Study on Network Structure and Heat Resistance in Air of Boron-Modified Phenolic Resin Aerogel
Abstract
1. Introduction
2. Experimentation
2.1. Materials
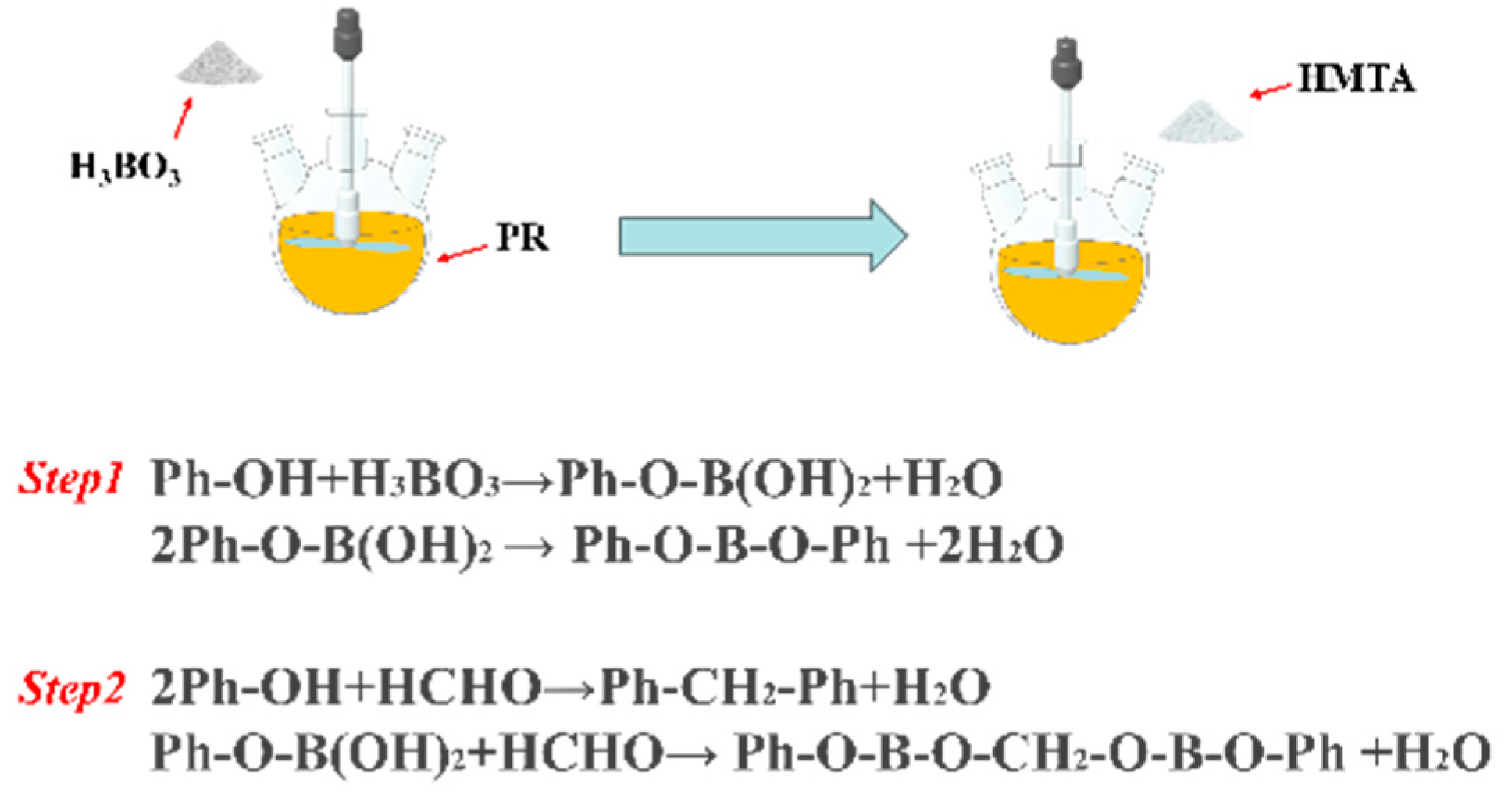
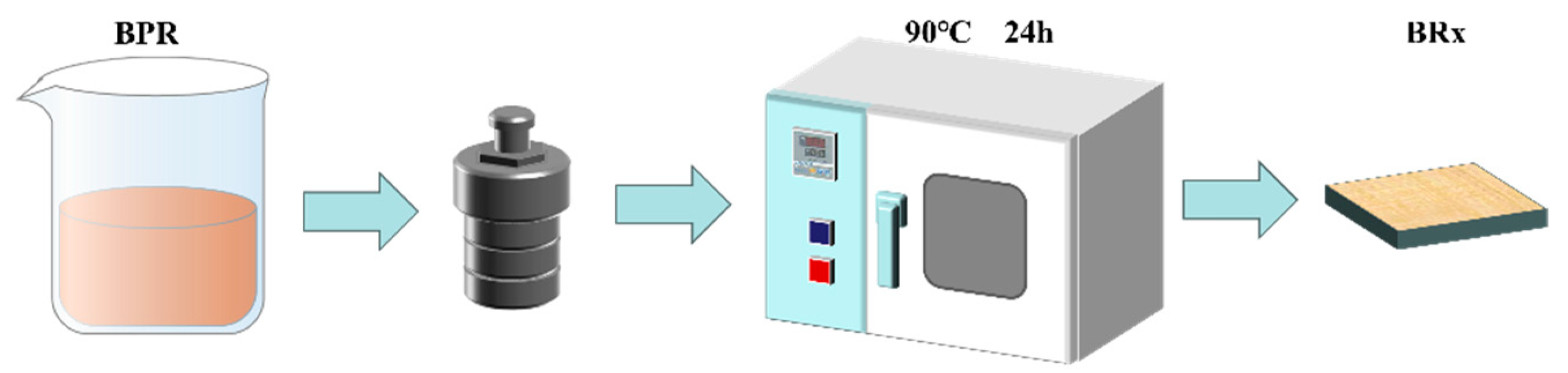
2.2. Preparation of Boron-Modified Phenolic Aerogel
2.3. Characterisation
3. Results and Discussion
3.1. Synthesis of Boron-Modified Phenolic Resin
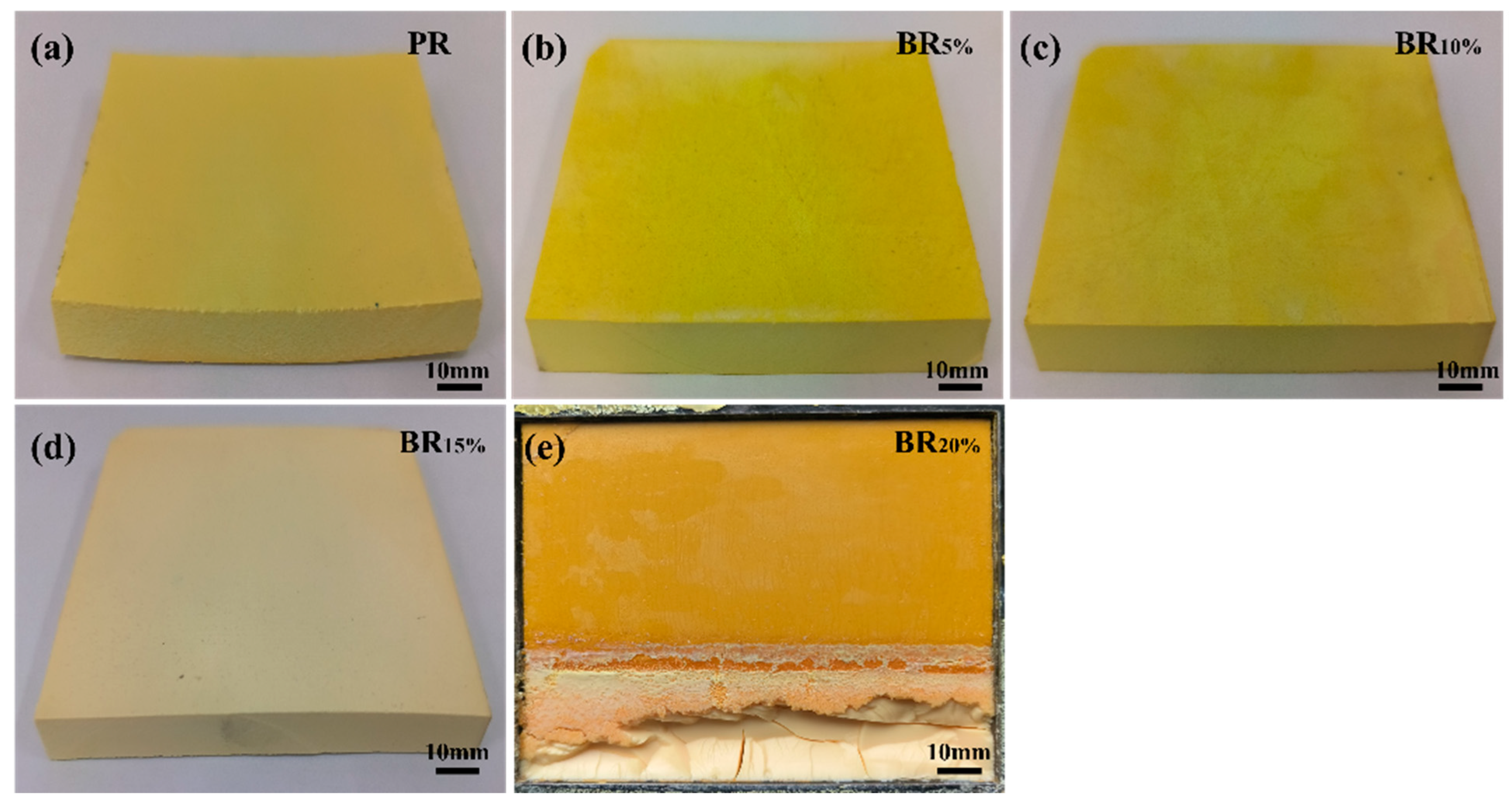
3.2. Formability of Boron-Modified Phenolic Aerogel
3.3. Microstructure of Boron-Modified Phenolic Aerogel
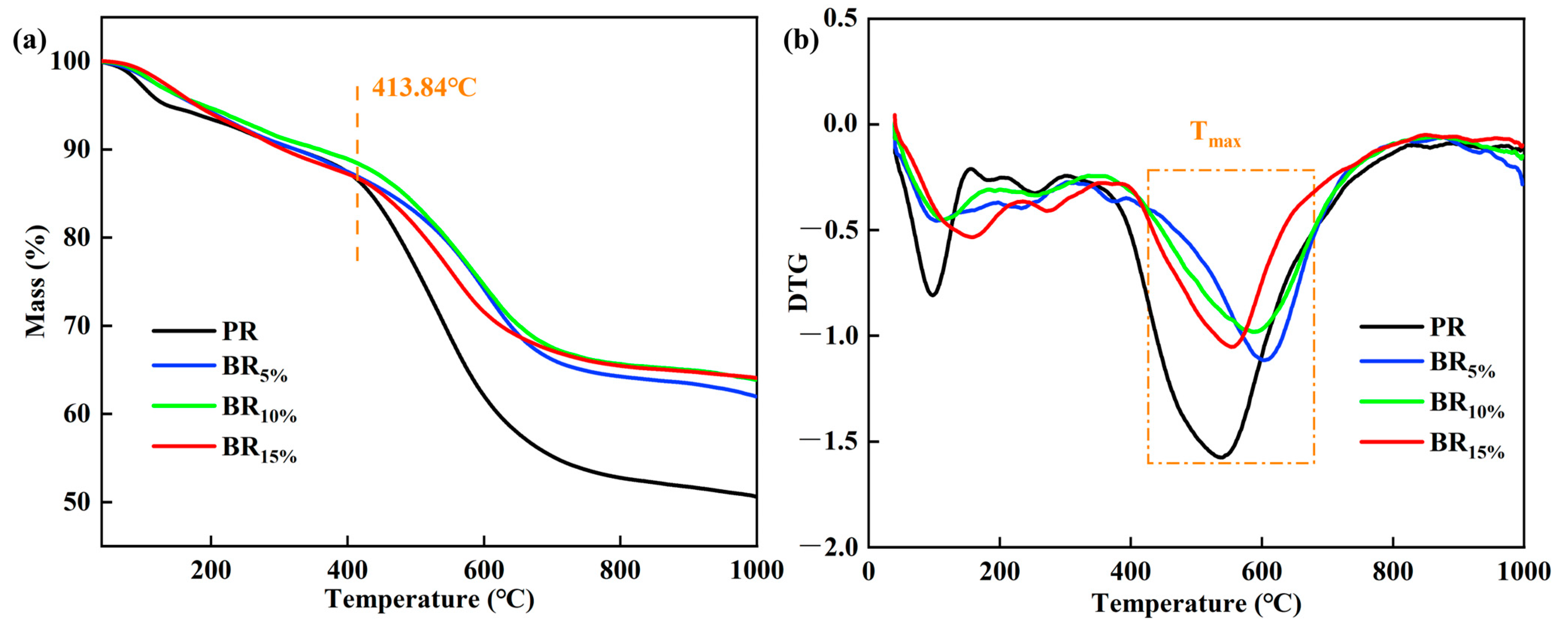
3.4. Thermal Stability of Boron-Modified Phenolic Aerogel in N2
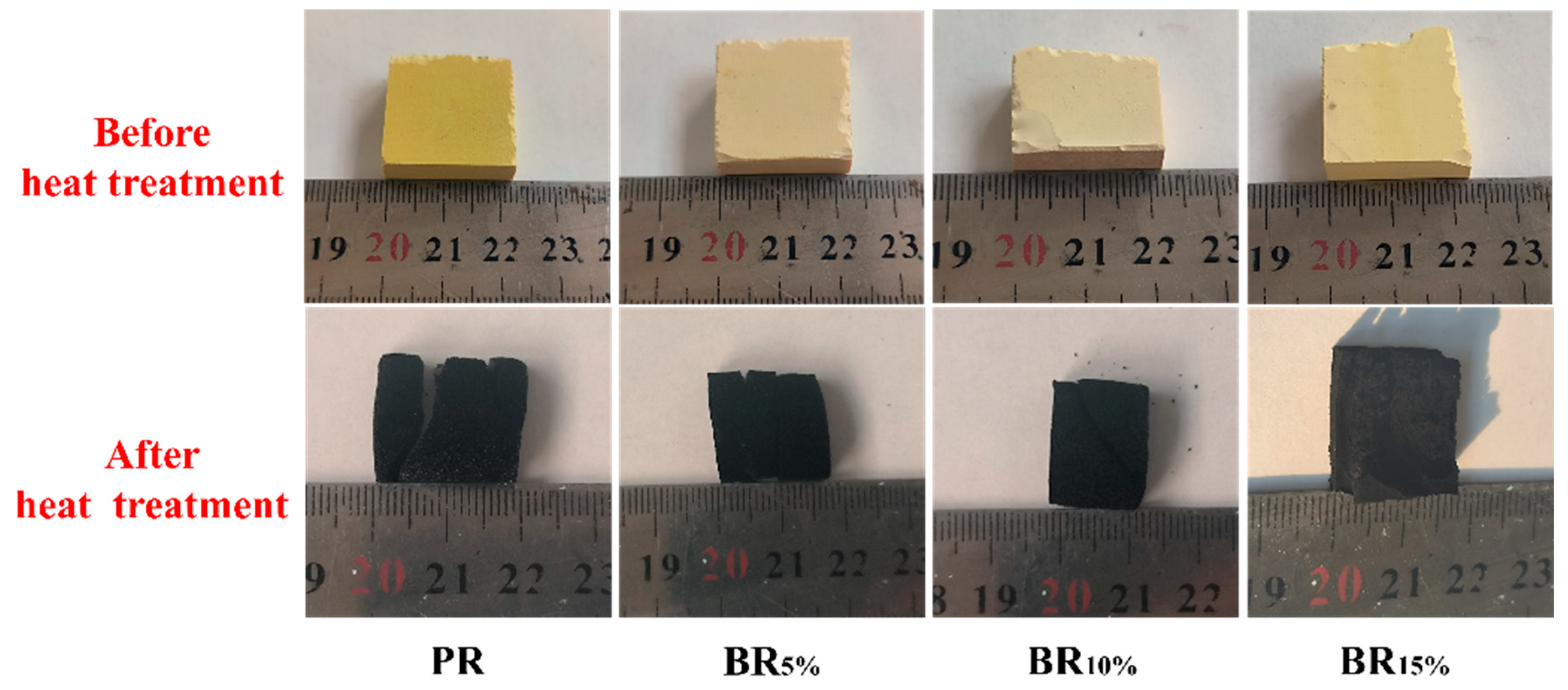
3.5. Heat Resistance in Air of Boron-Modified Phenolic Aerogel
3.6. Mechanical Properties of Phenolic Aerogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, C.; Wang, D.; Zhang, Y.; Li, K.; Ding, J. Research progress on preparation, modification, and application of phenolic aerogel. Nanotechnol. Rev. 2023, 12, 20230109. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, C.; Ding, J.; Zhuang, Y.; Li, Y.; Wang, C.; Huang, Z. A novel method for low-cost and rapid preparation of nanoporous phenolic aerogels and its performance regulation mechanism. Rev. Adv. Mater. Sci. 2022, 61, 817–828. [Google Scholar] [CrossRef]
- Han, M.; Zhang, X.; Wang, Z.; Silberschmidt, V.V.; Bi, Q. Thermo-mechanical coupling behavior of needle-punched carbon/carbon composites. Sci. Eng. Compos. Mater. 2024, 31, 20240045. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, Z. Advances in processing and ablation properties of carbon fiber reinforced ultra-high temperature ceramic composites. Rev. Adv. Mater. Sci. 2024, 63, 20240029. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Huang, H.; Pan, R.; Wu, C.; Yan, X.; Wang, H.; Pan, Y.; Hong, C.; Zhang, X. Multiscale, elastic, and low-density carbon fibre/siliconoxycarbide-phenolic interpenetrating aerogel nanocomposite for ablative thermal protection. Compos. Part B Eng. 2022, 245, 110212. [Google Scholar] [CrossRef]
- De Cesare, M.; Savino, L.; Ceglia, G.; Alfano, D.; Di Carolo, F.; French, A.D.; Rapagnani, D.; Gravina, S.; Cipullo, A.; Del Vecchio, A.; et al. Applied radiation physics techniques for diagnostic evaluation of the plasma wind and thermal protection system critical parameters in aerospace re-entry. Prog. Aerosp. Sci. 2020, 112, 100550. [Google Scholar] [CrossRef]
- Natali, M.; Kenny, J.M.; Torre, L. Science and technology of polymeric ablative materials for thermal protection systems and propulsion devices: A review. Prog. Mater. Sci. 2016, 84, 192–275. [Google Scholar] [CrossRef]
- Yin, R.; Cheng, H.; Hong, C.; Zhang, X. Synthesis and characterization of novel phenolic resin/silicone hybrid aerogel composites with enhanced thermal, mechanical and ablative properties. Compos. Part A Appl. Sci. Manuf. 2017, 101, 500–510. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, Y.; Huang, Y.; Tian, J.; Heng, Z.; Liang, M.; Chen, Y.; Zou, H. Nano Co-Continuous Structure Poly(imide-siloxane)/Phenolic Hybrid Resin Fabricated by Copolymerization for the Thermal Protection System. Ind. Eng. Chem. Res. 2023, 62, 10477–10486. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.; Huang, R.; Huang, M.; Liu, X.; Zhou, Z.; Guo, P.; Mao, Z.; Xu, X.; Wang, X. Elastic, antiflaming phenolic aerogels for advanced thermal protection at extreme environments. Compos. Commun. 2024, 48, 101889. [Google Scholar] [CrossRef]
- Pan, Y.; Jin, X.; Wang, H.; Huang, H.; Wu, C.; Yan, X.; Hong, C.; Zhang, X. Nano-TiO2 coated needle carbon fiber reinforced phenolic aerogel composite with low density, excellent heat-insulating and infrared radiation shielding performance. J. Mater. Sci. Technol. 2023, 152, 181–189. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, Z.; Zhu, X.; Ge, B.; Guo, F.; Men, X.; Liu, W. Influence of functional graphene as filler on the tribological behaviors of Nomex fabric/phenolic composite. Compos. Part A Appl. Sci. Manuf. 2013, 49, 157–164. [Google Scholar] [CrossRef]
- Wang, S.; Jing, X.; Wang, Y.; Si, J. High char yield of aryl boron-containing phenolic resins: The effect of phenylboronic acid on the thermal stability and carbonization of phenolic resins. Polym. Degrad. Stab. 2014, 99, 1–11. [Google Scholar] [CrossRef]
- Li, P.; Shi, M.; Deng, Z.; Han, P.; Yang, T.; Hu, R.; Dong, C.; Wang, R.; Ding, J. Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing. Nanotechnol. Rev. 2022, 11, 3031–3041. [Google Scholar] [CrossRef]
- Rao, G.R.; Srikanth, I.; Reddy, K.L. Effect of organo-modified montmorillonite nanoclay on mechanical, thermal and ablation behavior of carbon fiber/phenolic resin composites. Def. Technol. 2021, 17, 812–820. [Google Scholar] [CrossRef]
- Gane, P.; Dimic-Misic, K.; Hummel, M.; Welker, M.; Rentsch, S. Stochastic transient Liquid-Solid Phase Separation reveals multi-level Dispersion States of Particles in Suspension. Appl. Rheol. 2019, 29, 41–57. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, A.; Ge, T.; Liu, X.; Tang, X.; Li, Y. Research progress on modification of phenolic resin. Mater. Today Commun. 2021, 26, 101879. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, H.; Gao, X.; Wang, H.; Fang, G.; Wang, B.; Hong, C.; Meng, S. Insight into pyrolysis behavior of silicone-phenolic hybrid aerogel through thermal kinetic analysis and ReaxFF MD simulations. Chem. Eng. J. 2023, 458, 141480. [Google Scholar] [CrossRef]
- Xiao, J.; Fang, G.; Jin, X.; Wang, B.; Meng, S. A comprehensive study of pyrolysis characteristics of silicone-modified phenolic aerogel matrix Nanocomposites: Kinetic Analysis, ReaxFF MD Simulations, and ANN prediction. Chem. Eng. J. 2023, 472, 145049. [Google Scholar] [CrossRef]
- Huang, H.; Lv, Y.; Jin, X.; Wang, H.; Wu, C.; Pan, Y.; Yan, X.; Hong, C.; Han, W.; Zhang, X. Bifunctional silicone triggered long-range crosslinking phenolic aerogels with flexibility and thermal insulation for thermal regulation. Chem. Eng. J. 2023, 470, 144413. [Google Scholar] [CrossRef]
- Huang, H.; Hong, C.; Jin, X.; Wu, C.; Wang, W.; Wang, H.; Pan, Y.; Wu, S.; Yan, X.; Han, W.; et al. Facile fabrication of superflexible and thermal insulating phenolic aerogels backboned by silicone networks. Compos. Part A Appl. Sci. Manuf. 2023, 164, 107270. [Google Scholar] [CrossRef]
- Fu, H.; Qin, Y.; Peng, Z.; Dou, J.; Huang, Z. A novel co-continuous Si–Zr hybrid phenolic aerogel composite with excellent antioxidant ablation enabled by sea-island-like ceramic structure at high temperature. Ceram. Int. 2024, 50, 21008–21019. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Ye, L.; Zhao, T.; Li, H. Microphase separation regulation of polyurethane modified phenolic resin aerogel to enhance mechanical properties and thermal insulation. Chem. Eng. J. 2024, 495, 153532. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Z.; Zhang, Y.; Shen, C.; Wang, Y.; Zhan, L. High-energy B-O bonds enable the phenolic aerogel with enhanced thermal stability and low thermal conductivity. Appl. Surf. Sci. 2024, 669, 160459. [Google Scholar] [CrossRef]
- Wang, D.; Ding, J.; Wang, B.; Zhuang, Y.; Huang, Z. Synthesis and Thermal Degradation Study of Polyhedral Oligomeric Silsesquioxane (POSS) Modified Phenolic Resin. Polymers 2021, 13, 1182. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Hong, C.; Zhang, X.; Zeng, T. Lightweight chopped carbon fibre reinforced silica-phenolic resin aerogel nanocomposite: Facile preparation, properties and application to thermal protection. Compos. Part A Appl. Sci. Manuf. 2018, 112, 81–90. [Google Scholar] [CrossRef]
- Fitzer, E.; Schäfer, W. The effect of crosslinking on the formation of glasslike carbons from thermosetting resins. Carbon 1970, 8, 353–364. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Su, H.; Li, C.; Fan, W.; Jing, X. Enhanced thermal resistance and ablation properties of ethylene-propylene-diene monomer rubber with boron-containing phenolic resins. React. Funct. Polym. 2022, 170, 105136. [Google Scholar] [CrossRef]



| Samples | Phenolic Resin (g) | H3BO3 (g) | B (wt%) |
|---|---|---|---|
| PR | 100 | 0 | 0 |
| BR5% | 100 | 28.60 | 5 |
| BR10% | 100 | 57.20 | 10 |
| BR15% | 100 | 85.80 | 15 |
| BR20% | 100 | 114.40 | 20 |
| Samples | PR | BR5% | BR10% | BR15% | BR20% |
|---|---|---|---|---|---|
| Densities (g/cm3) | 0.537 | 0.507 | 0.511 | 0.642 | / |
| Volumetric shrinkage (%) | 34.72 | 20.02 | 18.35 | 20.06 | / |
| Samples | PR | BR5% | BR10% | BR15% |
|---|---|---|---|---|
| Mass residual rate (%) | 36.51 | 49.72 | 54.92 | 46.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Wang, D.; Wang, Q.; Chen, X.; Ding, J.; Yan, X. Study on Network Structure and Heat Resistance in Air of Boron-Modified Phenolic Resin Aerogel. Polymers 2025, 17, 860. https://doi.org/10.3390/polym17070860
Wu T, Wang D, Wang Q, Chen X, Ding J, Yan X. Study on Network Structure and Heat Resistance in Air of Boron-Modified Phenolic Resin Aerogel. Polymers. 2025; 17(7):860. https://doi.org/10.3390/polym17070860
Chicago/Turabian StyleWu, Tengfei, Degang Wang, Qin Wang, Xiaolong Chen, Jie Ding, and Xizhuo Yan. 2025. "Study on Network Structure and Heat Resistance in Air of Boron-Modified Phenolic Resin Aerogel" Polymers 17, no. 7: 860. https://doi.org/10.3390/polym17070860
APA StyleWu, T., Wang, D., Wang, Q., Chen, X., Ding, J., & Yan, X. (2025). Study on Network Structure and Heat Resistance in Air of Boron-Modified Phenolic Resin Aerogel. Polymers, 17(7), 860. https://doi.org/10.3390/polym17070860







