innovation of this study lies in the development of a composite fish collagen peptide emulsion (CHFRE), which optimizes the ratio of the three bioactive peptides. This formulation not only forms a protective film on the hair surface but also enhances the penetration of small-peptide molecules, thereby improving the repair efficacy within the hair structure.
Consequently, the design and development of novel protein-based, eco-friendly, and sustainable products for human hair restoration hold significant potential for future advancements. In this study, we aim to validate the hair fiber repair effects of the selected fish-derived collagen peptide formulations and enhance their practical application potential by incorporating them into an emulsion system.
4. Conclusions
Existing commercial haircare products generally exhibit limited repair efficacy (
Figure S10 and Table S5). While some products partially seal hair cuticles and smooth the hair, in some cases, they exacerbate hair roughness due to the excessive surfactant content. Additionally, although silicone-based formulations may enhance hair texture, they hinder the absorption of protein peptides and amino acids, thereby compromising internal structural repair. Most products fail to effectively stabilize the α-configuration or facilitate keratin reorganization, resulting in overall suboptimal restorative performance. Even when certain products increase amino acid content, their penetration and binding capacities remain insufficient, limiting their ability to provide deep repair and long-lasting nourishment.
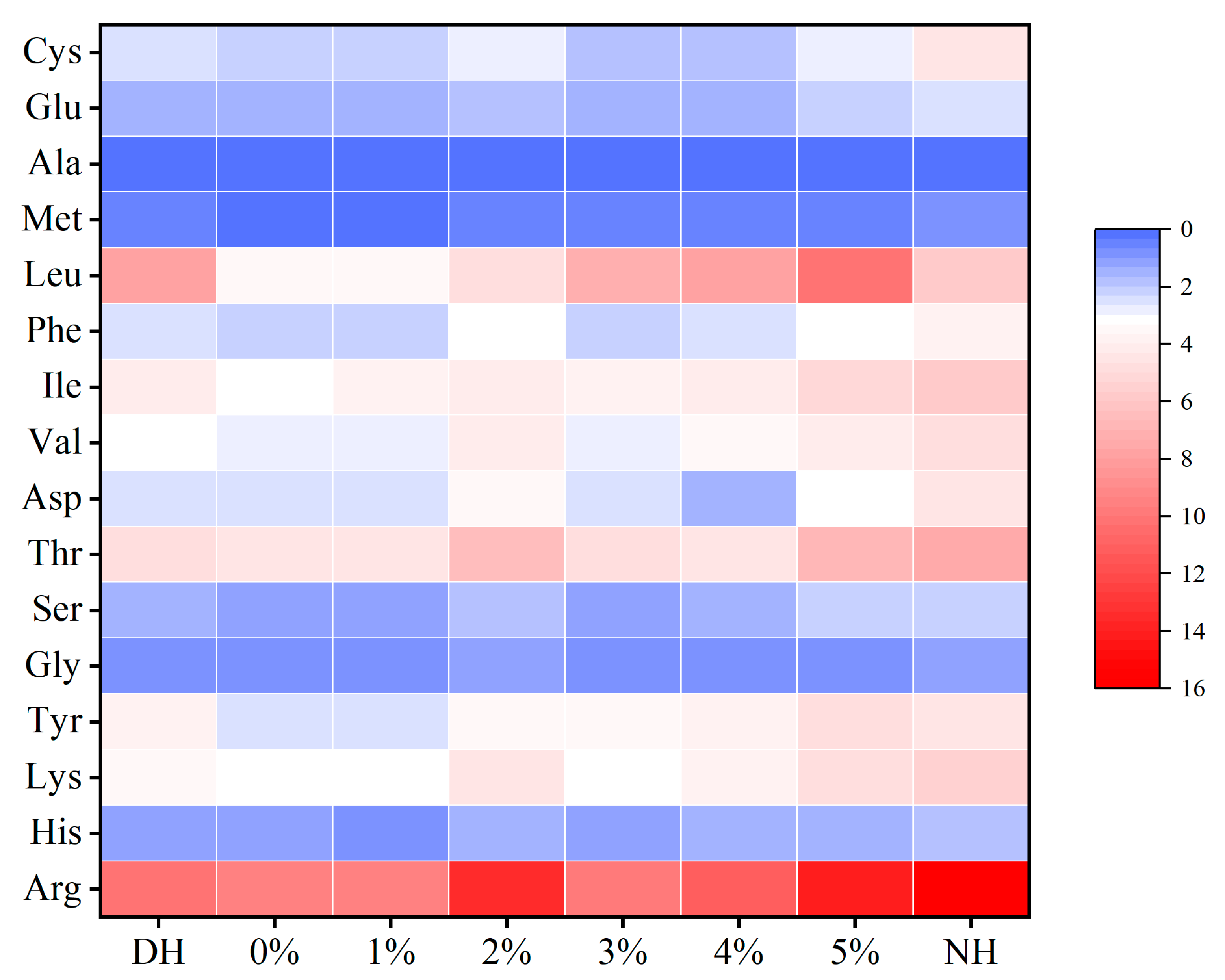
In comparison, the emulsion treatment described in this study exhibited exceptional restorative effects on DH, effectively reducing breakage, sealing hair cuticles, and filling pores caused by oxidative damage. The peptide/protein analogs in the emulsion were able to penetrate the cortical layer to varying degrees, enhancing protein absorption and optimizing its distribution, while the cystine derivatives progressively exhibited absorption characteristics similar to those of healthy hair. Following CHFRE treatment, the α-configuration content increased by 5.94%, and the RHC increased by 28.44%, significantly enhancing protein stability and bringing the keratin structure closer to that of healthy hair. Additionally, the transmittance of the S=O characteristic peak at 1042 cm−1 increased by 14.63%, further confirming a reduction in oxidative damage. Furthermore, total amino acid content increased by 16.77 g/100 g and hydrophobic amino acid content increased by 2.47 g/100 g, indicating improved hair affinity and keratin stability. Overall, CHFRE substantially improved the structural and chemical stability of damaged hair, enhanced protein absorption and uniform distribution, effectively mitigated oxidative damage, and increased amino acid content, making the hair more comparable to its healthy counterpart. This study provides valuable insights for the development of natural hair repair products with enhanced efficacy.
This study provides a novel approach to the efficient utilization of natural polymer proteins in the development of sustainable and eco-friendly haircare materials, contributing to the advancement of green beauty innovations and the sustainable use of resources.