Castor Oil-Based Epoxy Vitrimer Based on Dual Dynamic Network with Intrinsic Photothermal Self-Healing Capability
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Synthesis
2.2.1. Synthesis of Aromatic Schiff Base Curing Agent (ASB)
2.2.2. Synthesis of Castor Oil-Based Epoxy Resin (ASB–ECO)
2.3. Characterization and Test
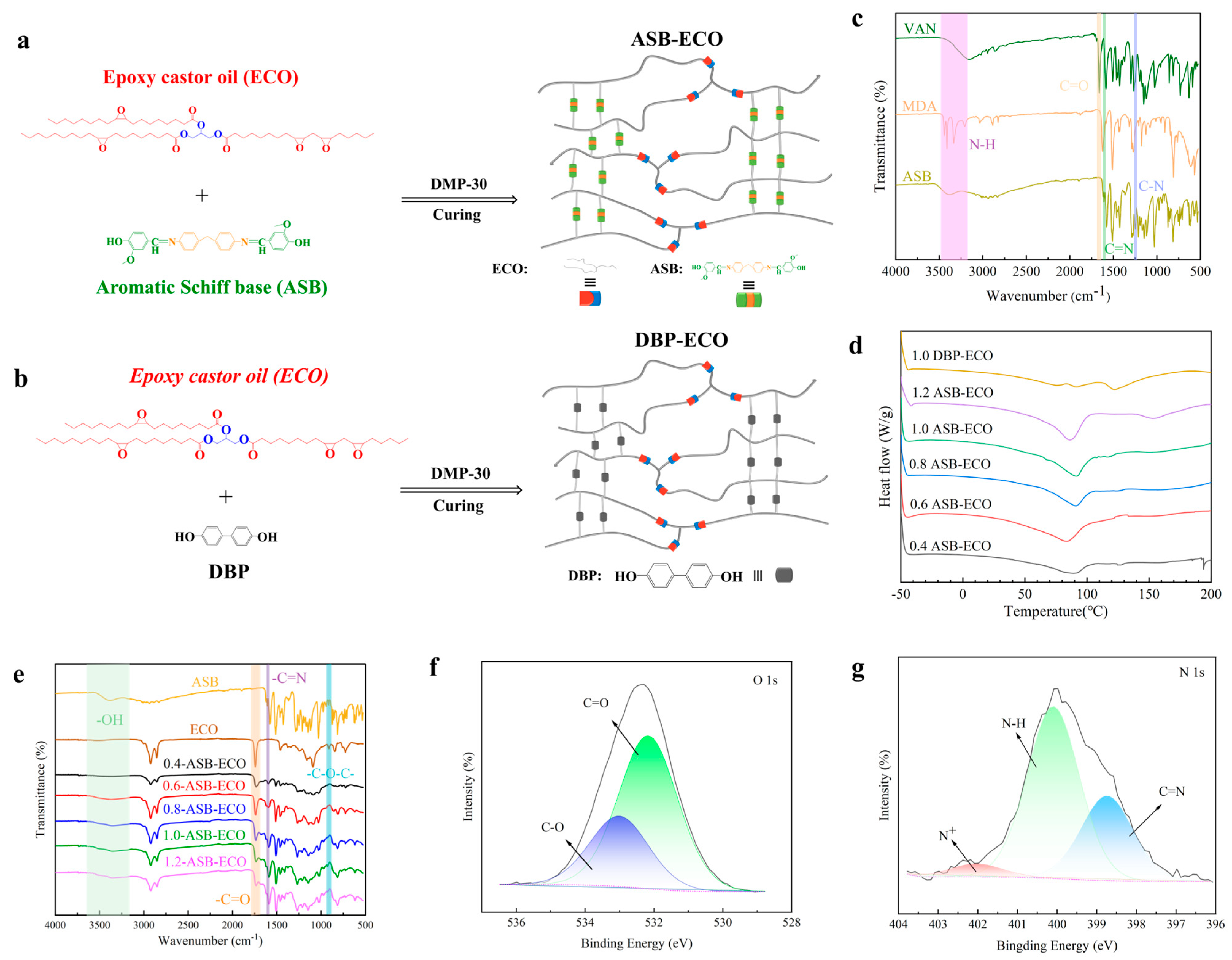
2.3.1. FTIR Characterization of ASB and ASB−ECO
2.3.2. 1H-NMR Characterization of ASB
2.3.3. Differential Scanning Calorimetry (DSC) Characterization of the ASB−ECO Mixture
2.3.4. Thermal Stability Test of ASB−ECO
2.3.5. Dynamic Temperature Analysis of ASB−ECO
2.3.6. Stress Relaxation Test of ASB−ECO
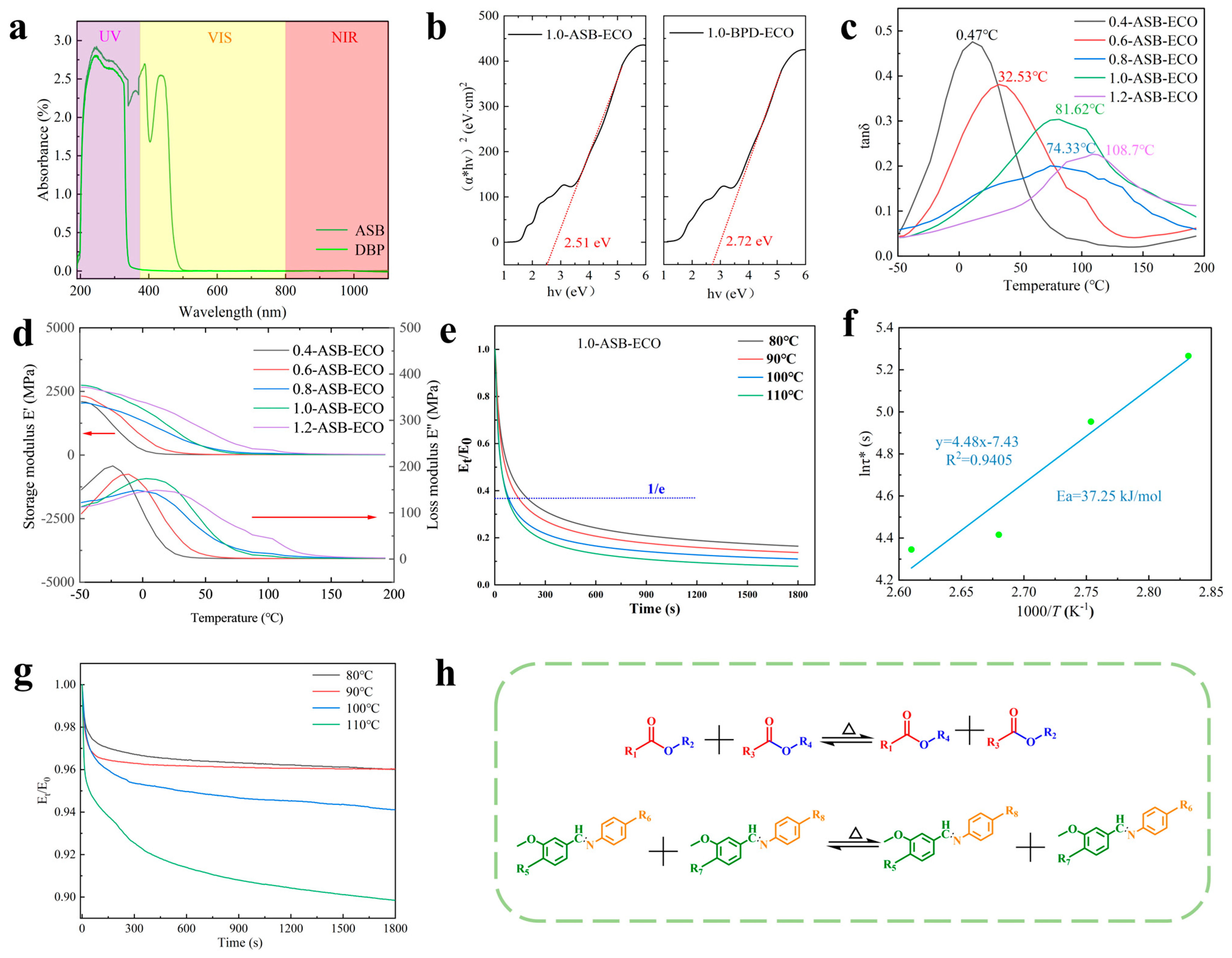
2.3.7. Ultraviolet–Visible (UV−Vis) Spectrophotometer Test
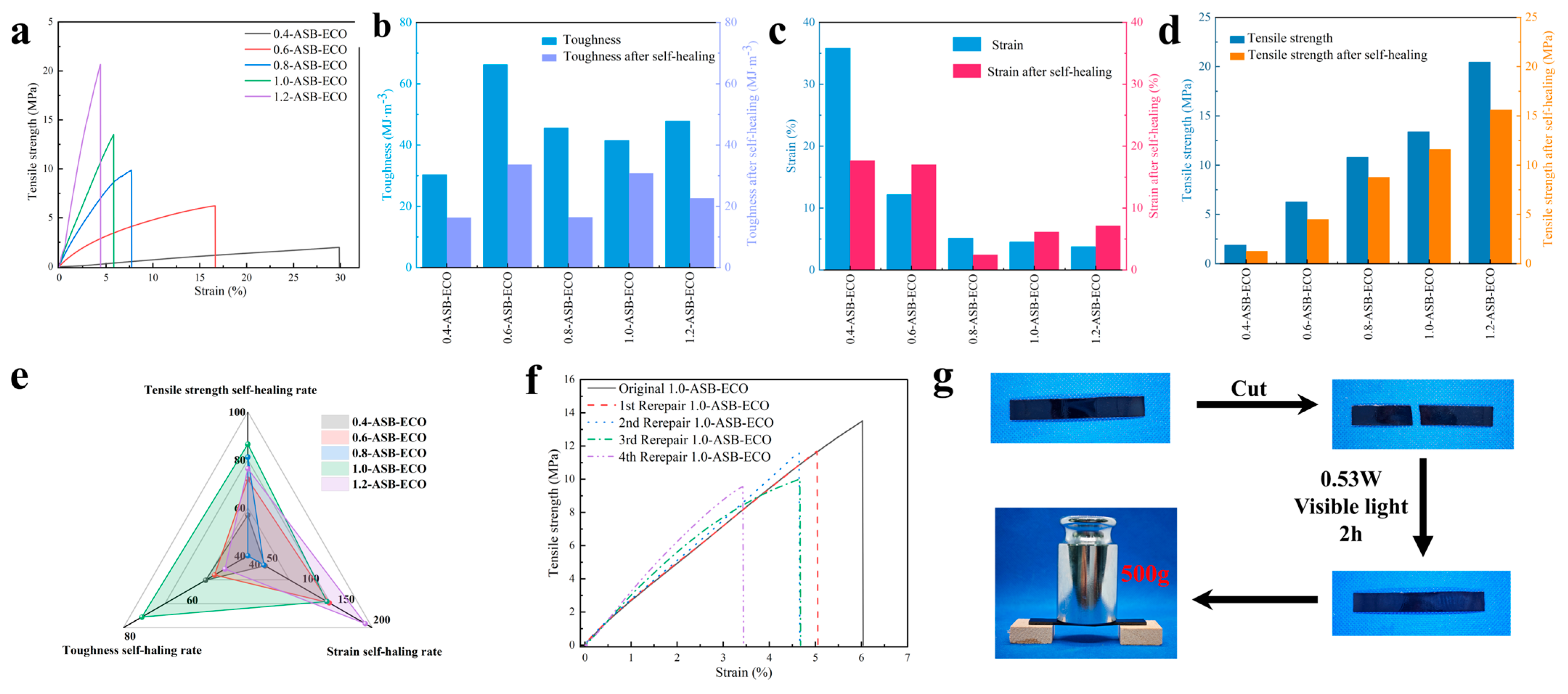
2.3.8. Mechanical and Self-Healing Performance Test of ASB−ECO
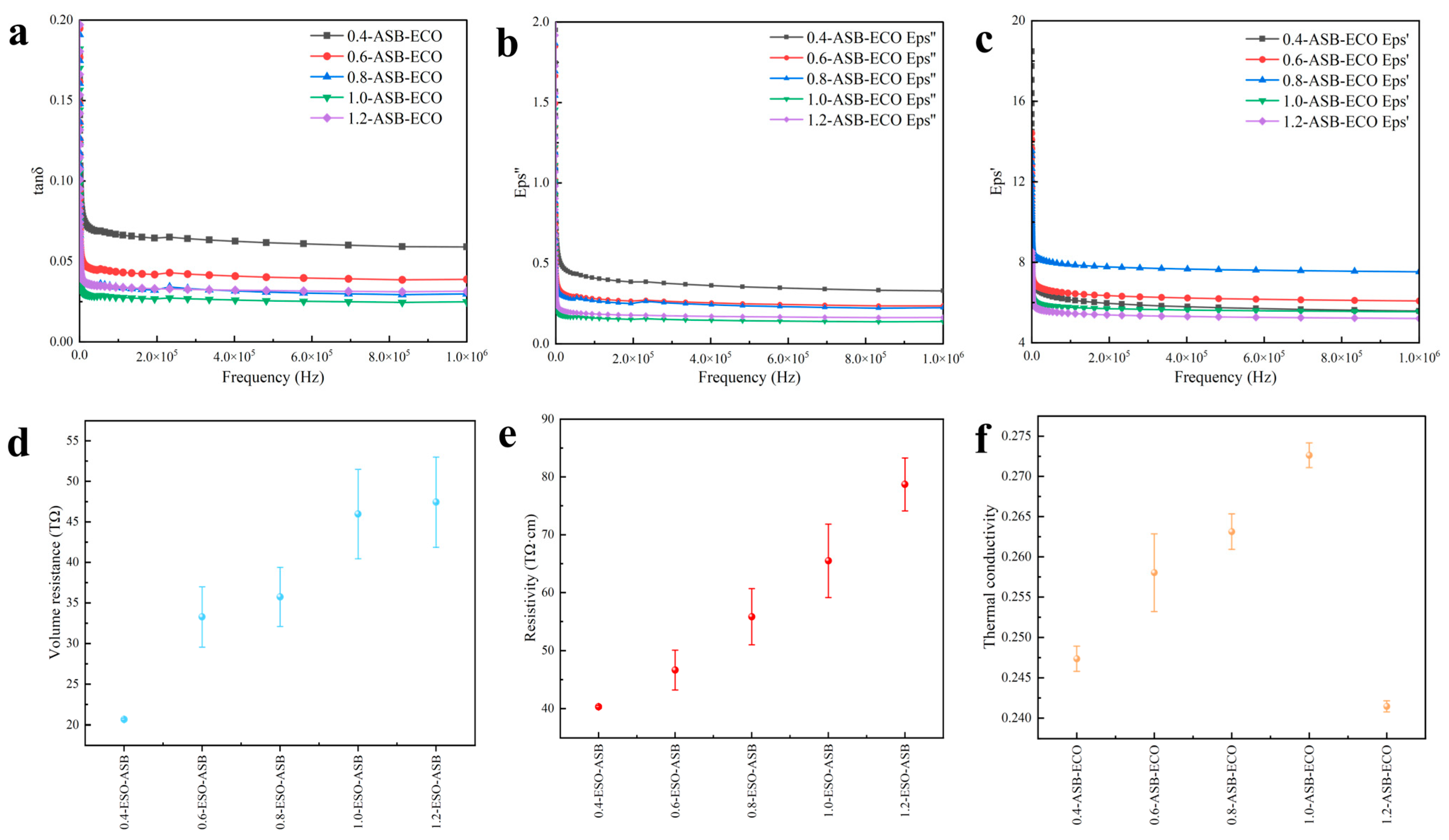
2.3.9. ASB−ECO Electrical Performance Test
3. Results and Discussion
3.1. Structure Characterization of ASB and ASB−ECO
3.2. Photothermal Effect and Thermomechanical Properties of ASB−ECO
3.2.1. Photothermal Effect of ASB−ECO
3.2.2. Viscoelastic Analysis of ASB−ECO
3.2.3. Stress Relaxation Analysis
3.3. ASB−ECO Mechanical Properties and Self-Healing Properties
3.4. Electrical and Thermal Conductivity of ASB−ECO
3.4.1. Dielectric Characteristics Analysis
3.4.2. Conductivity Characteristics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.Y.; On, S.Y.; Kim, J.; Lee, J.; Kim, T.-S.; Wardle, B.L.; Kim, S.S. Electronic Packaging Enhancement Engineered by Reducing the Bonding Temperature via Modified Cure Cycles. ACS Appl. Mater. Interfaces 2023, 15, 11024–11032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, T.; Lv, X.; Zhang, S.; Li, J.; Zhang, G.; Sun, R. High-Adhesion Epoxy-Based Solder Resist Dry Film for Integrated Circuits Enhanced by 2,2-Bis(hydroxymethyl)propionic Acid. ACS Appl. Polym. Mater. 2024, 6, 11987–11996. [Google Scholar] [CrossRef]
- Wu, X.; Hartmann, P.; Berne, D.; De Bruyn, M.; Cuminet, F.; Wang, Z.; Zechner, J.M.; Boese, A.D.; Placet, V.; Caillol, S.; et al. Closed-loop recyclability of a biomass-derived epoxy-amine thermoset by methanolysis. Science 2024, 384, eadj9989. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Zhang, J.; Dang, L.; Zhang, F.; Zhang, K.; Kong, Q.; Gu, J. Effect of the Structure of Epoxy Monomers and Curing Agents: Toward Making Intrinsically Highly Thermally Conductive and Low-Dielectric Epoxy Resins. JACS Au 2023, 3, 3424–3435. [Google Scholar] [CrossRef]
- Han, J.; Zhang, W.; Wei, M.; Zhu, Y.; Liu, X.; Li, X. Recyclable azine polyurethane thermosets and carbon fiber reinforced composites with excellent thermostability and mechanical properties. Compos. Part B Eng. 2024, 284, 111693. [Google Scholar] [CrossRef]
- Chen, M.; Luo, W.; Lin, S.; Zheng, B.; Zhang, H. Recyclable, reprocessable, self-healing elastomer-like epoxy vitrimer with low dielectric permittivity and its closed-loop recyclable carbon fiber reinforced composite. Compos. Part B Eng. 2023, 257, 110666. [Google Scholar] [CrossRef]
- Maes, S.; Badi, N.; Winne, J.M.; Du Prez, F.E. Taking dynamic covalent chemistry out of the lab and into reprocessable industrial thermosets. Nat. Rev. Chem. 2025, 9, 144–158. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Van Geem, K.M. Plastic waste recycling is gaining momentum. Science 2023, 381, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Ermis, H.; Collins, C.; Kumar Saha, S.; Murray, P. Beyond Visibility: Microorganisms for tackling plastic and microplastic problems for cleaner future. Chem. Eng. J. 2024, 497, 154585. [Google Scholar] [CrossRef]
- Qin, Z.-H.; Mou, J.-H.; Chao, C.Y.H.; Chopra, S.S.; Daoud, W.; Leu, S.-y.; Ning, Z.; Tso, C.Y.; Chan, C.K.; Tang, S.; et al. Biotechnology of Plastic Waste Degradation, Recycling, and Valorization: Current Advances and Future Perspectives. ChemSusChem 2021, 14, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhang, J.; Wu, C.; Luo, M.; Ye, B.; Zhang, H.; Wang, J.; Miao, M.; Li, T.; Zhang, D. Closed-Loop Recycling of Tough and Flame-Retardant Epoxy Resins. Macromolecules 2023, 56, 5290–5305. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Tian, F.; Yang, Y.; Wang, X.-L.; An, W.-L.; Zhao, X.; Xu, S.; Wang, Y.-Z. From waste epoxy resins to efficient oil/water separation materials via a microwave assisted pore-forming strategy. Mater. Horiz. 2019, 6, 1733–1739. [Google Scholar] [CrossRef]
- Wang, W.; Lu, X.; Li, Y.; Li, X.; Sun, H.; Sun, J. Mechanically Robust Reversibly Cross-Linked Polymers with Closed-Loop Recyclability for Use in Flexible Printed Circuit Boards. Adv. Funct. Mater. 2024, 34, 2401822. [Google Scholar] [CrossRef]
- Zhang, V.; Kang, B.; Accardo, J.V.; Kalow, J.A. Structure–Reactivity–Property Relationships in Covalent Adaptable Networks. J. Am. Chem. Soc. 2022, 144, 22358–22377. [Google Scholar] [CrossRef]
- Liu, J.; Bernaerts, K.V. Preparation of lignin-based imine vitrimers and their potential application as repairable, self-cleaning, removable and degradable coatings. J. Mater. Chem. A 2024, 12, 2959–2973. [Google Scholar] [CrossRef]
- Hu, Y.; Sha, Y.; Chen, L.; Ma, Y.; Huang, Q.; Zhang, M.; Jia, P.; Zhou, Y. Bio-based adaptable dynamically cross-linked networks and their composites: Multiple stimulus responses and potential electromagnetic shielding applications. J. Mater. Chem. A 2025, 13, 4317–4328. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Yang, Q.; Gong, P.; Park, C.B.; Li, G. Sustainable polyester vitrimer capable of fast self-healing and multiple shape-programming via efficient synthesis and configuration processing. J. Mater. Chem. A 2023, 11, 10912–10926. [Google Scholar] [CrossRef]
- He, W.; Huang, H.; Xie, L.; Wang, C.; Yu, J.; Lu, S.; Fan, H. The influence of self-crosslinked epoxidized castor oil on the properties of Poly (lactic acid) via dynamic vulcanization: Toughening effect, thermal properties and structures. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127517. [Google Scholar] [CrossRef]
- Esen, H.; Çayli, G. Epoxidation and polymerization of acrylated castor oil. Eur. J. Lipid Sci. Technol. 2016, 118, 959–966. [Google Scholar] [CrossRef]
- Chebotar, A.; Domnich, B.; Panchenko, Y.; Donchak, V.; Stetsyshyn, Y.; Voronov, A. Thermal behavior of polymers and copolymers based on plant oils with differing saturated and monounsaturated content. Polym. Int. 2025, 74, 255–263. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Y.; Xia, C.; Ren, Y.; Gao, J.; Xiang, Y.; Shi, S. Sustainable, recyclable and biodegradable castor oil-derived elastomers enabled by dynamic acetoacetyl formed amides. Chem. Eng. J. 2024, 491, 152195. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Song, F.; Shang, Q.; Hu, Y.; Jia, P.; Liu, C.; Hu, L.; Zhu, G.; Huang, J.; et al. Castor-oil-based, robust, self-healing, shape memory, and reprocessable polymers enabled by dynamic hindered urea bonds and hydrogen bonds. Chem. Eng. J. 2022, 429, 131848. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Hogerat, R.; Ghosal, K.; Shaheen-Mualim, M.; Farah, S. Renewable polyol-based biodegradable polyesters as greener plastics for industrial applications. Chem. Eng. J. 2023, 459, 141211. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Yuan, W.; Zhou, S.; You, S.; Zhu, J. Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green Chem. 2019, 21, 1484–1497. [Google Scholar] [CrossRef]
- Wu, J.; Yu, X.; Zhang, H.; Guo, J.; Hu, J.; Li, M.-H. Fully Biobased Vitrimers from Glycyrrhizic Acid and Soybean Oil for Self-Healing, Shape Memory, Weldable, and Recyclable Materials. ACS Sustain. Chem. Eng. 2020, 8, 6479–6487. [Google Scholar] [CrossRef]
- Bergoglio, M.; Reisinger, D.; Schlögl, S.; Griesser, T.; Sangermano, M. Sustainable Bio-Based UV-Cured Epoxy Vitrimer from Castor Oil. Polymers 2023, 15, 1024. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, W.; Yang, W.; Liu, X.; Lin, J.; Zhang, L.; He, S. High-Strength and Multi-Recyclable Epoxy Vitrimer Containing Dual-Dynamic Covalent Bonds Based on the Disulfide and Imine Bond Metathesis. ACS Sustain. Chem. Eng. 2023, 11, 14591–14600. [Google Scholar] [CrossRef]
- Luo, J.; Demchuk, Z.; Zhao, X.; Saito, T.; Tian, M.; Sokolov, A.P.; Cao, P.-F. Elastic vitrimers: Beyond thermoplastic and thermoset elastomers. Matter 2022, 5, 1391–1422. [Google Scholar] [CrossRef]
- Hao, Y.; Zhong, L.; Li, T.; Zhang, J.; Zhang, D. High-Performance Recyclable and Malleable Epoxy Resin with Vanillin-Based Hyperbranched Epoxy Resin Containing Dual Dynamic Bonds. ACS Sustain. Chem. Eng. 2023, 11, 11077–11087. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Liu, Y.-Y.; Weng, Y.; Li, Y.-D.; Zeng, J.-B. Sustainable Epoxy Vitrimers from Epoxidized Soybean Oil and Vanillin. ACS Sustain. Chem. Eng. 2020, 8, 15020–15029. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Des. 2020, 186, 108248. [Google Scholar] [CrossRef]
- Zhang, Y.; Yukiko, E.; Tadahisa, I. Bio-based vitrimers from divanillic acid and epoxidized soybean oil. RSC Sustain. 2023, 1, 543–553. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; He, J.; Li, Y.-D.; Zhao, X.-L.; Zeng, J.-B. Biobased, reprocessable and weldable epoxy vitrimers from epoxidized soybean oil. Ind. Crops Prod. 2020, 153, 112576. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.-L.; Huang, Z.-j.; Tang, Z.-c.; Sun, D.; Yang, J.; Qi, X.; Wang, Y. Bio-inspired polypyrrole nanowire arrays on melamine foam with high-performance photo/electro-thermal conversion for all-weather cleanup of crude oil. J. Mater. Chem. A 2025, 13, 8594–8607. [Google Scholar] [CrossRef]
- An, B.; Yang, M.; Shang, Y.; Sun, C.; Wang, S.; Qian, K.; Zou, X.; Dong, Q.; Shao, Y.; Chu, C.; et al. Broad-spectrum photothermal high-entropy alloy powders for efficient solar-driven antibacterial and dye degradation. J. Mater. Chem. A 2025, 13, 7999–8014. [Google Scholar] [CrossRef]
- Wang, J.; Lin, X.; Wang, R.; Lu, Y.; Zhang, L. Self-Healing, Photothermal-Responsive, and Shape Memory Polyurethanes for Enhanced Mechanical Properties of 3D/4D Printed Objects. Adv. Funct. Mater. 2023, 33, 2211579. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, H.; Zhang, Z.; Shao, Y.; Yu, Q.; Cao, X.; Pan, S.; Mu, X.; Gao, Z.; Wang, D.; et al. Self-healing polyurethane coatings based on dynamic chemical bond synergy under conditions of photothermal response. Chem. Eng. J. 2023, 474, 145811. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Zhai, M.; Wei, B.; Lyu, B.; Liu, L. Self-Healing and Recyclable Castor Oil-Based Epoxy Vitrimer Based on Dual Dynamic Bonds of Disulfide and Ester Bonds. ACS Appl. Polym. Mater. 2024, 6, 8399–8408. [Google Scholar] [CrossRef]
- Cao, L.; Gong, Z.; Liu, C.; Fan, J.; Chen, Y. Design and fabrication of mechanically strong and self-healing rubbers via metal-ligand coordination bonds as dynamic crosslinks. Compos. Sci. Technol. 2021, 207, 108750. [Google Scholar] [CrossRef]
- Feng, H.; Wang, W.; Wang, T.; Zhang, L.; Li, W.; Hou, J.; Chen, S. Preparation of dynamic polyurethane networks with UV-triggered photothermal self-healing properties based on hydrogen and ion bonds for antibacterial applications. J. Mater. Sci. Technol. 2023, 133, 89–101. [Google Scholar] [CrossRef]
- Wang, G.; Tang, Z.; Gao, Y.; Liu, P.; Li, Y.; Li, A.; Chen, X. Phase Change Thermal Storage Materials for Interdisciplinary Applications. Chem. Rev. 2023, 123, 6953–7024. [Google Scholar] [CrossRef]
- Chen, X.; Yang, N.; Wang, Y.; He, H.; Wang, J.; Wan, J.; Jiang, H.; Xu, B.; Wang, L.; Yu, R.; et al. Highly Efficient Photothermal Conversion and Water Transport during Solar Evaporation Enabled by Amorphous Hollow Multishelled Nanocomposites. Adv. Mater. 2022, 34, 2107400. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, J.; Gao, L.; Zhang, B.; Jiang, J.; Hu, J. Recyclable, reprocessable, self-adhered and repairable carbon fiber reinforced polymers using full biobased matrices from camphoric acid and epoxidized soybean oil. Green Chem. 2021, 23, 2763–2772. [Google Scholar] [CrossRef]
- Li, J.; Ju, B.; Zhang, S. Catalyst-free, sustainable epoxy vitrimers from epoxidized soybean oil and natural sugar alcohols. Ind. Crops Prod. 2023, 205, 117466. [Google Scholar] [CrossRef]
- Sangaletti, D.; Ceseracciu, L.; Marini, L.; Athanassiou, A.; Zych, A. Biobased boronic ester vitrimer resin from epoxidized linseed oil for recyclable carbon fiber composites. Resour. Conserv. Recycl. 2023, 198, 107205. [Google Scholar] [CrossRef]
- Li, C.; Ju, B.; Zhang, S. Fully bio-based hydroxy ester vitrimer synthesized by crosslinking epoxidized soybean oil with doubly esterified starch. Carbohydr. Polym. 2023, 302, 120442. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, M.; Shi, L.; Lei, Q.; Zhang, S.; Zhang, L.; Lyu, B.; Zhao, S.; Ma, J.; Thakur, V.K. Sustainable castor oil-based vitrimers: Towards new materials with reprocessability, self-healing, degradable and UV-blocking characteristics. Ind. Crops Prod. 2023, 193, 116210. [Google Scholar] [CrossRef]
| Sample Codes | ASB (g) | DBP (g) | ECO (g) |
|---|---|---|---|
| 0.4-ASB–ECO | 3.72 | 0 | 10 |
| 0.6-ASB–ECO | 5.29 | 0 | 10 |
| 0.8-ASB–ECO | 7.65 | 0 | 10 |
| 1.0-ASB–ECO | 9.32 | 0 | 10 |
| 1.2-ASB–ECO | 11.18 | 0 | 10 |
| 1.0-DBP–ECO | 0 | 7.44 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Zhu, H.; Chen, K.; Jin, T.; Wang, Z.; Luo, Z.; Wang, J.; Sun, H.; Wei, S.; Gao, Z. Castor Oil-Based Epoxy Vitrimer Based on Dual Dynamic Network with Intrinsic Photothermal Self-Healing Capability. Polymers 2025, 17, 897. https://doi.org/10.3390/polym17070897
Shao Y, Zhu H, Chen K, Jin T, Wang Z, Luo Z, Wang J, Sun H, Wei S, Gao Z. Castor Oil-Based Epoxy Vitrimer Based on Dual Dynamic Network with Intrinsic Photothermal Self-Healing Capability. Polymers. 2025; 17(7):897. https://doi.org/10.3390/polym17070897
Chicago/Turabian StyleShao, Yingqing, Haoxin Zhu, Kang Chen, Tianyi Jin, Zhiwen Wang, Zhixin Luo, Jinhui Wang, Haoyuan Sun, Shuangying Wei, and Zhenhua Gao. 2025. "Castor Oil-Based Epoxy Vitrimer Based on Dual Dynamic Network with Intrinsic Photothermal Self-Healing Capability" Polymers 17, no. 7: 897. https://doi.org/10.3390/polym17070897
APA StyleShao, Y., Zhu, H., Chen, K., Jin, T., Wang, Z., Luo, Z., Wang, J., Sun, H., Wei, S., & Gao, Z. (2025). Castor Oil-Based Epoxy Vitrimer Based on Dual Dynamic Network with Intrinsic Photothermal Self-Healing Capability. Polymers, 17(7), 897. https://doi.org/10.3390/polym17070897







