Flexible Waterborne Polyurethane-Bacterial Cellulose Films for Real-Time Physiological Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the WPU/BC Film and Flexible Electrode
2.3. Fabrication of the LM-WPU/BC Electrode
2.4. ECG Signal Acquisition Module
2.5. Characterization
2.5.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.5.2. X-Ray Photoelectron Spectroscopy (XPS)
2.5.3. Morphology Characterization
2.5.4. Water Contact Angle (WCA) Analysis
2.5.5. Thermal Properties
2.5.6. Recording of Human ECG Signal
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, Y.; Wang, K.; Gong, S.; Chen, H.; Dong, Y.; Gao, Q.; Liu, C.; Li, J. Flexible, shape-editable wood-based functional materials with acetal linkages. Chem. Commun. 2024, 60, 12702–12705. [Google Scholar] [CrossRef]
- Afanasenkau, D.; Kalinina, D.; Lyakhovetskii, V.; Tondera, C.; Gorsky, O.; Moosavi, S.; Pavlova, N.; Merkulyeva, N.; Kalueff, A.V.; Minev, I.R. Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces. Nat. Biomed. Eng. 2020, 4, 1010–1022. [Google Scholar] [CrossRef]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L. Electronic dura mater for long-term multimodal neural interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Mariello, M.; Kim, K.; Wu, K.; Lacour, S.P.; Leterrier, Y. Recent advances in encapsulation of flexible bioelectronic implants: Materials, technologies, and characterization methods. Adv. Mater. 2022, 34, 2201129. [Google Scholar] [CrossRef]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah-Varnosfaderani, M.; Daniel, W.F.; Everhart, M.H.; Pandya, A.A.; Liang, H.; Matyjaszewski, K.; Dobrynin, A.V.; Sheiko, S.S. Mimicking biological stress–strain behaviour with synthetic elastomers. Nature 2017, 549, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ma, L.; Han, X.; Xun, X.; Li, T.; Duan, K.; Hu, X.; Wan, Y.; Ao, H. A facile, biosynthetic design strategy for high-performance multifunctional bacterial cellulose-based dressing. Compos. Part B 2022, 238, 109945. [Google Scholar] [CrossRef]
- Fang, S.; Hu, Y.H. Emerging approaches of utilizing trees to produce advanced structural and functional materials. Chem. Commun. 2024, 60, 7663–7671. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.; Maniura-Weber, K.; Siqueira, G.; Salentinig, S. Virus pH-dependent interactions with cationically modified cellulose and their application in water filtration. Small 2021, 17, 2100307. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Chen, C.; Kong, W.; Zhu, S.; Dai, J.; Diaz, A.J.; Hitz, E.; Solares, S.D.; Li, T. Transparent, anisotropic biofilm with aligned bacterial cellulose nanofibers. Adv. Funct. Mater. 2018, 28, 1707491. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Liu, T.; Puglia, D.; Kenny, J.M.; Xu, P.; Zhang, R.; Ma, P. Multiple structure reconstruction by dual dynamic crosslinking strategy inducing self-reinforcing and toughening the polyurethane/nanocellulose elastomers. Adv. Funct. Mater. 2023, 33, 2213294. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Guan, Q.-F.; Ling, Z.-C.; Han, Z.-M.; Yang, H.-B.; Yu, S.-H. Ultra-strong, ultra-tough, transparent, and sustainable nanocomposite films for plastic substitute. Matter 2020, 3, 1308–1317. [Google Scholar] [CrossRef]
- Feng, Z.; Li, M.; Jin, X.; Zheng, Y.; Liu, J.; Zhao, L.; Wang, Y.; Li, H.; Zuo, D. Design and characterization of plasticized bacterial cellulose/waterborne polyurethane composite with antibacterial function for nasal stenting. Regener. Biomater. 2020, 7, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghosh, B.D.; Goh, K.L.; Muthoka, R.M.; Kim, J. Modulation of interfacial interactions toward strong and tough cellulose nanofiber-based transparent thin films with antifogging feature. Carbohydr. Polym. 2022, 278, 118974. [Google Scholar] [CrossRef]
- Su, T.; Liu, N.; Lei, D.; Wang, L.; Ren, Z.; Zhang, Q.; Su, J.; Zhang, Z.; Gao, Y. Flexible MXene/bacterial cellulose film sound detector based on piezoresistive sensing mechanism. ACS Nano 2022, 16, 8461–8471. [Google Scholar] [CrossRef]
- Lv, P.; Zhou, H.; Zhao, M.; Li, D.; Lu, K.; Wang, D.; Huang, J.; Cai, Y.; Lucia, L.A.; Wei, Q. Highly flexible, transparent, and conductive silver nanowire-attached bacterial cellulose conductors. Cellulose 2018, 25, 3189–3196. [Google Scholar] [CrossRef]
- Jang, J.Y.; Byun, Y.; You, S.; Kim, S.; Lee, D.-M.; Kim, S.-W.; Son, S.U. Polyurethanes synthesized using biomass-derived furan diols as sustainable triboelectric materials. Chem. Commun. 2024, 60, 9741–9744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhang, H.; Su, Z.; Zhang, X.; Zhou, H.; Yu, L.; Chen, C.; Wang, X. A biodegradable, waterproof, and thermally processable cellulosic bioplastic enabled by dynamic covalent modification. Adv. Mater. 2023, 35, 2301398. [Google Scholar] [CrossRef] [PubMed]
- Urbina, L.; Alonso-Varona, A.; Saralegi, A.; Palomares, T.; Eceiza, A.; Corcuera, M.Á.; Retegi, A. Hybrid and biocompatible cellulose/polyurethane nanocomposites with water-activated shape memory properties. Carbohydr. Polym. 2019, 216, 86–96. [Google Scholar] [CrossRef]
- Ji, J.; Liu, N.; Tian, Y.; Zhai, H.; Zhao, S.; Liu, G.; Wei, Y.; Feng, L. Ultralong Inhibition of heterogeneous ice nucleation by robust Anti-Freezing coating with Self-Lubricating ionic salts layer. Chem. Eng. J. 2023, 474, 145537. [Google Scholar] [CrossRef]
- Ji, J.; Liu, N.; Tian, Y.; Li, X.; Zhai, H.; Zhao, S.; Liu, Y.; Liu, G.; Wei, Y.; Feng, L. Transparent polyurethane coating with synergistically enhanced antibacterial mechanism composed of low surface free energy and biocide. Chem. Eng. J. 2022, 445, 136716. [Google Scholar] [CrossRef]
- Zhou, N.; Ji, J.; Qu, R.; Feng, X.; Song, X.; Chen, M.; Chen, F.; Ma, Z.; Wei, Y. Permeable and Durable Liquid-Metal Fiber Mat as Implantable Physiological Electrodes with Long-Term Biocompatibility. Adv. Mater. 2025, 37, 2413728. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Q.; Zhou, N.; Zhuang, Q.; Ng, S.-W.; Zheng, Z. Stretchable and conductive fibers fabricated by a continuous method for wearable devices. Cell Rep. Phys. Sci. 2023, 4, 101300. [Google Scholar] [CrossRef]
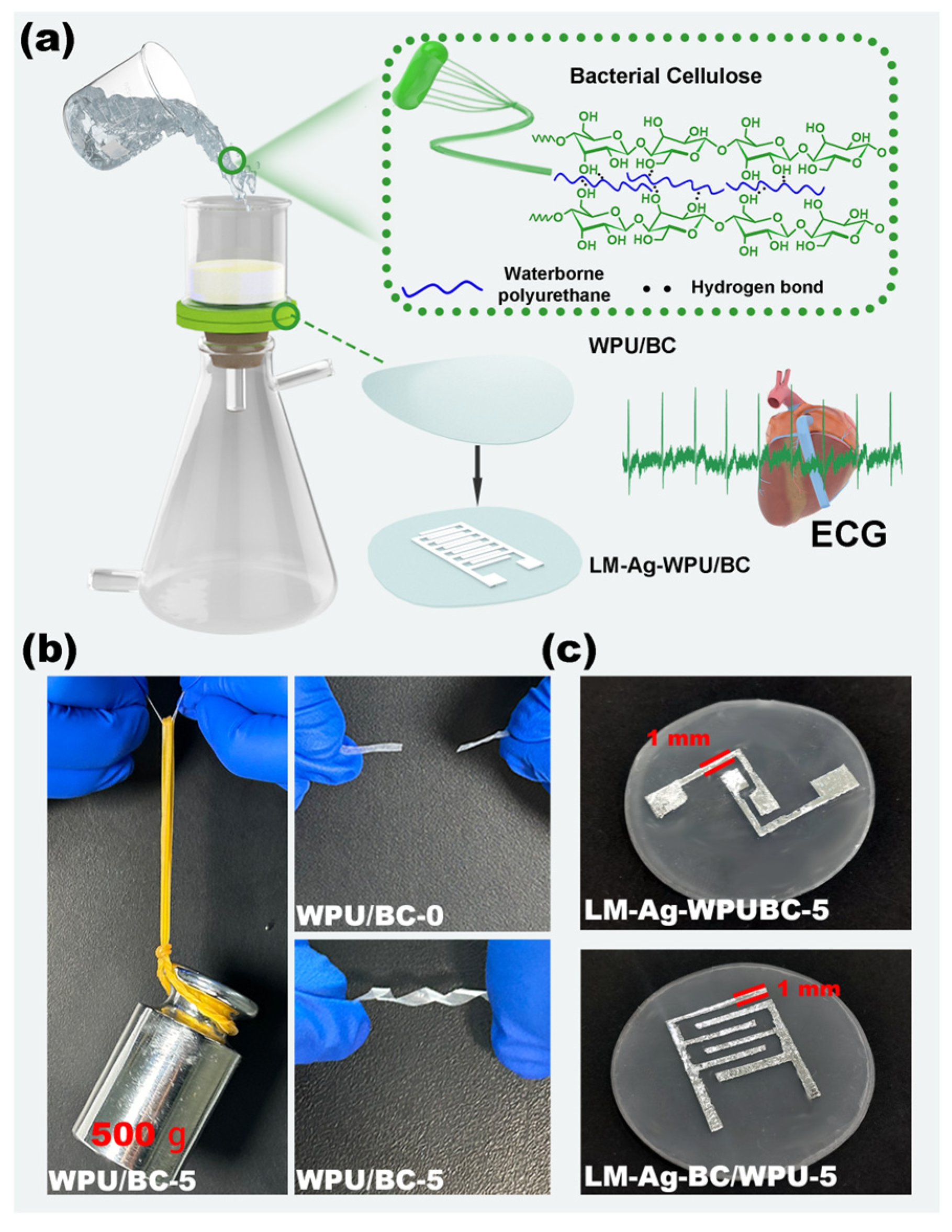

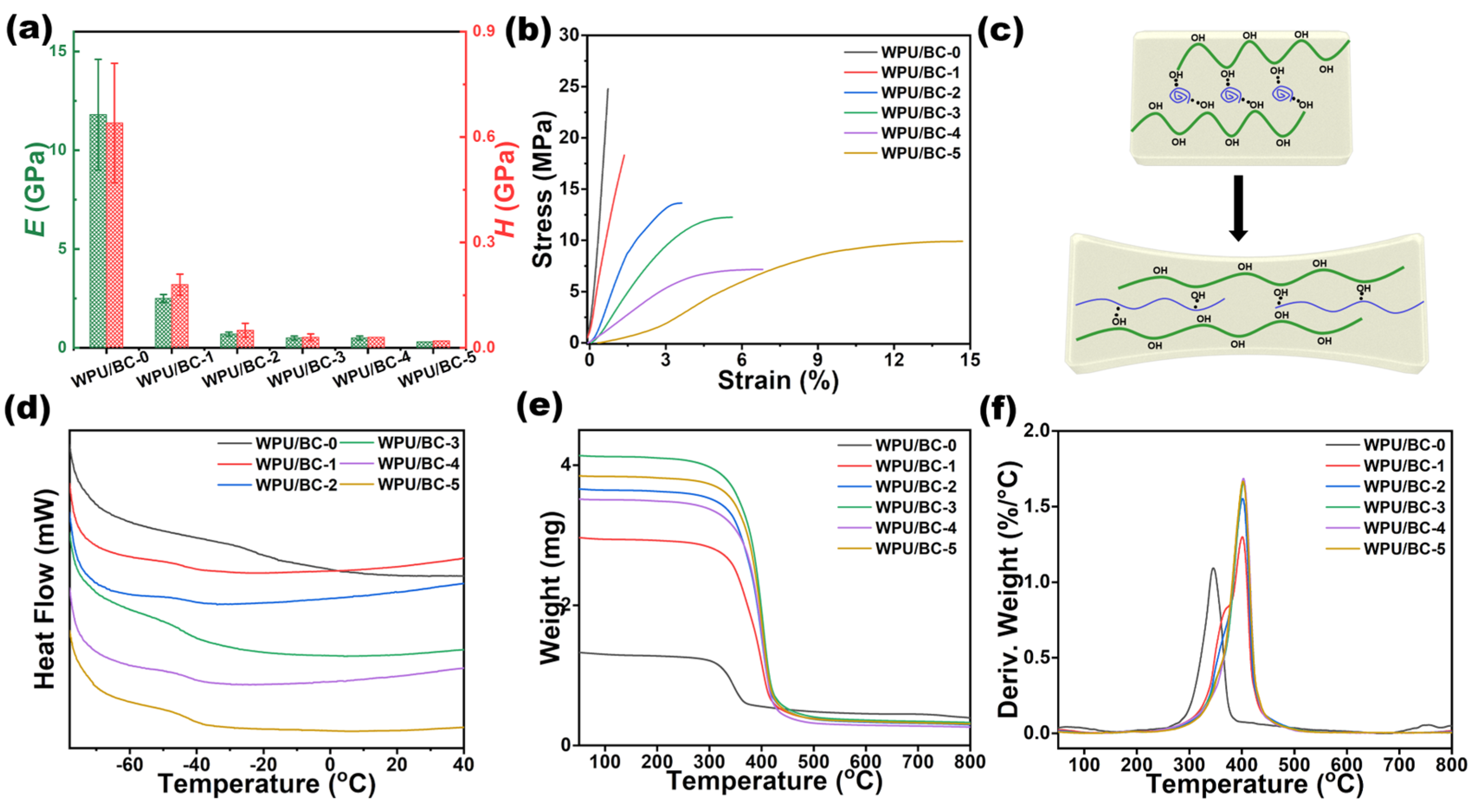
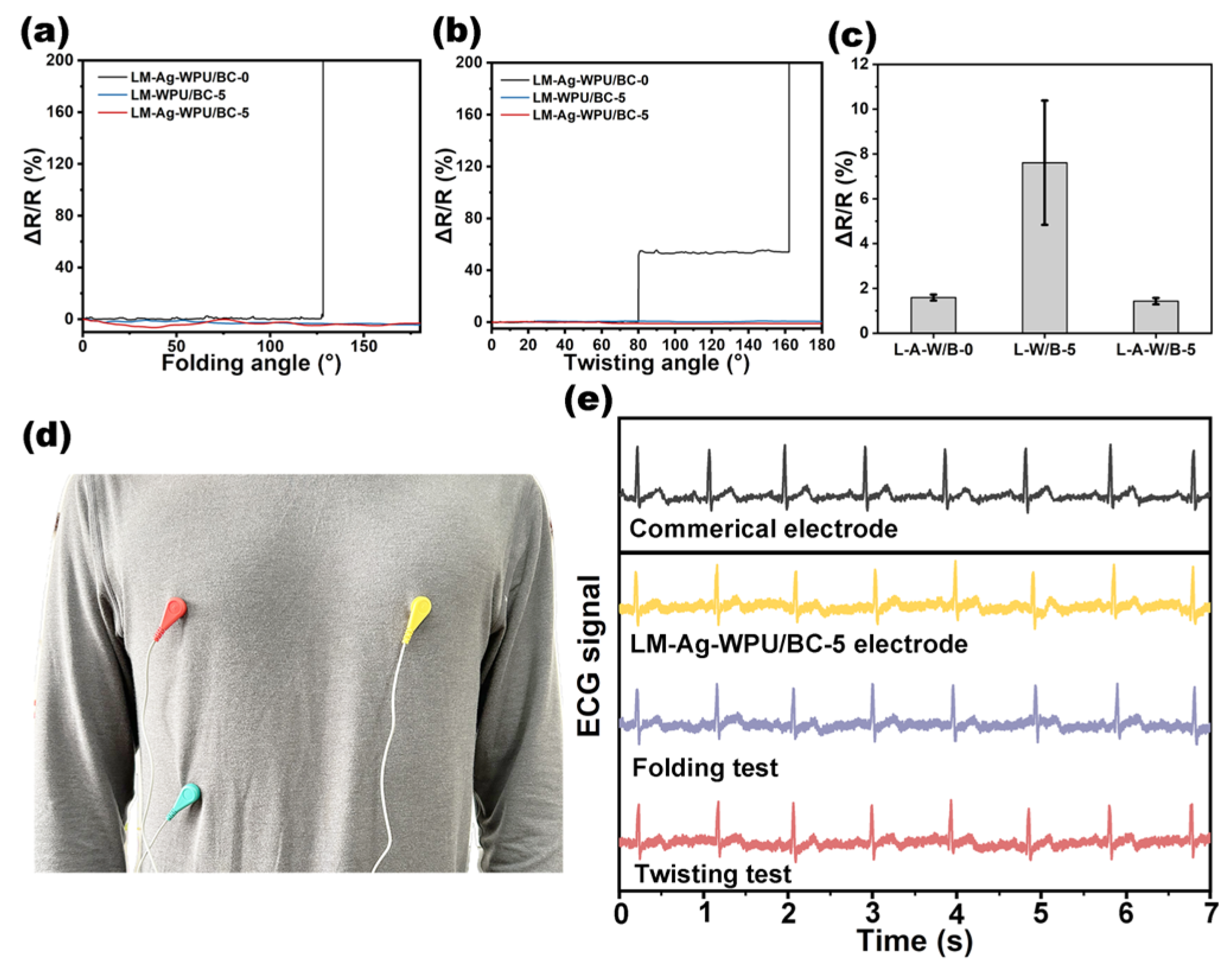
| WPU (6 wt%, g) | BC (6 wt%, g) | H2O (g) | Mixture (g) | Samples |
|---|---|---|---|---|
| 0 | 10 | 10 | 10.0 | WPU/BC-0 |
| 2 | 10 | 10 | 9.2 | WPU/BC-1 |
| 4 | 10 | 10 | 8.6 | WPU/BC-2 |
| 6 | 10 | 10 | 8.1 | WPU/BC-3 |
| 8 | 10 | 10 | 7.8 | WPU/BC-4 |
| 10 | 10 | 10 | 7.5 | WPU/BC-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Cao, C.; Qu, R.; Zhou, N.; He, E.; Wu, M.; Xiang, H.; Ma, Z.; Liu, G.; Wei, Y. Flexible Waterborne Polyurethane-Bacterial Cellulose Films for Real-Time Physiological Monitoring. Polymers 2025, 17, 787. https://doi.org/10.3390/polym17060787
Ji J, Cao C, Qu R, Zhou N, He E, Wu M, Xiang H, Ma Z, Liu G, Wei Y. Flexible Waterborne Polyurethane-Bacterial Cellulose Films for Real-Time Physiological Monitoring. Polymers. 2025; 17(6):787. https://doi.org/10.3390/polym17060787
Chicago/Turabian StyleJi, Jiujiang, Changyong (Chase) Cao, Ruixiang Qu, Ningjing Zhou, Enjian He, Mingrui Wu, Huacui Xiang, Zhijun Ma, Guojun Liu, and Yen Wei. 2025. "Flexible Waterborne Polyurethane-Bacterial Cellulose Films for Real-Time Physiological Monitoring" Polymers 17, no. 6: 787. https://doi.org/10.3390/polym17060787
APA StyleJi, J., Cao, C., Qu, R., Zhou, N., He, E., Wu, M., Xiang, H., Ma, Z., Liu, G., & Wei, Y. (2025). Flexible Waterborne Polyurethane-Bacterial Cellulose Films for Real-Time Physiological Monitoring. Polymers, 17(6), 787. https://doi.org/10.3390/polym17060787









