Integration of Complexed Caffeic Acid into Poly(Lactic Acid)-Based Biopolymer Blends by Supercritical CO2-Assisted Impregnation and Foaming: Processing, Structural and Thermal Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formation of Inclusion Complexes (IC) Based on β-CD/CA
2.3. PLA and PLA/PBAT Films Preparation by Extrusion Process
2.4. Supercritical Impregnation of Inclusion Complex
2.5. Supercritical Foaming of PLA and Blends
2.6. Encapsulation Efficiency
2.7. Quantification of Active Agent in Impregnated Films
2.8. Characterization of Inclusion Complexes and Materials Obtained (Films and Foams)
2.8.1. Nuclear Magnetic Resonance (NMR)
2.8.2. Attenuated Total Reflectance Fourier Transforms Infrared (ATR-FTIR) Spectroscopy
2.8.3. Morphological Properties
2.8.4. Thermogravimetric Analysis (TGA)
2.8.5. Differential Scanning Calorimetry (DSC)
2.9. Statistical Analysis
3. Results and Discussions
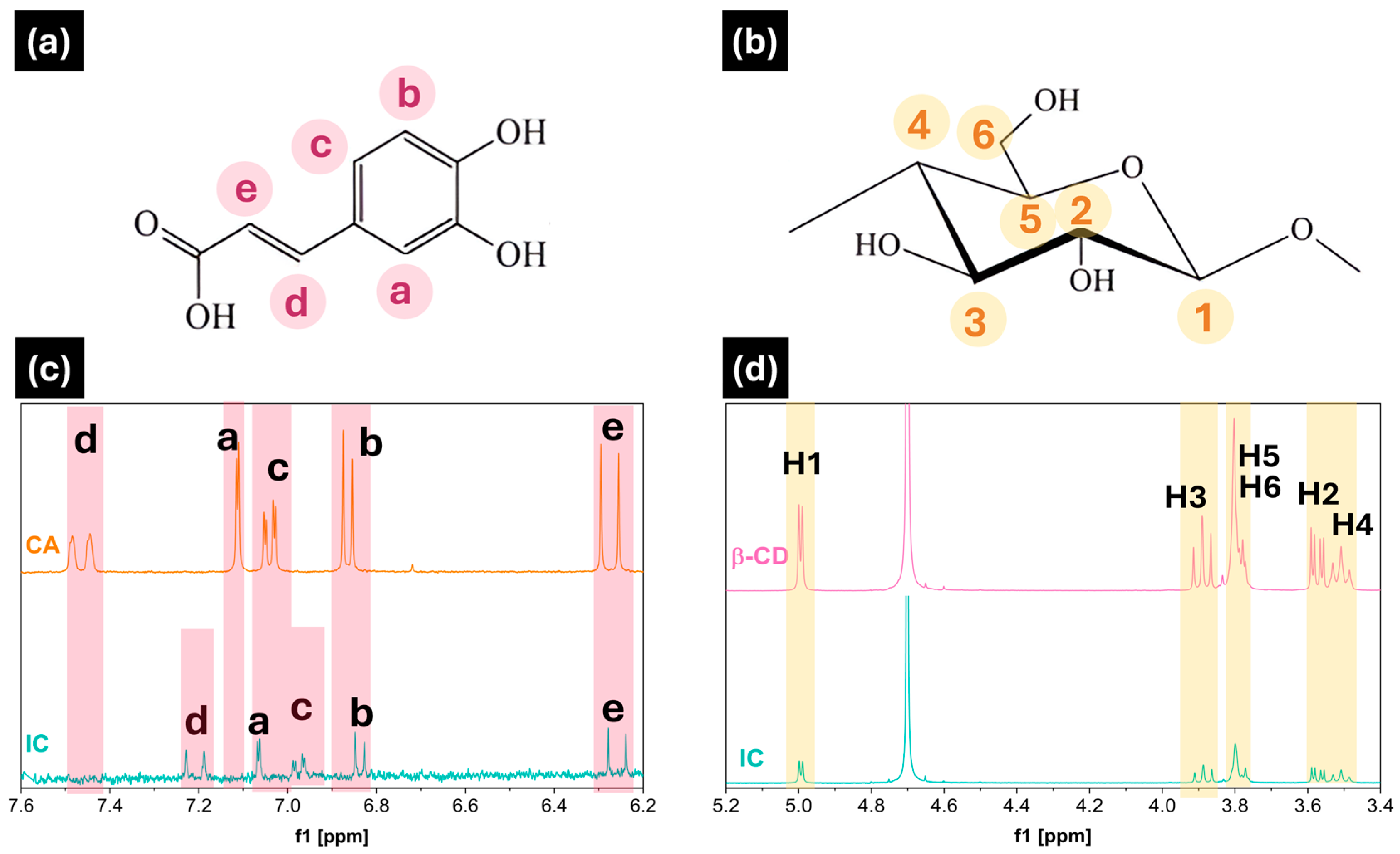
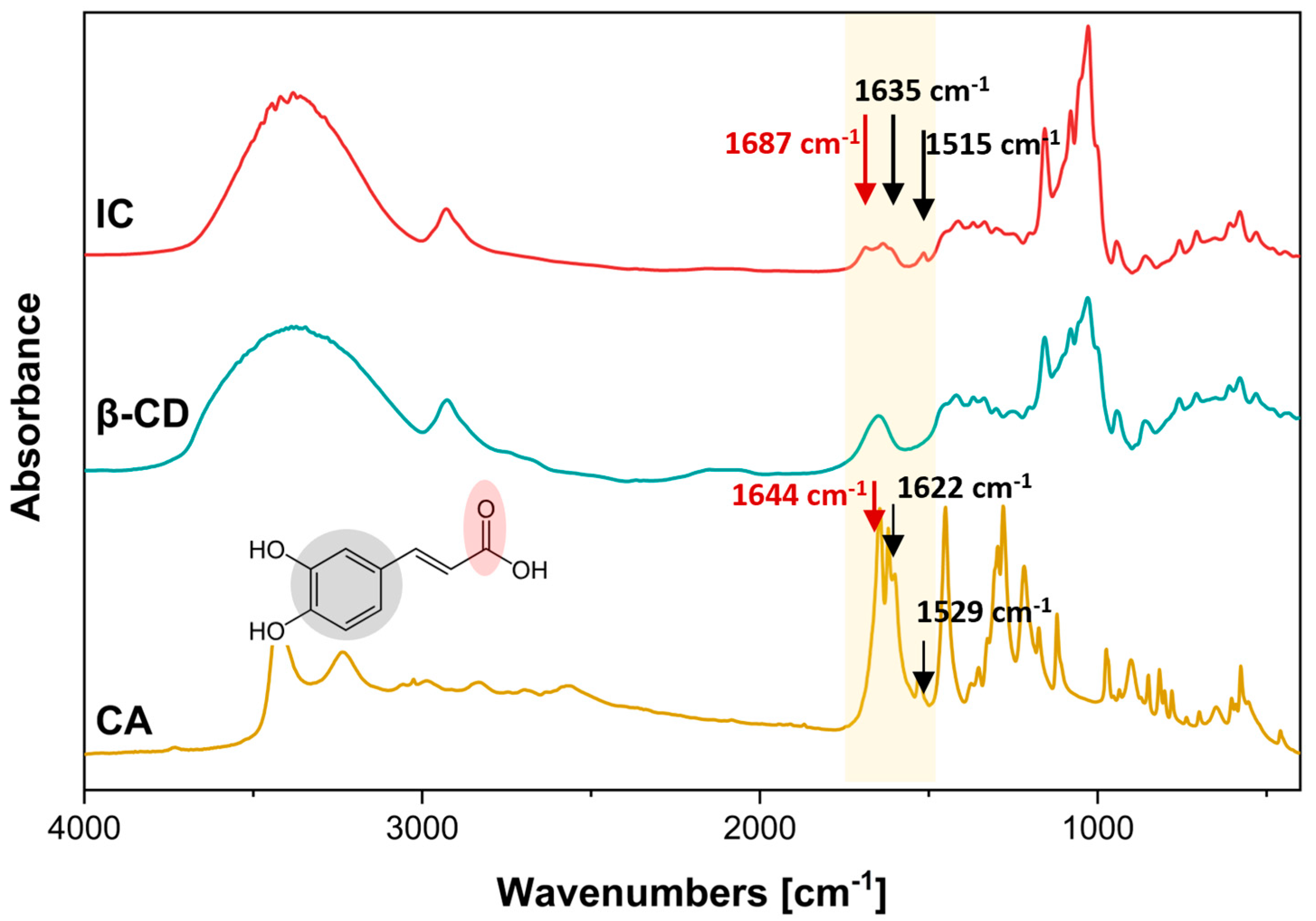
3.1. Characterization of Inclusion Complex IC
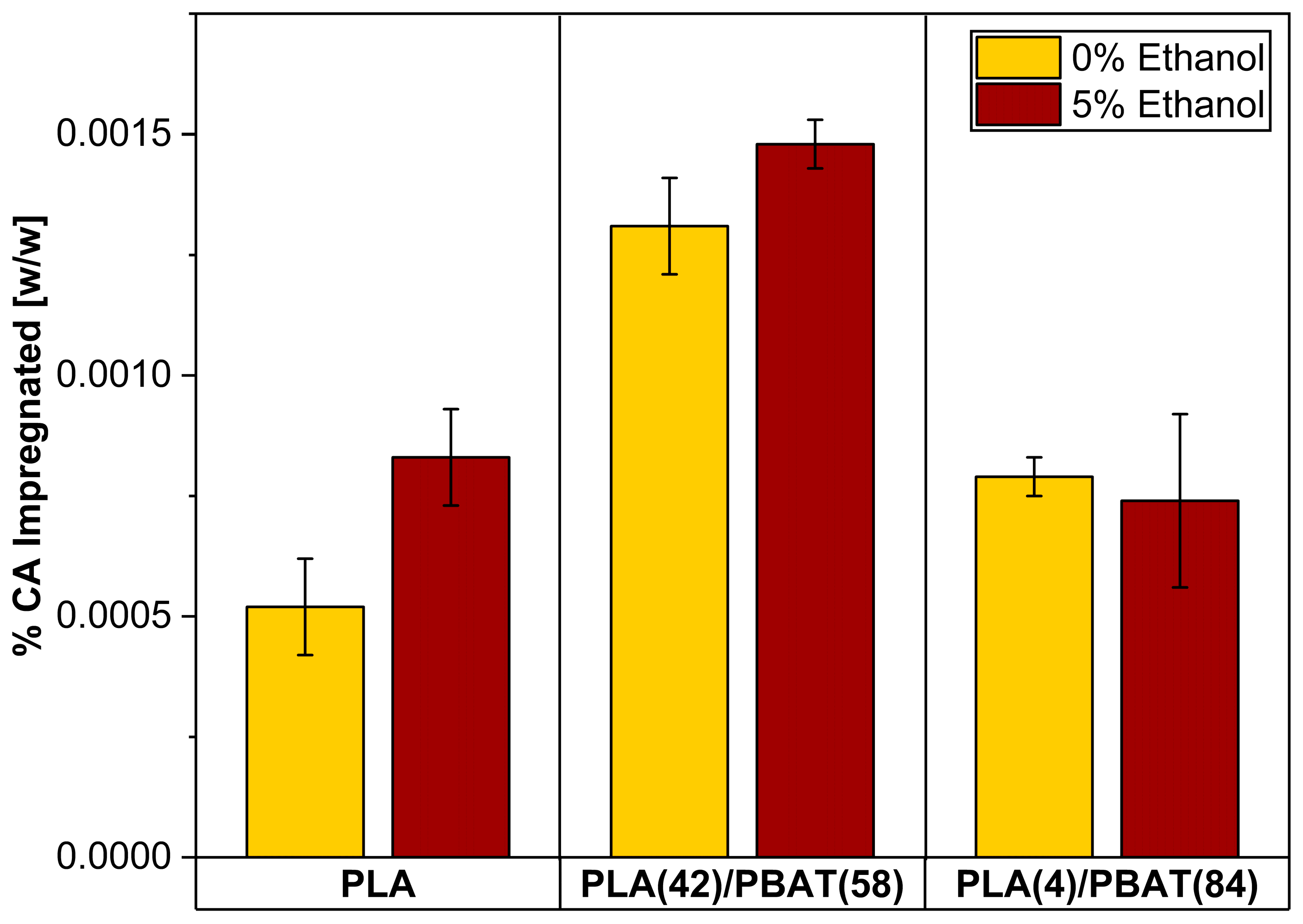
3.2. Quantification of Caffeic Acid (CA) in Impregnated Films and Foams
3.3. Characterization of the Materials Obtained (Films and Foams)
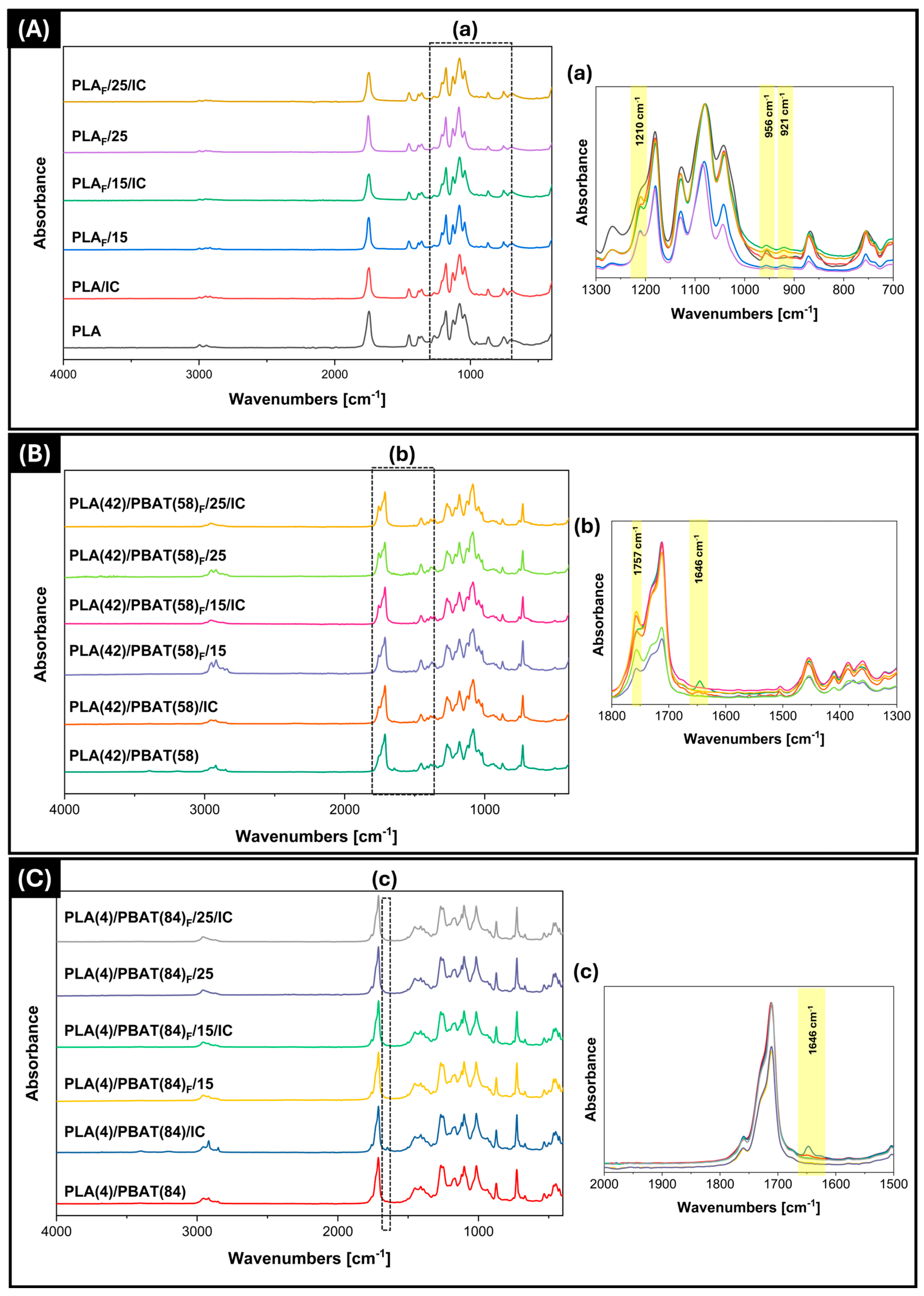
3.3.1. ATR-FTIR Analysis
3.3.2. Thermogravimetric Analysis (TGA)
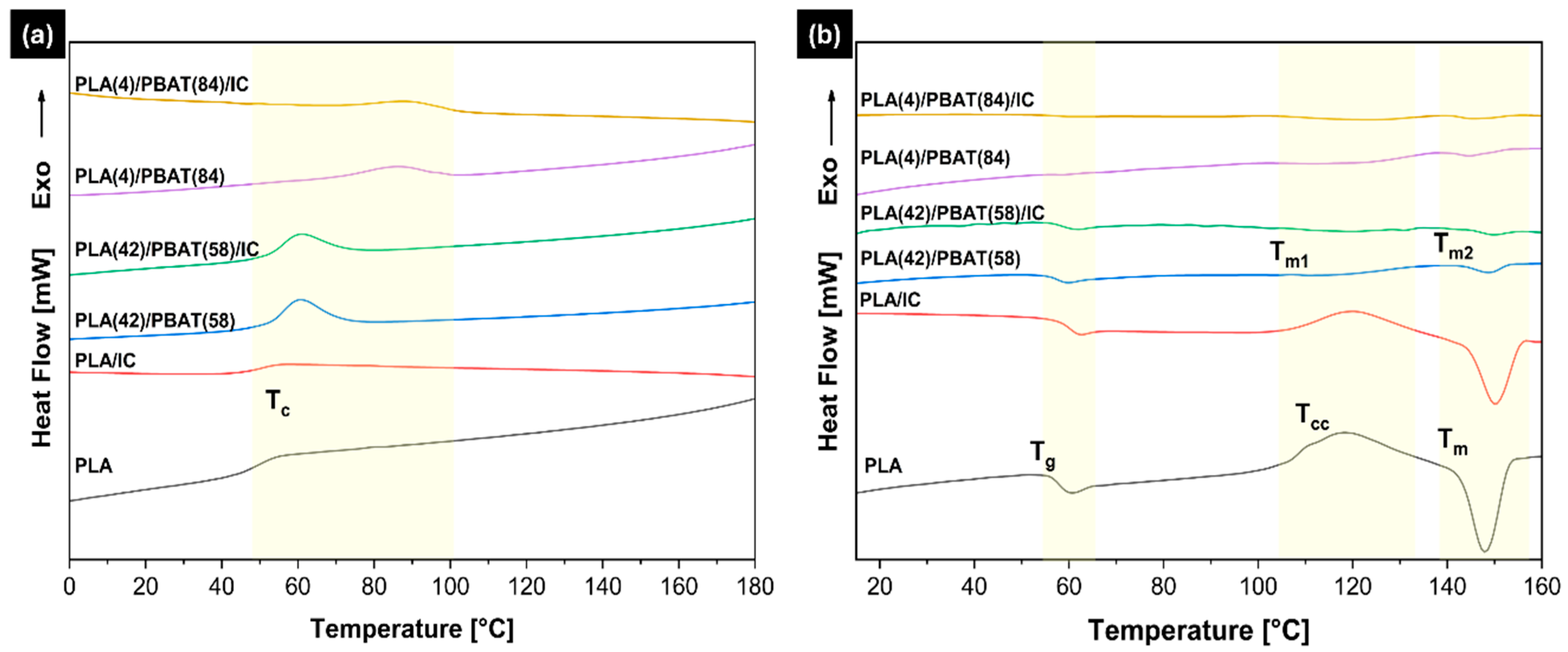
3.3.3. Differential Scanning Calorimetry (DSC)
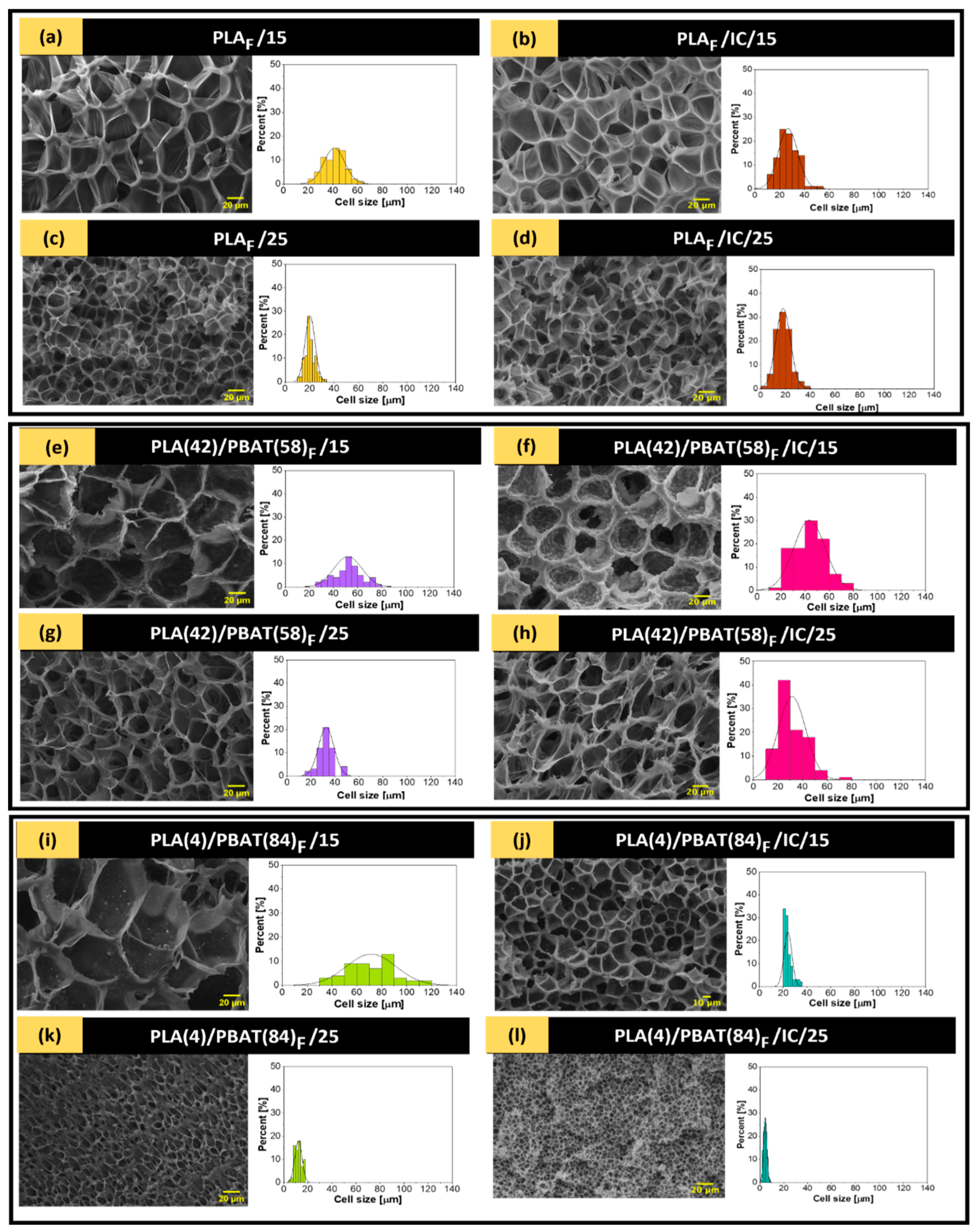
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to Nanocellular Polymer Foams: Progress (2004–2015) and Future Directions—A Review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Desole, M.P.; Gisario, A.; Fedele, L.; Aversa, C.; Barletta, M. Life Cycle Assessment of Secondary Packaging: Expanded Polystyrene versus Bioplastic-Coated Corrugated Cardboard. Sustain. Prod. Consum. 2024, 46, 11–28. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Barrow, C.J.; Adhikari, B. The Future of Bioplastics in Food Packaging: An Industrial Perspective. Food Packag. Shelf Life 2024, 43, 101279. [Google Scholar] [CrossRef]
- Ingrao, C.; Lo Giudice, A.; Bacenetti, J.; Mousavi Khaneghah, A.; Sant’Ana, A.S.; Rana, R.; Siracusa, V. Foamy Polystyrene Trays for Fresh-Meat Packaging: Life-Cycle Inventory Data Collection and Environmental Impact Assessment. Food Res. Int. 2015, 76, 418–426. [Google Scholar] [CrossRef]
- Parker, K.; Garancher, J.-P.; Shah, S.; Fernyhough, A. Expanded Polylactic Acid—An Eco-Friendly Alternative to Polystyrene Foam. J. Cell. Plast. 2011, 47, 233–243. [Google Scholar] [CrossRef]
- Chai, J.; Wang, G.; Zhao, J.; Zhang, A.; Shi, Z.; Wei, C.; Zhao, G. Microcellular PLA/PMMA Foam Fabricated by CO2foaming with Outstanding Shape-Memory Performance. J. CO2 Util. 2021, 49, 101553. [Google Scholar] [CrossRef]
- Miranda-Valdez, I.Y.; Coffeng, S.; Zhou, Y.; Viitanen, L.; Hu, X.; Jannuzzi, L.; Puisto, A.; Kostiainen, M.A.; Mäkinen, T.; Koivisto, J.; et al. Foam-Formed Biocomposites Based on Cellulose Products and Lignin. Cellulose 2023, 30, 2253–2266. [Google Scholar] [CrossRef]
- Nechita, P.; Năstac, S.M. Overview on Foam Forming Cellulose Materials for Cushioning Packaging Applications. Polymers 2022, 14, 1963. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (Lactic Acid) Foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Oluwabunmi, K.; D’Souza, N.A.; Zhao, W.; Choi, T.Y.; Theyson, T. Compostable, Fully Biobased Foams Using PLA and Micro Cellulose for Zero Energy Buildings. Sci. Rep. 2020, 10, 17771. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Biopolymer Composites: Synthesis, Properties, and Applications. Int. J. Mol. Sci. 2022, 23, 2257. [Google Scholar] [CrossRef] [PubMed]
- de Souza, F.M.; Desai, Y.; Gupta, R.K. Introduction to Polymeric Foams. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1439, pp. 1–23. [Google Scholar]
- Li, L.; Xu, D.; Bai, S.; Chen, N.; Wang, Q. Progress in Preparation of High-performance and Multi-functional Polymer Foams. J. Polym. Sci. 2024, 62, 3122–3136. [Google Scholar] [CrossRef]
- Osman, M.A.; Virgilio, N.; Rouabhia, M.; Mighri, F. Polylactic Acid (PLA) Foaming: Design of Experiments for Cell Size Control. Mater. Sci. Appl. 2022, 13, 63–77. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Cao, H.; Wu, J.; Wang, F.; Wang, Y. The Crack Propagation Behaviour of CO2 Fracturing Fluid in Unconventional Low Permeability Reservoirs: Factor Analysis and Mechanism Revelation. Processes 2025, 13, 159. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wu, J.; Li, X.; Li, H.; Cheng, Y. Wellhead Stability During Development Process of Hydrate Reservoir in the Northern South China Sea: Evolution and Mechanism. Processes 2024, 13, 40. [Google Scholar] [CrossRef]
- Chang, C.J.; Venkatesan, M.; Cho, C.J.; Chung, P.Y.; Chandrasekar, J.; Lee, C.H.; Wang, H.T.; Wong, C.M.; Kuo, C.C. Thermoplastic Starch with Poly(Butylene Adipate-Co-Terephthalate) Blends Foamed by Supercritical Carbon Dioxide. Polymers 2022, 14, 1952. [Google Scholar] [CrossRef] [PubMed]
- Faba, S.; Arrieta, M.P.; Romero, J.; Agüero, Á.; Torres, A.; Martínez, S.; Rayón, E.; Galotto, M.J. Biodegradable Nanocomposite Poly(Lactic Acid) Foams Containing Carvacrol-Based Cocrystal Prepared by Supercritical CO2 Processing for Controlled Release in Active Food Packaging. Int. J. Biol. Macromol. 2024, 254, 127793. [Google Scholar] [CrossRef]
- Dejene, B.K.; Gudayu, A.D. Eco-Friendly Packaging Innovations: Integrating Natural Fibers and ZnO Nanofillers in Polylactic Acid (PLA) Based Green Composites—A Review. Polym.-Plast. Technol. Mater. 2024, 63, 1645–1681. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable Biopolymers for Active Packaging: Demand, Development and Directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Sosik, A.; Małkowska, A.; Zasada, L.; Michalska-Sionkowska, M. Chitosan-Based Films Enriched by Caffeic Acid with Poly(Ethylene Glycol)—A Physicochemical and Antibacterial Properties Evaluation. Int. J. Biol. Macromol. 2021, 192, 728–735. [Google Scholar] [CrossRef]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the Physical Properties and Biological Activity of Chitosan Films Grafted with Gallic Acid and Caffeic Acid: A Comparison Study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Puglia, D. Antioxidant Packaging Films Based on Ethylene Vinyl Alcohol Copolymer (EVOH) and Caffeic Acid. Molecules 2020, 25, 3953. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Song, Q.-Y.; Su, J.-C.; Tang, W.; Song, J.-G.; Huang, X.-J.; An, J.; Li, Y.-L.; Ye, W.-C.; Wang, Y. Caffeic Acid Oligomers from Mesona Chinensis and Their In Vitro Antiviral Activities. Fitoterapia 2020, 144, 104603. [Google Scholar] [CrossRef]
- Yu, S.-H.; Hsieh, H.-Y.; Pang, J.-C.; Tang, D.-W.; Shih, C.-M.; Tsai, M.-L.; Tsai, Y.-C.; Mi, F.-L. Active Films from Water-Soluble Chitosan/Cellulose Composites Incorporating Releasable Caffeic Acid for Inhibition of Lipid Oxidation in Fish Oil Emulsions. Food Hydrocoll. 2013, 32, 9–19. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Zhang, L.; Chao, J. Preparation and Spectral Investigation of Inclusion Complex of Caffeic Acid with Hydroxypropyl-β-Cyclodextrin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 1891–1895. [Google Scholar] [CrossRef]
- Pei, F.; Han, P.; Zhou, Z.; Fang, D.; Mariga, A.M.; Yang, W.; Ma, N.; Hu, Q. The Characteristics of the Film Assembled by Caffeic Acid-Grafted-Chitosan/Polylactic Acid and Its Effect on the Postharvest Quality of Agaricus Bisporus. Food Packag. Shelf Life 2022, 32, 100828. [Google Scholar] [CrossRef]
- Kfoury, M.; Hădărugă, N.G.; Hădărugă, D.I.; Fourmentin, S. Cyclodextrins as Encapsulation Material for Flavors and Aroma. In Encapsulations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 127–192. ISBN 9780128043073. [Google Scholar]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation Strategies to Enhance the Antibacterial Properties of Essential Oils in Food System. Food Control. 2021, 123, 107856. [Google Scholar] [CrossRef]
- Faba, S.; Arrieta, M.P.; Agüero, Á.; Torres, A.; Romero, J.; Rojas, A.; Galotto, M.J. Processing Compostable PLA/Organoclay Bionanocomposite Foams by Supercritical CO2 Foaming for Sustainable Food Packaging. Polymers 2022, 14, 4394. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and Plasticization Effects of Carvacrol in Biodegradable Poly(Lactic Acid) and Poly(Butylene Adipate Terephthalate) Blend Films for Bakery Packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Lopresti, F.; Botta, L.; Scaffaro, R.; Bilello, V.; Settanni, L.; Gaglio, R. Antibacterial Biopolymeric Foams: Structure–Property Relationship and Carvacrol Release Kinetics. Eur. Polym. J. 2019, 121, 109298. [Google Scholar] [CrossRef]
- Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals 2023, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.F.; dos Santos Niculau, E.; de Oliveira, D.A.B.; de Assis, M.W.V.; Oliveira, M.N. Preparation, Physicochemical Characterization and Computational Studies of Plectranthus Ornatus Codd Essential Oil/β-Cyclodextrin Inclusion Complex. J. Mol. Struct. 2023, 1285, 135476. [Google Scholar] [CrossRef]
- Torres, L.H.; de Carvalho, M.Z.; Melo, P.S.; de Paula, E.; Saczk, A.A.; Pinto, L.M.A. Characterization and Cytotoxicity of a Benzocaine Inclusion Complex. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 9–15. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, B.; Wang, Y.; Liu, C.; Shen, C. Effect of Different Sterilization Methods on the Properties of Commercial Biodegradable Polyesters for Single-Use, Disposable Medical Devices. Mater. Sci. Eng. C 2019, 105, 110041. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Gammino, M.; La Mantia, F.P. Effect of an Organoclay on the Photochemical Transformations of a PBAT/PLA Blend and Morpho-Chemical Features of Crosslinked Networks. Polym. Degrad. Stab. 2021, 187, 109549. [Google Scholar] [CrossRef]
- Muñoz-Shugulí, C.; Rodríguez-Mercado, F.; Mascayano, C.; Herrera, A.; Bruna, J.E.; Guarda, A.; Galotto, M.J. Development of Inclusion Complexes With Relative Humidity Responsive Capacity as Novel Antifungal Agents for Active Food Packaging. Front. Nutr. 2022, 8, 799779. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of Processing Conditions on the Physical, Chemical and Transport Properties of Polylactic Acid Films Containing Thymol Incorporated by Supercritical Impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Mistretta, M.C.; La Mantia, F.P.; Titone, V.; Botta, L.; Pedeferri, M.; Morreale, M. Effect of Ultraviolet and Moisture Action on Biodegradable Polymers and Their Blend. J. Appl. Biomater. Funct. Mater. 2020, 18, 228080002092665. [Google Scholar] [CrossRef]
- Rivera, P.; Torres, A.; Romero, J.; Alarcón, Á.; Martínez, S.; Arrieta, M.P.; Rodríguez-Mercado, F.; Galotto, M.J. Effect of Operational Variables on Supercritical Foaming of Caffeic Acid-Loaded Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blends for the Development of Sustainable Materials. Polymers 2024, 16, 948. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Sojoudiasli, H.; Park, C.B.; Carreau, P.J.; Heuzey, M.C.; Kamal, M.R. Mechanical and Bead Foaming Behavior of PLA-PBAT and PLA-PBSA Blends with Different Morphologies. Eur. Polym. J. 2017, 90, 231–244. [Google Scholar] [CrossRef]
- Arrua, D.; Strumia, M.C.; Nazareno, M.A. Immobilization of Caffeic Acid on a Polypropylene Film: Synthesis and Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 9228–9234. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.d.P.; Serafini, M.R.; de Carvalho, Y.M.B.G.; Soares Santana, D.V.; Lima, B.S.; Quintans-Júnior, L.J.; Marreto, R.N.; de Aquino, T.M.; Sabino, A.R.; Scotti, L.; et al. Kinetic and Physical-Chemical Study of the Inclusion Complex of β-Cyclodextrin Containing Carvacrol. J. Mol. Struct. 2016, 1125, 323–330. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R.; Gmbh, F.K. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Kuska, R.; Milovanovic, S.; Frerich, S.; Ivanovic, J. Thermal Analysis of Polylactic Acid under High CO2 Pressure Applied in Supercritical Impregnation and Foaming Process Design. J. Supercrit. Fluids 2019, 144, 71–80. [Google Scholar] [CrossRef]
- ASTM D792; Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM International: West Conshohocken, PA, USA, 2020.
- Pilla, S.; Kim, S.G.; Auer, G.K.; Gong, S.; Park, C.B. Microcellular Extrusion Foaming of Poly(Lactide)/Poly(Butylene Adipate-Co-Terephthalate) Blends. Mater. Sci. Eng. C 2010, 30, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, P.; Wang, X.; Wang, F.; Wu, Y. Effects of Poly(Butyleneadipate-Co-Terephthalate) as a Macromolecular Nucleating Agent on the Crystallization and Foaming Behavior of Biodegradable Poly(Lactic Acid). Cell. Polym. 2017, 36, 75–96. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Konteles, S.; Mourtzinos, I.; Troullidou, E.; Chiou, A.; Karathanos, V.T. Encapsulation of Complex Extracts in β-Cyclodextrin: An Application to Propolis Ethanolic Extract. J. Microencapsul. 2009, 26, 603–613. [Google Scholar] [CrossRef]
- Chao, J.-B.; Tong, H.-B.; Li, Y.-F.; Zhang, L.-W.; Zhang, B.-T. Investigation on the Inclusion Behavior of Caffeic Acid with Cyclodextrin. Supramol. Chem. 2008, 20, 461–466. [Google Scholar] [CrossRef]
- Pinho, E.; Soares, G.; Henriques, M. Evaluation of Antibacterial Activity of Caffeic Acid Encapsulated by β -Cyclodextrins. J. Microencapsul. 2015, 32, 804–810. [Google Scholar] [CrossRef]
- Kfoury, M.; Geagea, C.; Ruellan, S.; Greige-Gerges, H.; Fourmentin, S. Effect of Cyclodextrin and Cosolvent on the Solubility and Antioxidant Activity of Caffeic Acid. Food Chem. 2019, 278, 163–169. [Google Scholar] [CrossRef]
- Herrera, A.; Rodríguez, F.J.; Bruna, J.E.; Abarca, R.L.; Galotto, M.J.; Guarda, A.; Mascayano, C.; Sandoval-Yáñez, C.; Padula, M.; Felipe, F.R.S. Antifungal and Physicochemical Properties of Inclusion Complexes Based on β-Cyclodextrin and Essential Oil Derivatives. Food Res. Int. 2019, 121, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, R.; Inoue, Y.; Murata, I.; Kanamoto, I. Effect of Antioxidant Activity of Caffeic Acid with Cyclodextrins Using Ground Mixture Method. Asian J. Pharm. Sci. 2018, 13, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Simsek, T.; Simsek, S.; Mayer, C.; Rasulev, B. Combined Computational and Experimental Study on the Inclusion Complexes of β-Cyclodextrin with Selected Food Phenolic Compounds. Struct. Chem. 2019, 30, 1395–1406. [Google Scholar] [CrossRef]
- Narayanan, V.; Alam, M.; Ahmad, N.; Balakrishnan, S.B.; Ganesan, V.; Shanmugasundaram, E.; Rajagopal, B.; Thambusamy, S. Electrospun Poly (Vinyl Alcohol) Nanofibers Incorporating Caffeic Acid/Cyclodextrins through the Supramolecular Assembly for Antibacterial Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 249, 119308. [Google Scholar] [CrossRef]
- Trindade, G.G.G.; Thrivikraman, G.; Menezes, P.P.; França, C.M.; Lima, B.S.; Carvalho, Y.M.B.G.; Souza, E.P.B.S.S.; Duarte, M.C.; Shanmugam, S.; Quintans-Júnior, L.J.; et al. Carvacrol/β-Cyclodextrin Inclusion Complex Inhibits Cell Proliferation and Migration of Prostate Cancer Cells. Food Chem. Toxicol. 2019, 125, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, J.; Wang, X.; Wang, X.; Liu, R.; Fu, X.; Li, Y.; Xue, J.; Li, X.; Zhang, R.; et al. Preparation and Properties of Cyclodextrin Inclusion Complexes of Hyperoside. Molecules 2022, 27, 2761. [Google Scholar] [CrossRef]
- Mangrulkar, S.; Shah, P.; Navnage, S.; Mazumdar, P.; Chaple, D. Phytophospholipid Complex of Caffeic Acid: Development, In Vitro Characterization, and In Vivo Investigation of Antihyperlipidemic and Hepatoprotective Action in Rats. AAPS PharmSciTech 2021, 22, 28. [Google Scholar] [CrossRef]
- Li, R.; Bao, R.; Yang, Q.X.; Wang, Q.L.; Adu-Frimpong, M.; Wei, Q.Y.; Elmurat, T.; Ji, H.; Yu, J.N.; Xu, X.M. [6]-Shogaol/β-CDs Inclusion Complex: Preparation, Characterisation, in Vivo Pharmacokinetics, and in Situ Intestinal Perfusion Study. J. Microencapsul. 2019, 36, 500–512. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing an Essential Oil Component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef]
- Zeren, S.; Sahin, S.; Sumnu, G. Encapsulation of Caffeic Acid in Carob Bean Flour and Whey Protein-Based Nanofibers via Electrospinning. Foods 2022, 11, 1860. [Google Scholar] [CrossRef]
- Sambasevam, K.; Mohamad, S.; Sarih, N.; Ismail, N. Synthesis and Characterization of the Inclusion Complex of β-Cyclodextrin and Azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.B.; Shim, J.-J. Solubility in Supercritical Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429122088. [Google Scholar]
- Bitencourt, R.G.; Palma, A.M.; Coutinho, J.A.P.; Cabral, F.A.; Meirelles, A.J.A. Solubility of Caffeic Acid in CO2+ ethanol: Experimental and Predicted Data Using Cubic Plus Association Equation of State. J. Supercrit. Fluids 2018, 138, 238–246. [Google Scholar] [CrossRef]
- Shi, X.; Qin, J.; Wang, L.; Ren, L.; Rong, F.; Li, D.; Wang, R.; Zhang, G. Introduction of Stereocomplex Crystallites of PLA for the Solid and Microcellular Poly(Lactide)/Poly(Butylene Adipate-: Co-Terephthalate) Blends. RSC Adv. 2018, 8, 11850–11861. [Google Scholar] [CrossRef] [PubMed]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Olive Leaf Extract to Obtain Bioactive Films Effective in Cherry Tomato Preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Rojas, A.; Cerro, D.; Torres, A.; Galotto, M.J.; Guarda, A.; Romero, J. Supercritical Impregnation and Kinetic Release of 2-Nonanone in LLDPE Films Used for Active Food Packaging. J. Supercrit. Fluids 2015, 104, 76–84. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S. Supercritical CO2 Impregnation of PLA / PCL Fi Lms with Natural Substances for Bacterial Growth Control in Food Packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of Cinnamaldehyde into Cassava Starch Biocomposite Films Using Supercritical Fluid Technology for the Development of Food Active Packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef]
- Machado, N.D.; Mosquera, J.E.; Martini, R.E.; Goñi, M.L.; Gañán, N.A. Supercritical CO2-Assisted Impregnation/Deposition of Polymeric Materials with Pharmaceutical, Nutraceutical, and Biomedical Applications: A Review (2015–2021). J. Supercrit. Fluids 2022, 191, 105763. [Google Scholar] [CrossRef]
- Da Silva, C.V.; Pereira, V.J.; Rosa, P.T.V.; Cabral-Albuquerque, E.C.M.; Vieira De Melo, S.A.B.; Costa, G.M.N.; Dias, A.M.A.; De Sousa, H.C.; Braga, M.E.M. Effect of ScCO2 Sorption Capacity on the Total Amount of Borage Oil Loaded by ScCO2 Impregnation/Deposition into a Polyurethane-Based Wound Dressing. J. Supercrit. Fluids 2016, 115, 1–9. [Google Scholar] [CrossRef]
- Pai, A.J.; Sarojini, B.K.; Harshitha, K.R.; Shivarama Holla, B.; Lobo, A.G. Spectral, Morphological and Optical Studies on Bischalcone Doped Polylactic Acid (PLA) Thin Films as Luminescent and UV Radiation Blocking Materials. Opt. Mater. 2019, 90, 145–151. [Google Scholar] [CrossRef]
- Moliner, C.; Finocchio, E.; Arato, E.; Ramis, G.; Lagazzo, A. Influence of the Degradation Medium on Water Uptake, Morphology, and Chemical Structure of Poly(Lactic Acid)-Sisal Bio-Composites. Materials 2020, 13, 3974. [Google Scholar] [CrossRef]
- Ramezani, M.; Amoozegar, M.A.; Ventosa, A. Screening and Comparative Assay of Poly-Hydroxyalkanoates Produced by Bacteria Isolated from the Gavkhooni Wetland in Iran and Evaluation of Poly-β-Hydroxybutyrate Production by Halotolerant Bacterium Oceanimonas sp. GK1. Ann. Microbiol. 2015, 65, 517–526. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical Impregnation of Cinnamaldehyde into Polylactic Acid as a Route to Develop Antibacterial Food Packaging Materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.C.; Ribeiro, K.C.; Martini, F.A.; Barrioni, B.R.; Santos, J.P.F.; Melo Carvalho, B. PBAT/PLA/Cellulose Nanocrystals Biocomposites Compatibilized with Polyethylene Grafted Maleic Anhydride (PE-g-MA). J. Appl. Polym. Sci. 2021, 138, 151342. [Google Scholar] [CrossRef]
- Meaurio, E.; López-Rodríguez, N.; Sarasua, J.R. Infrared Spectrum of Poly(L-Lactide): Application to Crystallinity Studies. Macromolecules 2006, 39, 9291–9301. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J.; Bigger, S.W. Effect of Supercritical CO2 and Olive Leaf Extract on the Structural, Thermal and Mechanical Properties of an Impregnated Food Packaging Film. J. Supercrit. Fluids 2019, 145, 181–191. [Google Scholar] [CrossRef]
- Ma, F.; Wang, B.; Leng, X.; Wang, Y.; Sun, Z.; Wang, P.; Sang, L.; Wei, Z. Biodegradable PBAT/PLA/CaCO3 Blowing Films with Enhanced Mechanical and Barrier Properties: Investigation of Size and Content of CaCO3 Particles. Macromol. Mater. Eng. 2022, 307, 2200135. [Google Scholar] [CrossRef]
- Rocha, D.B.; Souza de Carvalho, J.; de Oliveira, S.A.; dos Santos Rosa, D. A New Approach for Flexible PBAT/PLA/CaCO3 Films into Agriculture. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Teamsinsungvon, A.; Jarapanyacheep, R.; Ruksakulpiwat, Y.; Jarukumjorn, K. Melt Processing of Maleic Anhydride Grafted Poly(Lactic Acid) and Its Compatibilizing Effect on Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blend and Their Composite. Polym. Sci. Ser. A 2017, 59, 384–396. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Huang, S.-Y.; Chen, Y.-F.; Kuo, M.-T.; Chiang, T.-Y.; Chang, C.-Y.; Wang, Y.-H. Heat Treatment Effects on the Mechanical Properties and Morphologies of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate) Blends. Int. J. Polym. Sci. 2013, 2013, 951696. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, G.; Liu, Y.; Ma, Z.; Jing, Z.; Fan, X. Microcellular Foaming of Polylactide and Poly(Butylene Adipate-co-terphathalate) Blends and Their CaCO3 Reinforced Nanocomposites Using Supercritical Carbon Dioxide. Polym. Adv. Technol. 2016, 27, 550–560. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Tanrattanakul, V.; Phusunti, N. Mechanical Characteristics and Thermal Behaviours of Polylactide Blend Films: Influence of Nucleating Agent and Poly(Butylenes Adipate-Co-Terephthalate). Plast. Rubber Compos. 2016, 45, 333–345. [Google Scholar] [CrossRef]
- Byun, Y.; Rodriguez, K.; Han, J.H.; Kim, Y.T. Improved Thermal Stability of Polylactic Acid (PLA) Composite Film via PLA–β-Cyclodextrin-Inclusion Complex Systems. Int. J. Biol. Macromol. 2015, 81, 591–598. [Google Scholar] [CrossRef]
- Hao, A.; Geng, Y.; Xu, Q.; Lu, Z.; Yu, L. Study of Different Effects on Foaming Process of Biodegradable PLA/Starch Composites in Supercritical/Compressed Carbon Dioxide. J. Appl. Polym. Sci. 2008, 109, 2679–2686. [Google Scholar] [CrossRef]
- Zhang, L.; Zhen, W.; Zhou, Y. Structure and Properties of Poly(Lactic Acid)/Poly(Lactic Acid)-α-Cyclodextrin Inclusion Compound Composites. J. Polym. Eng. 2017, 37, 897–909. [Google Scholar] [CrossRef]
- Wang, J.; Chai, J.; Wang, G.; Zhao, J.; Zhang, D.; Li, B.; Zhao, H.; Zhao, G. Strong and Thermally Insulating Polylactic Acid/Glass Fiber Composite Foam Fabricated by Supercritical Carbon Dioxide Foaming. Int. J. Biol. Macromol. 2019, 138, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2—Assisted Production of PLA and PLGA Foams for Controlled Thymol Release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Mi, J.; Du, Z.; Wang, X.; Zhang, C. Effect of the Crystallization of Modified Polybutylene Terephthalate on Its Foaming Using Supercritical CO2: Transition from Microcellular to Nanocellular Foam. J. Supercrit. Fluids 2022, 181, 105463. [Google Scholar] [CrossRef]
- Moo-Tun, N.M.; Iñiguez-Covarrubias, G.; Valadez-Gonzalez, A. Assessing the Effect of PLA, Cellulose Microfibers and CaCO3 on the Properties of Starch-Based Foams Using a Factorial Design. Polym. Test. 2020, 86, 106482. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Chen, X.; Liu, S.; Zhang, C. Flexibly Controlling the Polycrystallinity and Improving the Foaming Behavior of Polylactic Acid via Three Strategies. ACS Omega 2022, 7, 6248–6260. [Google Scholar] [CrossRef] [PubMed]
- Rezvanpanah, E.; Ghaffarian Anbaran, S.R. The Effect of Crystallinity on Cell Growth in Semi-Crystalline Microcellular Foams by Solid-State Process: Modeling and Numerical Simulation. Mater. Res. Express 2017, 4, 115305. [Google Scholar] [CrossRef]
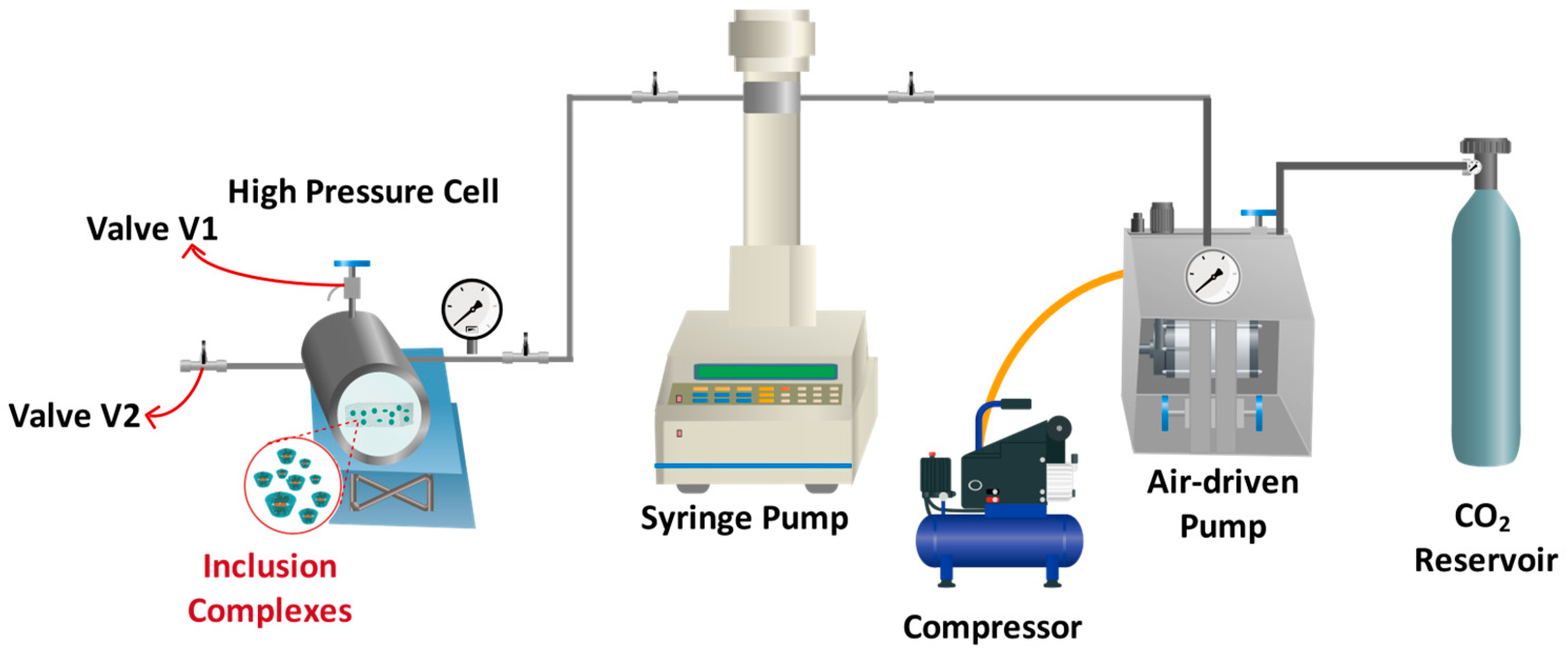


| Sample | Pressure [MPa] | Depressurization Rate [MPa/min] | Cosolvent Concentration [wt%] |
|---|---|---|---|
| PLA/IC | 15 | 1 | 0; 5 |
| PLA(42)/PBAT (58)/IC | 12 | 1 | 0; 5 |
| PLA(4)/PBAT(84)/IC | 12 | 0.1 | 0; 5 |
| δFree | δComplex | Δδ | |
|---|---|---|---|
| Ha | 7.110 | 7.150 | −0.04 |
| Hb | 6.875 | 6.848 | 0.027 |
| Hc | 7.033 | 6.940 | 0.093 |
| Hd | 7.445 | 7.220 | 0.225 |
| He | 6.295 | 6.367 | −0.072 |
| H-1 | 5.088 | 5.087 | 0.001 |
| H-2 | 3.680 | 3.678 | 0.002 |
| H-3 | 3.978 | 3.976 | 0.002 |
| H-4 | 3.598 | 3.597 | 0.001 |
| H-5 | 3.868 | 3.860 | 0.008 |
| H-6 | 3.892 | 3.888 | 0.004 |
| Sample | Type | Pressure [MPa] | % CA [w/w] |
|---|---|---|---|
| PLA/IC | Film | - | 0.00096 ± 0.0001 |
| PLA/15/IC | Foam | 15 | 0.00085 ± 0.0001 |
| PLA/25/IC | Foam | 25 | 0.00085 ± 0.0001 |
| PLA(42)/PBAT(58)/IC | Film | - | 0.0015 ± 0.0001 |
| PLA(42)/PBAT(58)/15/IC | Foam | 15 | 0.0013 ± 0.0001 |
| PLA(42)/PBAT(58)/25/IC | Foam | 25 | 0.0014 ± 0.0002 |
| PLA(4)/PBAT(84)/IC | Film | - | 0.00082 ± 0.0001 |
| PLA(4)/PBAT(84)/15/IC | Foam | 15 | 0.00082 ± 0.0001 |
| PLA(4)/PBAT(84)/25/IC | Foam | 25 | 0.00073 ± 0.0001 |
| Sample | Pressure [MPa] | Initial Degradation Temperature [°C] | Maximum Degradation Temperature [°C] | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| PLA | 344.9 ± 0.9 a,b,c | N.D. | N.D. | 361.8 ± 0.9 a,b | N.D. | N.D. | |
| PLA/IC | 348.1 ± 1.2 a | N.D. | N.D. | 363.2 ± 0.7 a | N.D. | N.D. | |
| PLAF | 15 | 341.8 ±2.3 a,b,c,d | N.D. | N.D. | 360.4 ± 1.3 a,b | N.D. | N.D. |
| PLAF/IC | 348.2 ± 0.7 a | N.D. | N.D. | 362.9 ± 0.5 a | N.D. | N.D. | |
| PLAF | 25 | 346.9 ± 1.7 a,b | N.D. | N.D. | 362.6 ± 0.4 a | N.D. | N.D. |
| PLAF/IC | 347.5 ± 0.5 a,b | N.D. | N.D. | 362.9 ± 0.1 a | N.D. | N.D. | |
| PLA(42)/PBAT(58) | 337.3 ± 3.3 c,d | 388.7 ± 1.1 d | N.D. | 358.1 ± 0.9 a,b | 400.1 ± 2.9 j | N.D. | |
| PLA(42)/PBAT(58)/IC | 340.2 ± 0.1 b,c,d | 392.2 ± 0.2 a | N.D. | 357.7 ± 1.2 a,b | 403.3 ± 0.1 f | N.D. | |
| PLA(42)/PBAT(58)F | 15 | 337.7 ± 1.2 c,d | 387.2 ± 1.9 e | N.D. | 357.1 ± 0.2 a,b | 399.2 ± 0.2 k | N.D. |
| PLA(42)/PBAT(58)F/IC | 336.3 ± 0.1 d | 391.1 ± 2.1 c | N.D. | 355.5 ± 3.1 b | 402.6 ± 0.3 h | N.D. | |
| PLA(42)/PBAT(58)F | 25 | 335.3 ± 2.6 d | 383.9 ± 5.5 f | N.D. | 355.6 ± 2.3 a,b | 400.8 ± 1.9 l | N.D. |
| PLA(42)/PBAT(58)F/IC | 338.2 ± 1.3 c,d | 391.1 ± 0.1 b | N.D. | 356.9 ± 3.6 a,b | 400.2 ± 3.5 i | N.D. | |
| PLA(4)/PBAT(84) | 314.5 ± 0.6 e,f | 378.6 ± 0.7 k | 555.5 ± 5.5 a | 328.9 ± 0.9 c | 403.5 ± 0.3 d | 586.5 ± 0.5 a | |
| PLA(4)/PBAT(84)/IC | 314.6 ± 0.3 e,f | 378.9 ± 0.1 j | 563.3 ± 9.3 a | 328.1 ± 0.2 c,d | 404.6 ± 0.5 a | 597.2 ± 6.8 a | |
| PLA(4)/PBAT(84)F | 15 | 310.2 ± 0.5 f | 378.1 ± 3.9 l | 552.9 ± 11.7 a | 322.0 ± 2.1 d | 402.8 ± 1.7 g | 592.7 ± 13.3 a |
| PLA(4)/PBAT(84)F/IC | 310.6 ± 1.5 f | 379.2 ± 0.1 h | 559.1 ± 4.9 a | 327.2 ± 1.2 c,d | 403.9 ± 0.1 b | 592.2 ± 3.0 a | |
| PLA(4)/PBAT(84)F | 25 | 320.3 ± 5.2 e | 384.7 ± 5.3 g | 559.6 ± 7.1 a | 326.6 ± 3.5 c,d | 403.3 ± 1.0 e | 597.8 ± 6.3 a |
| PLA(4)/PBAT(84)F/IC | 315.3 ± 1.8 e,f | 379.1 ± 0.1 i | 562.2 ± 6.9 a | 329.3 ± 1.3 c | 403.7 ± 0.4 c | 590.7 ± 1.4 a | |
| Sample | Tg PBAT [°C] | Tg PLA [°C] | Tm PBAT [°C] | Hm PBAT [J/g] | Tm PLA [°C] | Hm PLA [J/g] | Xc PLA [%] |
|---|---|---|---|---|---|---|---|
| PLA | N.D. | 58.1 ± 0,2 a | N.D. | N.D. | 148.2 ± 0.2 b | 29.2 ± 0.4 a | 4.1 ± 0.2 b |
| PLA(42)/PBAT(58) | −31.3 ± 0.1 c | 57.4 ± 1.1 a | 115.1 ± 0.1 d | 7.1 ± 0.4 a | 148.9 ± 0.2 b | 2.2 ± 0.1 b | 5.7 ± 0.2 b |
| PLA(4)/PBAT(84) | −32.0 ± 2.6 d | 56.6 ± 0.3 a | 123.4 ± 0.1 b | 4.4 ± 0.2 c | 145.8 ± 0.1 c | 1.5 ± 0.1 b | 39.0 ± 2.6 a |
| PLA/IC | N.D. | 58.5 ± 0.3 a | N.D. | N.D. | 149.6 ± 0.8 a,b | 28.7 ± 0.5 a | 4.0 ± 0.2 b |
| PLA(42)/PBAT(58)/IC | −29.5 ± 0.3 b | 56.9 ± 0.2 a | 120.2 ± 0.7 c | 3.7 ± 0.4 d | 151.1 ± 0.4 a | 1.3 ± 0.1 b | 3.2 ± 0.1 b |
| PLA(4)/PBAT(84)/IC | −27.9 ± 0.1 a | 56.9 ± 0.7 a | 123.8 ± 0.6 a | 4.3 ± 0.1 b | 145.6 ± 0.1 c | 1.4 ± 0.1 b | 37.1 ± 2.3 a |
| Sample | Pressure [MPa] | Tm PLA [°C] | ΔHm [J/g] PLA | %Xc PLA |
|---|---|---|---|---|
| PLAF | 15 | 153.5 ± 0.6 a,b | 46.1 ± 4.3 a | 49.2 ± 4.6 b,c |
| PLA(42)/PBAT(58)F | 152.5 ± 0.1 a,b,c | 9.9 ± 0.7 d | 25.1 ± 1.8 g | |
| PLA(4)/PBAT(84)F | 150.9 ± 0.2 b,c,d | 2.0 ± 0.1 e | 52.1 ± 2.3 a,b | |
| PLAF | 25 | 148.9 ± 0.4 d,e,f | 33.0 ± 0.4 b | 35.2 ± 0.4 e,f |
| PLA(42)/PBAT(58)F | 147.2 ± 0.5 e,f | 15.2 ± 2.3 c | 38.7 ± 5.9 d,e | |
| PLA(4)/PBAT(84)F | 147.3 ± 0.2 e,f | 1.0 ± 0.1 e | 27.8 ± 2.6 f,g | |
| PLAF/IC | 15 | 155.1 ± 0.9 a | 43.5 ± 1.2 a | 46.5 ± 1.3 b,c,d |
| PLA(42)/PBAT(58)F/IC | 153.4 ± 3.1 a,b | 17.3 ± 2.3 c | 43.9 ± 5.8 c,d | |
| PLA(4)/PBAT(84)F/IC | 150.8 ± 1.1 b,c,d | 2.2 ± 0.1 e | 59.8 ± 3.4 a | |
| PLAF/IC | 25 | 146.4 ± 1.3 f | 32.4 ± 2.7 b | 34.6 ± 6.4 e,f |
| PLA(42)/PBAT(58)F/IC | 147.2 ± 0.1 e,f | 15.6 ± 0.1 c | 39.6 ± 0.3 d,e | |
| PLA(4)/PBAT(84)F/IC | 149.8 ± 3.3 c,d,e | 1.1 ± 0.1 e | 28.0 ± 1.5 f,g |
| Sample | Pressure [MPa] | d [μm] | ρf [kg/m3] | NC [×1011 Cell/cm3] | ER |
|---|---|---|---|---|---|
| PLA | 15 | 41.1 ± 8.4 | 104.7 ± 4.8 | 1.3 | 9.1 |
| PLA(42)/PBAT(58) | 52.0 ± 11.6 | 269.6 ± 1.5 | 0.5 | 3.3 | |
| PLA(4)/PBAT(84) | 72.9 ± 19.9 | 203.6 ± 8.7 | 0.2 | 5.0 | |
| PLA | 25 | 20.3 ± 4.1 | 197.5 ± 10.6 | 9.5 | 4.8 |
| PLA(42)/PBAT(58) | 32.7 ± 6.4 | 140.1 ± 15.6 | 2.5 | 6.4 | |
| PLA(4)/PBAT(84) | 12.2 ± 2.9 | 295.5 ± 46.7 | 39.0 | 3.4 | |
| PLA/IC | 15 | 26.7 ± 7.9 | 319.5 ± 9.8 | 3.6 | 3.3 |
| PLA(42)/PBAT(58)/IC | 43.7 ± 7.2 | 380.5 ± 38.2 | 0.7 | 3.3 | |
| PLA(4)/PBAT(84)/IC | 23.9 ± 3.3 | 273.0 ± 37.4 | 5.2 | 2.6 | |
| PLA/IC | 25 | 18.1 ± 5.9 | 181.1 ± 14.5 | 14.0 | 5.7 |
| PLA(42)/PBAT(58)/IC | 31.1 ± 6.3 | 152.4 ± 4.2 | 2.8 | 3.5 | |
| PLA(4)/PBAT(84)/IC | 4.5 ± 1.4 | 335.9 ± 21.4 | 689.8 | 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, P.; Torres, A.; Pacheco, M.; Romero, J.; Arrieta, M.P.; Rodríguez-Mercado, F.; Bruna, J. Integration of Complexed Caffeic Acid into Poly(Lactic Acid)-Based Biopolymer Blends by Supercritical CO2-Assisted Impregnation and Foaming: Processing, Structural and Thermal Characterization. Polymers 2025, 17, 803. https://doi.org/10.3390/polym17060803
Rivera P, Torres A, Pacheco M, Romero J, Arrieta MP, Rodríguez-Mercado F, Bruna J. Integration of Complexed Caffeic Acid into Poly(Lactic Acid)-Based Biopolymer Blends by Supercritical CO2-Assisted Impregnation and Foaming: Processing, Structural and Thermal Characterization. Polymers. 2025; 17(6):803. https://doi.org/10.3390/polym17060803
Chicago/Turabian StyleRivera, Patricia, Alejandra Torres, Miguel Pacheco, Julio Romero, Marina P. Arrieta, Francisco Rodríguez-Mercado, and Julio Bruna. 2025. "Integration of Complexed Caffeic Acid into Poly(Lactic Acid)-Based Biopolymer Blends by Supercritical CO2-Assisted Impregnation and Foaming: Processing, Structural and Thermal Characterization" Polymers 17, no. 6: 803. https://doi.org/10.3390/polym17060803
APA StyleRivera, P., Torres, A., Pacheco, M., Romero, J., Arrieta, M. P., Rodríguez-Mercado, F., & Bruna, J. (2025). Integration of Complexed Caffeic Acid into Poly(Lactic Acid)-Based Biopolymer Blends by Supercritical CO2-Assisted Impregnation and Foaming: Processing, Structural and Thermal Characterization. Polymers, 17(6), 803. https://doi.org/10.3390/polym17060803









