Study of the Influence of Chitosan-Wrapped Carbon Nanotubes on Biopolymer Film Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Film Samples Preparation
2.2. Film Samples Characterization
2.2.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.2.2. Thickness
2.2.3. Ionic Exchange Capacity (IEC)
2.2.4. Swelling and Solubility
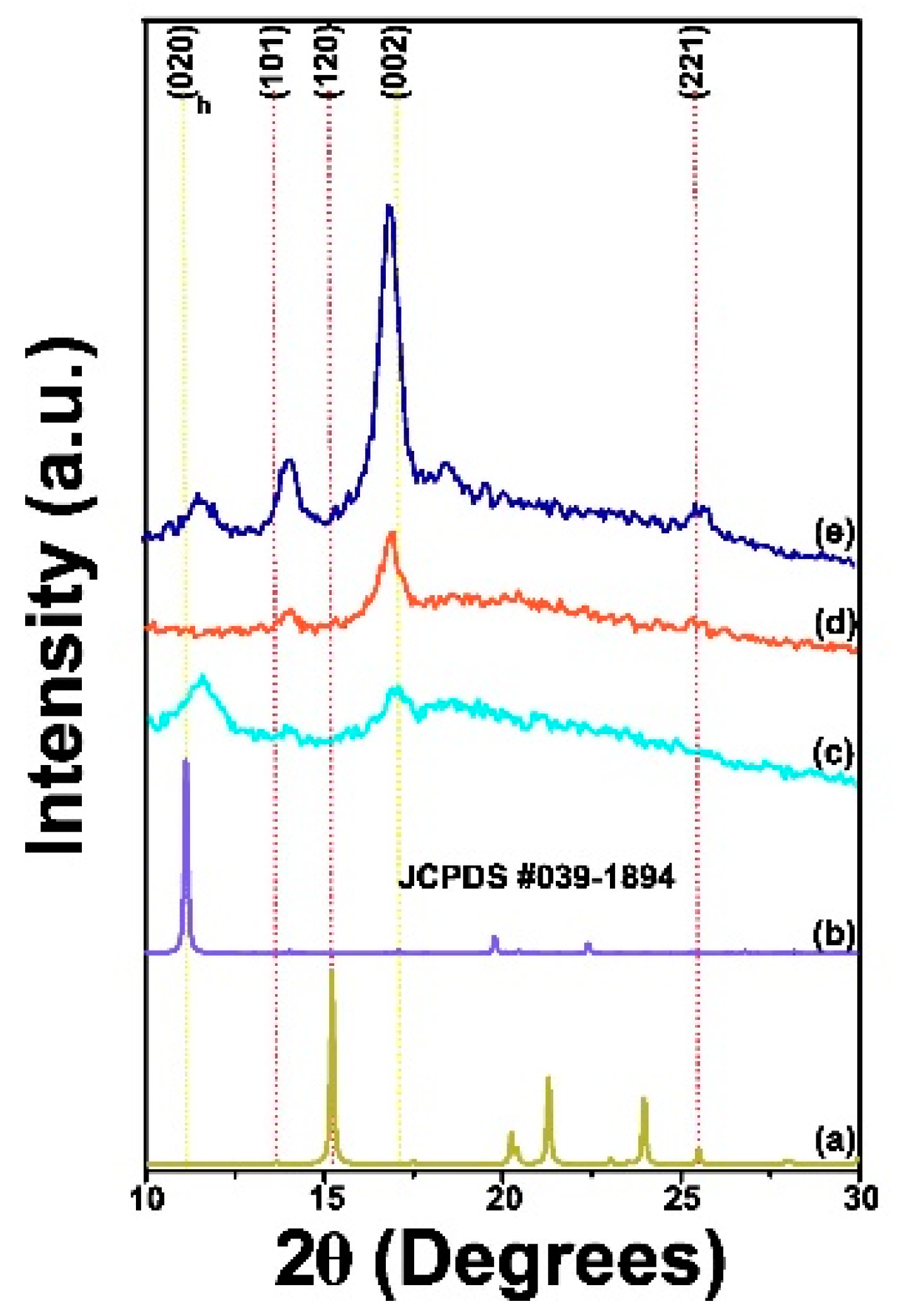
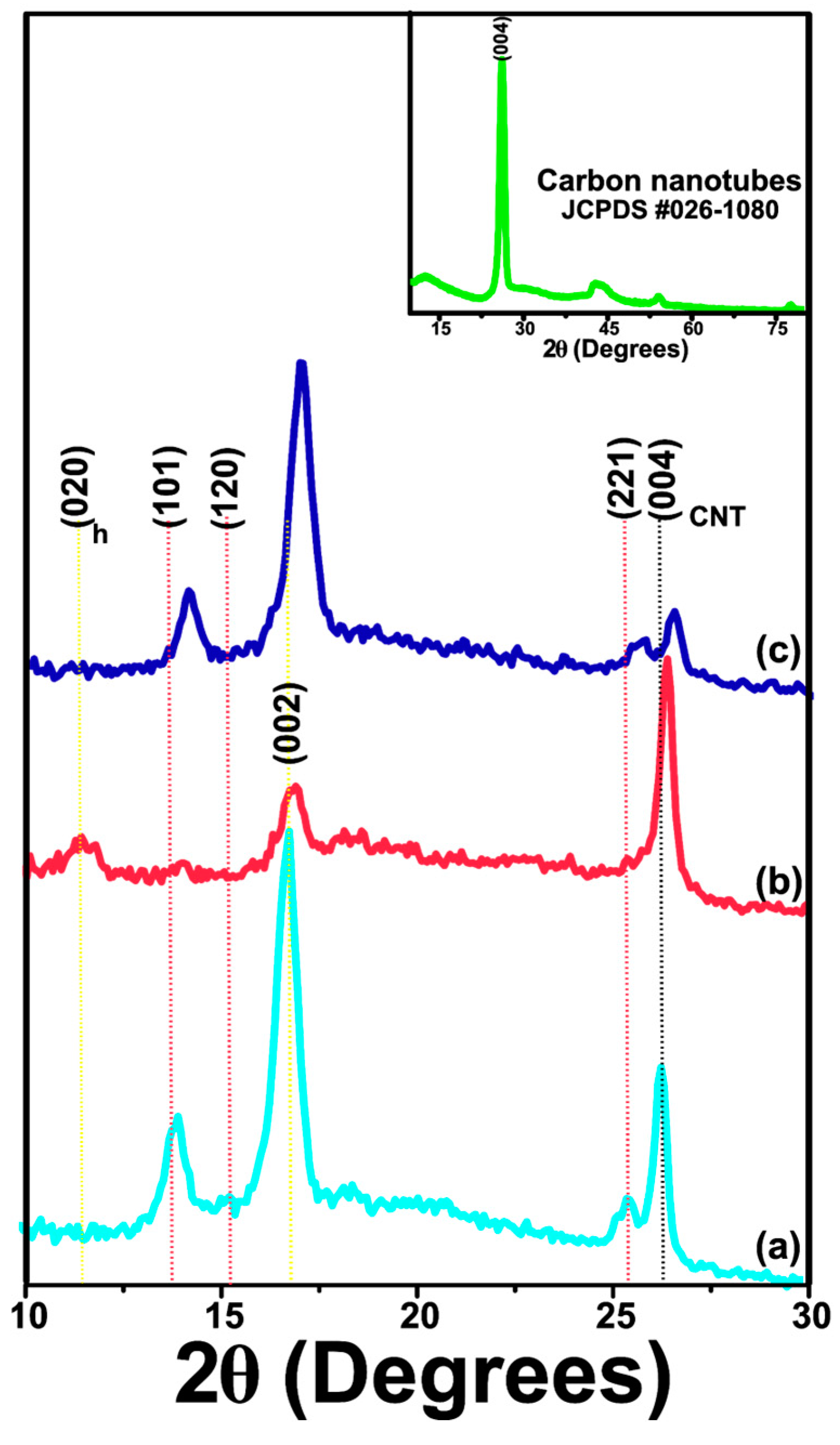
2.2.5. XRD Analysis
2.2.6. X-Ray Photoelectron Spectroscopy (XPS)
2.2.7. Mechanical Characterization
2.2.8. SEM Analysis
2.2.9. 4-Probe Measurements
2.2.10. Photoluminescence (PL) Studies
3. Results
3.1. Chemical Composition, Structural Characterization, Physical, Mechanical and Optical Properties of Film Samples
3.1.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.1.2. Thickness
3.1.3. Ion Exchange Capacity (IEC)
3.1.4. Degree of Swelling and Solubility
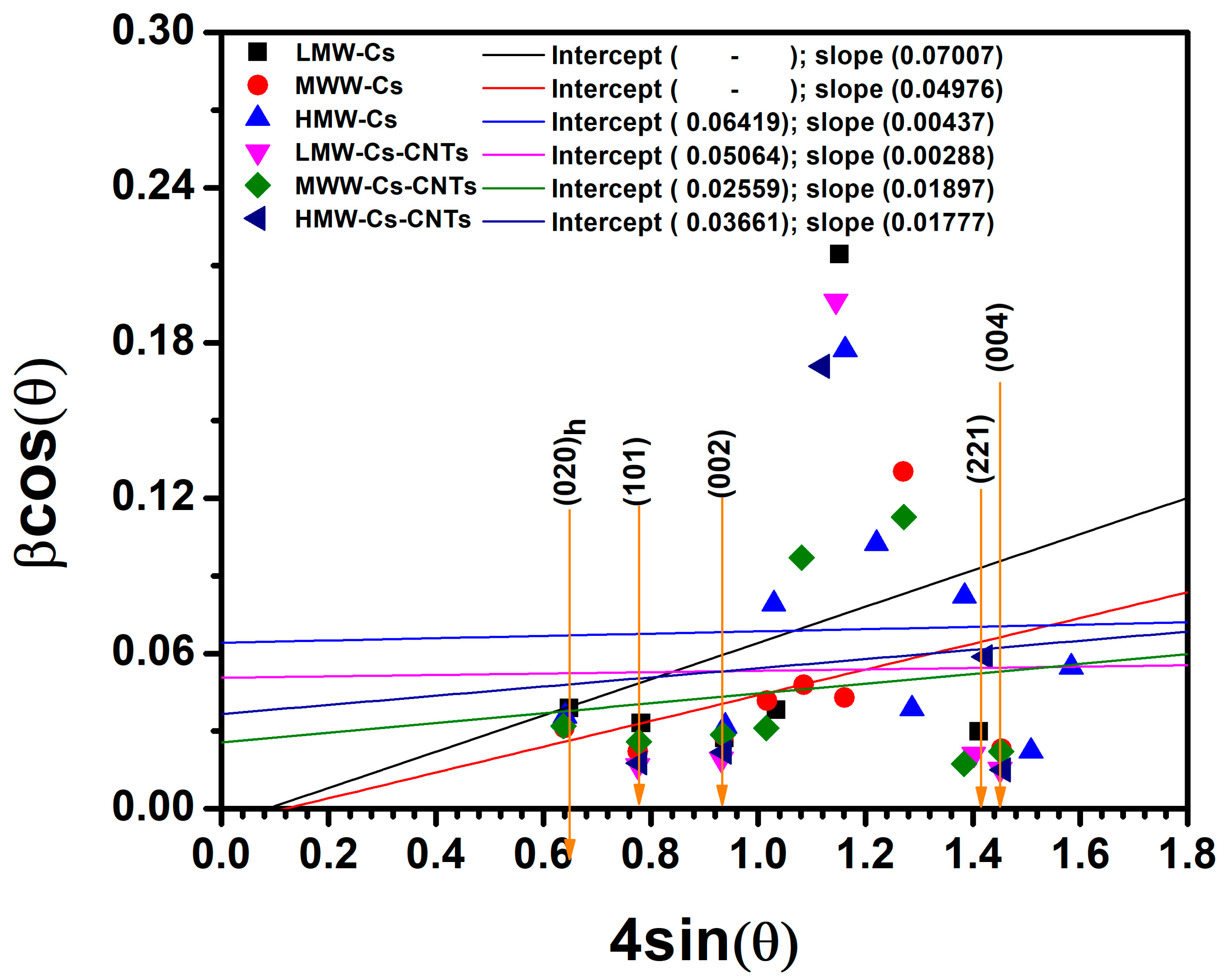
3.1.5. XRD Analysis
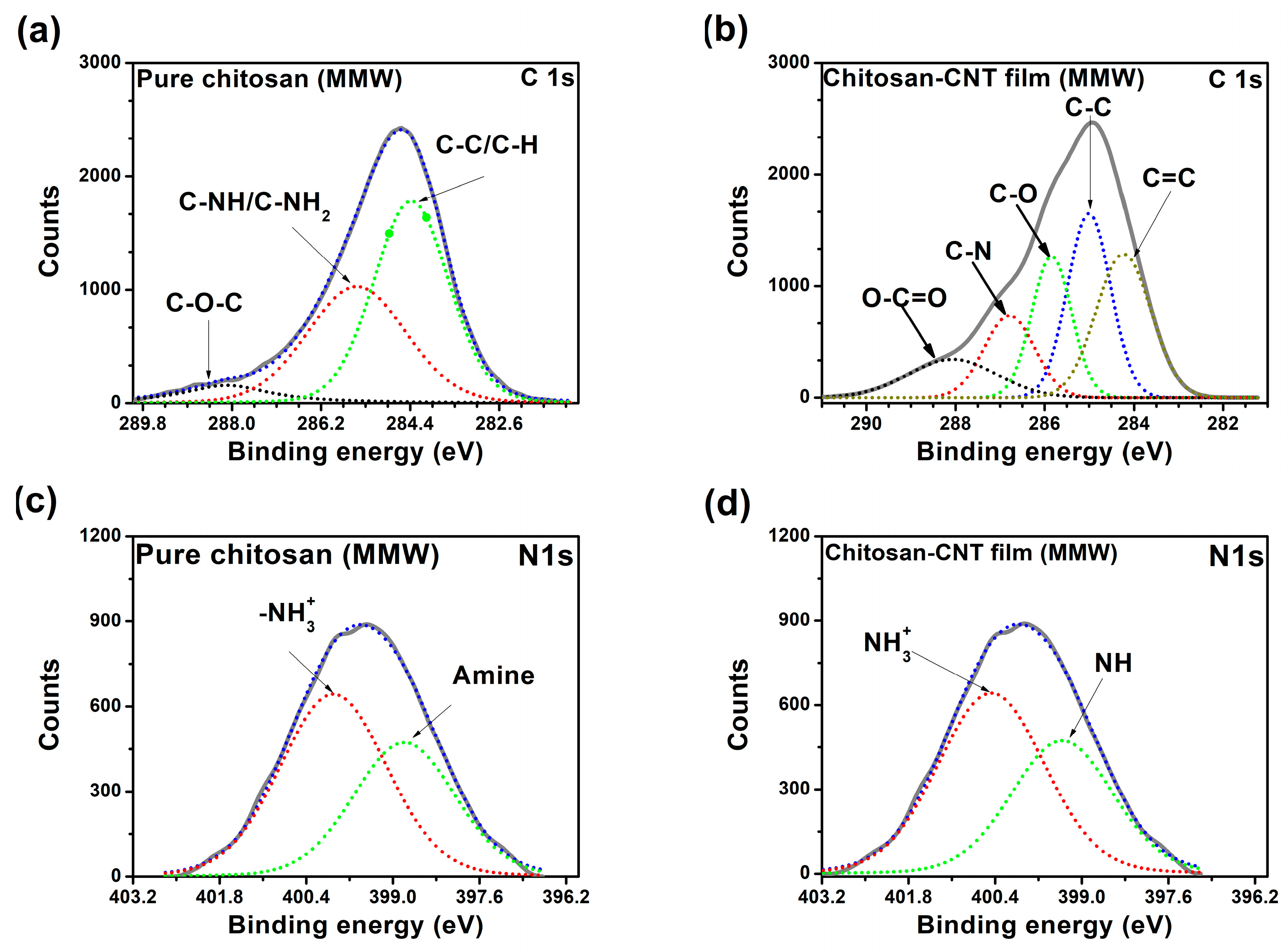
3.1.6. X-Ray Photoelectron Spectroscopy (XPS)
3.1.7. Mechanical Properties
3.1.8. Scanning Electron Microscopy (SEM) Analysis
3.1.9. Four-Probe Measurements
3.1.10. Photoluminescence (PL) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cs | Chitosan |
| CNTs | Carbon nanotubes |
| LMW-Cs | Low-molecular-weight chitosan |
| MMW-Cs | Medium-molecular-weight chitosan |
| HMW-Cs | High-molecular-weight chitosan |
| LMW-Cs/CNTs | Low-molecular-weight chitosan/carbon nanotubes |
| MMW-Cs/CNTs | Medium-molecular-weight chitosan/carbon nanotubes |
| HMW-Cs/CNTs | High-molecular-weight chitosan/carbon nanotubes |
References
- Kosowska, K.; Domalik-Pyzik, P.; Krok-Borkowicz, M.; Chłopek, J. Synthesis and Characterization of Chitosan/Reduced Graphene Oxide Hybrid Composites. Materials 2019, 12, 2077. [Google Scholar] [CrossRef]
- El-Araby, A.; Janati, W.; Ullah, R.; Ercisli, S.; Errachidi, F. Chitosan, chitosan derivatives, and chitosan-based nanocomposites: Eco-friendly materials for advanced applications (a review). Front. Chem. 2024, 11, 1327426. [Google Scholar] [CrossRef]
- Slyusarenko, N.; Gerasimova, M.; Plotnikov, A.; Gaponik, N.; Slyusareva, E. Photoluminescence properties of self-assembled chitosan-based composites containing semiconductor nanocrystals. Phys. Chem. Chem. Phys. 2019, 21, 4831–4838. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Du, Y. Novel water-soluble chitosan derivatives/quantum dots nanocomposite: Synthesis, characterization and photoluminescence properties. J. Nanosci. Nanotechnol. 2009, 9, 6866–6875. [Google Scholar] [CrossRef]
- Javed, M.; Hussain, S.; Riaz, M.; Asghar, A.; Syed, S.; Barkaat, S.; Suleman, M.; Idrees, M.; Ashraf, F.; Faizan, M.; et al. Synthesis and characterization of nanoparticles derived from chitosan-based biopolymer; their photocatalytic and anti-termite potential. Dig. J. Nanomater. Biostruct. 2021, 16, 1607–1618. [Google Scholar] [CrossRef]
- González-Martínez, J.R.; López-Oyama, A.B.; Del Ángel-López, D.; García-Guendulain, C.; Rodríguez-González, E.; Pulido-Barragan, E.U.; Barffuson-Domínguez, F.; Magallanes-Vallejo, A.G.; Mogica-Cantú, P.J. Influence of Reduced Graphene Oxide and Carbon Nanotubes on the Structural, Electrical, and Photoluminescent Properties of Chitosan Films. Polymers 2024, 16, 1827. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Maliyekkal, M.S.; Deepti, K.; Chaudhari, K.; Xavier, P.L.; Pradeep, T. Transparent, luminescent, antibacterial and patternable film forming composites of graphene oxide/reduced graphene oxide. ACS Appl. Mater. Interfaces 2011, 3, 2643–2654. [Google Scholar] [CrossRef]
- Meriem, F.; Mekhzoum, M.E.M.; Qaiss, A.; Bouhfid, R. Bionanocomposite Materials Based on Chitosan Reinforced with Nanocrystalline Cellulose and Organo-Modified Montmorillonite. In Nanoclay Reinforced Polymer Composites; Springer: Singapore, 2016; pp. 167–194. [Google Scholar] [CrossRef]
- Pan, X.; Ren, W.; Gu, L.; Wang, G.; Liu, Y. Photoluminescence from Chitosan for Bio-Imaging. Aust. J. Chem. 2014, 67, 1422. [Google Scholar] [CrossRef]
- Falamarzpour, P.; Anbaran, S.R.G. Synergistic effect of carbon nanotubes on chitosan-graphene oxide supramolecular structure. J. Polym. Res. 2022, 29, 211. [Google Scholar] [CrossRef]
- Rungrotmongkol, T.; Arsawang, U.; Iamsamai, C.; Vongachariya, A.; Dubas, S.T.; Ruktanonchai, U.; Soottitantawat, A.; Hannongbua, S. Increased dispersion and solubility of carbon nanotubes noncovalently modified by the polysaccharide biopolymer, chitosan: MD simulations. Chem. Phys. Lett. 2011, 507, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Pan, F.; Sun, H.; Lu, L.; Jiang, Z. Novel nanocomposite pervaporation membranes composed of poly(vinyl alcohol) and chitosan-wrapped carbon nanotube. J. Membr. Sci. 2007, 300, 13–19. [Google Scholar] [CrossRef]
- Liu, Y.; Chipot, C.; Shao, X.; Cai, W. Free-Energy Landscape of the Helical Wrapping of a Carbon Nanotube by a Polysaccharide. J. Phys. Chem. C 2011, 115, 1851–1856. [Google Scholar] [CrossRef]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaidya, S.S.; Yadav, M.D. Carbon nanotubes in biomedical applications: Current status, promises, and challenges. Carbon. Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef]
- García-García, D.J.; Pérez-Sánchez, G.F.; Hernández-Cocoletzi, H.; Sánchez-Arzubide, M.G.; Luna-Guevara, M.L.; Rubio-Rosas, E.; Krishnamoorthy, R.; Morán-Raya, C. Chitosan Coatings Modified with Nanostructured ZnO for the Preservation of Strawberries. Polymers 2023, 15, 3772. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Rodríguez Llanos, J.H.; De Oliveira Vercik, L.C.; Vercik, A. Physical Properties of Chitosan Films Obtained after Neutralization of Polycation by Slow Drip Method. J. Biomater. Nanobiotechnol. 2015, 6, 15. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: Conshohocken, PA, USA, 2018.
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films: Effect of the type of solvent acid. LWT 2021, 135, 109984. [Google Scholar]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Carson, L.; Kelly-Brown, C.; Stewart, M.; Oki, A.; Regisford, G.; Luo, Z.; Bakhmutov, V.I. Synthesis and characterization of chitosan–carbon nanotube composites. Mater. Lett. 2009, 63, 617–620. [Google Scholar] [CrossRef]
- Saputra, E.; Tjahjaningsih, W.; Abdillah, A.A. Characteristics of edible film from chitosan as biodegradable packaging. IOP Conf. Ser. Earth Environ. Sci. 2021, 718, 012078. [Google Scholar]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films. J. Biomed. Mater. Res. A 2014, 102, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.Á.V.; Horn, M.M.; Martins, V.C.A.; Plepis, A.M.G. Single-wall carbon nanotubes-chitosan nanocomposites: Surface wettability, mechanical and thermal properties. Mater. Werkst. 2021, 52, 400–408. [Google Scholar] [CrossRef]
- Nhung, L.T.T.; Kim, I.Y.; Yoon, Y.S. Quaternized Chitosan-Based Anion Exchange Membrane Composited with Quaternized Poly(vinylbenzyl chloride)/Polysulfone Blend. Polymers 2020, 12, 2714. [Google Scholar] [CrossRef]
- Lewandowska, K.; Szulc, M. Rheological and Film-Forming Properties of Chitosan Composites. Int. J. Mol. Sci. 2022, 23, 8763. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Yasin, T.; Hassan, S.; Riaz, M.; Nawaz, M. Chitosan/CNTs green nanocomposite membrane: Synthesis, swelling and polyaromatic hydrocarbons removal. Mater. Sci. Eng. C 2015, 46, 359–365. [Google Scholar] [CrossRef]
- de Arruda, M.N.; de Souza, C.B.; Eugênio, K.F.R.D.M.S.; Gonçalves, E.S. Influence of swelling level on charge transmission of chitosan and reduced graphene oxide film electrodes. Mater. Chem. Phys. 2020, 255, 123623. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Okuyama, K.; Noguchi, K.; Miyazawa, T.; Yui, T.; Ogawa, K. Molecular and Crystal Structure of Hydrated Chitosan. Macromolecules 1997, 30, 5849–5855. [Google Scholar]
- Naito, P.K.; Ogawa, Y.; Sawada, D.; Nishiyama, Y.; Iwata, T.; Wada, M. X-ray crystal structure of anhydrous chitosan at atomic resolution. Biopolymers 2016, 105, 361–368. [Google Scholar] [CrossRef]
- Facchinatto, W.M.; Santos, D.M.D.; Fiamingo, A.; Bernardes-Filho, R.; Campana-Filho, S.P.; Azevedo, E.R.D.; Colnago, L.A. Evaluation of chitosan crystallinity: A high-resolution solid-state NMR spectroscopy approach. Carbohydr. Polym. 2020, 250, 116891. [Google Scholar] [CrossRef]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.; Kuskov, T.; Rychkov, D.; Lomovskii, O.; Bychkov, A. Mechanical Amorphization of Chitosan with Different Molecular Weights. Polymers 2022, 14, 4438. [Google Scholar] [CrossRef]
- Jayaram, P.; Pradyumnan, P.P.; Karazhanov, S.Z. Micro-strain, dislocation density and surface chemical state analysis of multication thin films. Phys. B Condens. Matter 2016, 501, 140–145. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar] [CrossRef]
- Magesh, G.; Bhoopathi, G.; Nithya, N.; Arun, A.P.; Kumar, E.R. Effect of Biopolymer Blend Matrix on Structural, Optical and Biological Properties of Chitosan–Agar Blend ZnO Nanocomposites. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1528–1539. [Google Scholar] [CrossRef]
- Pavlov, I.S.; Galiakhmetova, L.K.; Kudreyko, A.A.; Dmitriev, S.V. Mobility of dislocations in carbon nanotube bundles. Mater. Today Commun. 2024, 40, 110094. [Google Scholar] [CrossRef]
- Suarez-Martinez, I.; Savini, G.; Zobellil, A.; Heggie, M. Dislocations in carbon nanotube walls. J. Nanosci. Nanotechnol. 2007, 7, 3417–3420. [Google Scholar] [CrossRef]
- Wamuo, O.; Wu, Y.; Hsu, S.L.; Paul, C.W.; Eodice, A.; Huang, K.-Y.; Chen, M.-H.; Chang, Y.-H.; Lin, J.-L. Effects of chain configuration on the crystallization behavior of polypropylene based copolymers. Polymer 2017, 116, 342–349. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.M. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.B.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Greenfeld, I.; Wagner, H.D. Toughness of carbon nanotubes conforms to classic fracture mechanics. Sci. Adv. 2016, 2, e1500969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xiao, K.; Zhang, W.; Lei, X.; Bai, Y.; Wang, S.; Zhang, P.; Gao, F.; Wang, C.; Xu, W.; et al. Fabricating bio-inspired high impact resistance carbon nanotube network films for multi-protection under an extreme environment. Nano Res. 2024, 17, 7793–7802. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, W.; Zhang, Z.; Zhang, D.; Guo, Z.; Ren, P.; Liu, F. Effect of Nano-Silica and Sorbitol on the Properties of Chitosan-Based Composite Films. Polymers 2023, 15, 4015. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Zhang, W.D.; Tong, Y.J. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef]
- Khan, A.; Vu, K.D.; Chauve, G.; Bouchard, J.; Riedl, B.; Lacroix, M. Optimization of microfluidization for the homogeneous distribution of cellulose nanocrystals (CNCs) in biopolymeric matrix. Cellulose 2014, 21, 3457–3468. [Google Scholar] [CrossRef]
- Zhang, J.P.; Wang, A.Q. Synergistic effects of Na+-montmorillonite and multi-walled carbon nanotubes on mechanical properties of chitosan film. Express Polym. Lett. 2009, 3, 302–308. [Google Scholar] [CrossRef]
- Lau, Y.-T.R.; Yamaguchi, M.; Li, X.; Bando, Y.; Golberg, D.; Winnik, F.M. Facile and Mild Strategy Toward Biopolymer-Coated Boron Nitride Nanotubes via a Glycine-Assisted Interfacial Process. J. Phys. Chem. C 2013, 117, 19568–19576. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Chen, X.; Xin, J.H. Decoration of carbon nanotubes with chitosan. Carbon 2005, 43, 3178–3180. [Google Scholar] [CrossRef]
- Molla-Abbasi, P.; Ghaffarian, S.R. Decoration of carbon nanotubes by chitosan in a nanohybrid conductive polymer composite for detection of polar vapours. RSC Adv. 2014, 4, 30906–30913. [Google Scholar] [CrossRef]
- Lau, C.; Cooney, M.J.; Atanassov, P. Conductive Macroporous Composite Chitosan−Carbon Nanotube Scaffolds. Langmuir 2008, 24, 7004–7010. [Google Scholar] [CrossRef]
- Ramadhan, Z.R.; Han, J.W.; Lee, D.J.; Entifar, S.A.N.; Hong, J.; Yun, C.; Kim, Y.H. Surface-functionalized silver nanowires on chitosan biopolymers for highly robust and stretchable transparent conducting films. Mater. Res. Lett. 2019, 7, 124–130. [Google Scholar] [CrossRef]
- Rahman, S.U.; Bilal, S.; ul Haq Ali Shah, A. Synthesis and Characterization of Polyaniline-Chitosan Patches with Enhanced Stability in Physiological Conditions. Polymers 2020, 12, 2870. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Z.; Zhao, L.; Li, L. Degradable, ultra-flexible, transparent and conductive film made of assembling CuNWs on chitosan. Opt. Mater. 2022, 123, 111752. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Pulido-Barragán, E.U.; Rodríguez-González, E.; López-Oyama, A.B.; Morales-Cepeda, A.B.; Castro-Guerrero, C.F.; Heinze, T.; Koschella, A. Photoluminescence enhancement after thermal treatment of cellulose from different sources. Cellulose 2024, 31, 6611–6623. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, L.; Li, W.; Tian, D.; Xu, W. Enhancing Persistent Luminescence of Cellulose by Dehydration for Label-Free Time-Resolved Imaging. ACS Sustain. Chem. Eng. 2021, 9, 17420–17426. [Google Scholar] [CrossRef]
- Shimamoto, D.; Muramatsu, H.; Hayashi, T.; Kim, Y.A.; Endo, M.; Park, J.S.; Saito, R.; Terrones, M.; Dresselhaus, M.S. Strong and stable photoluminescence from the semiconducting inner tubes within double walled carbon nanotubes. Appl. Phys. Lett. 2009, 94, 083106. [Google Scholar] [CrossRef]
- Vega-Cázarez, C.A.; López-Cervantes, J.; Sánchez-Machado, D.I.; Madera-Santana, T.J.; Soto-Cota, A.; Ramírez-Wong, B. Preparation and Properties of Chitosan–PVA Fibers Produced by Wet Spinning. J. Polym. Environ. 2018, 26, 946–958. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Elashmawi, I.S.; Labeeb, S. Chitosan filler effects on the experimental characterization, spectroscopic investigation and thermal studies of PVA/PVP blend films. Phys. B Condens. Matter 2010, 405, 2021–2027. [Google Scholar] [CrossRef]
- Rance, G.A.; Marsh, D.H.; Nicholas, R.J.; Khlobystov, A.N. UV–vis absorption spectroscopy of carbon nanotubes: Relationship between the π-electron plasmon and nanotube diameter. Chem. Phys. Lett. 2010, 493, 19–23. [Google Scholar] [CrossRef]
- Joseph, S.; John, A.O.; Mugwang’a, F.K.; Katana, G.G. Tuning the Band Gap Energy of Reduced Graphene Oxide Using Biopolymer Chitosan for High Power and Frequency Device Applications. Am. J. Polym. Sci. Eng. 2019, 7, 8–19. [Google Scholar]

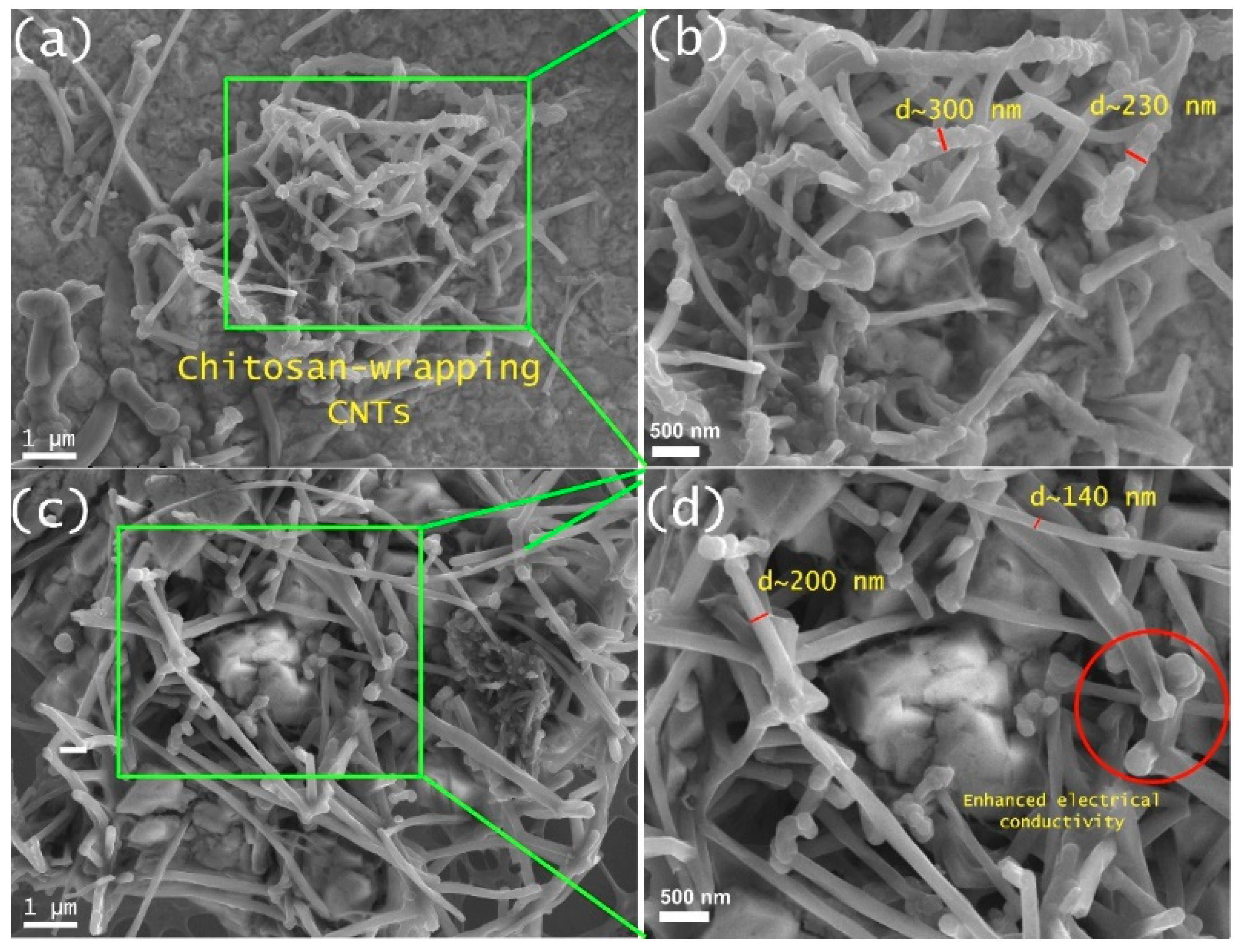
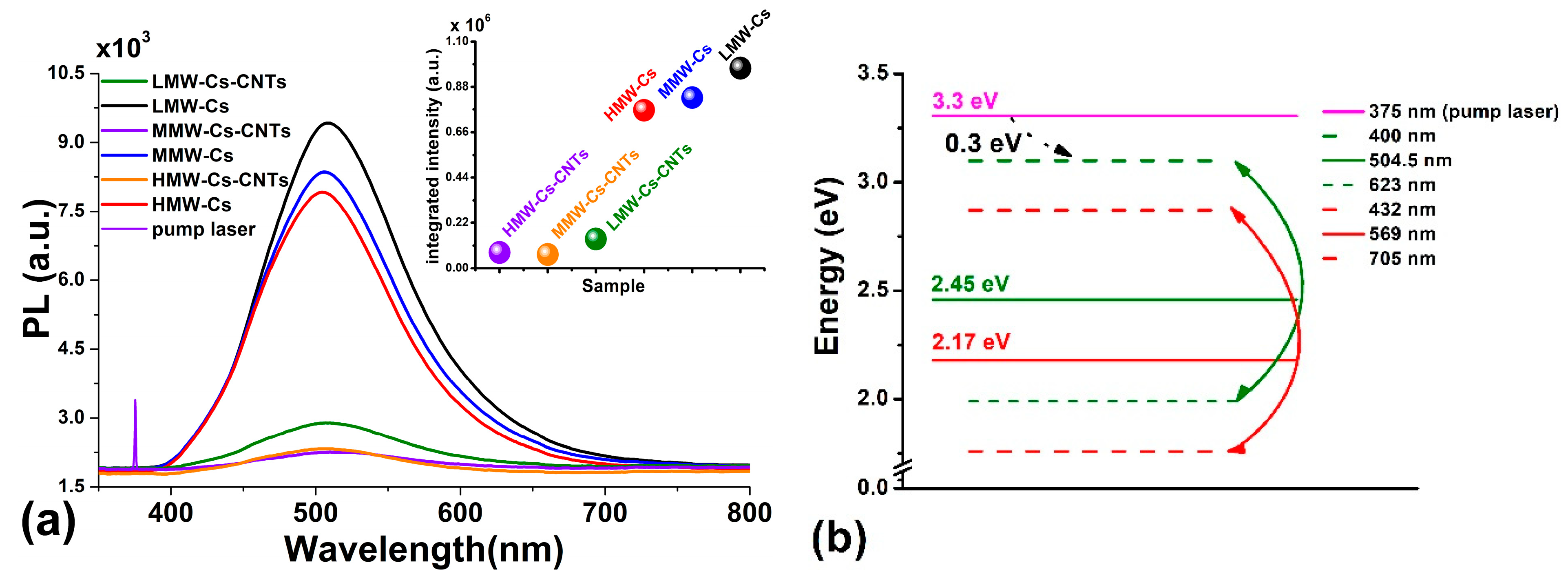
| Films | Thickness (µm) |
|---|---|
| LMW-Cs | 66 |
| LMW-Cs/CNTs | 84 |
| MMW-Cs | 54 |
| MMW-Cs/CNTs | 66 |
| HMW-Cs | 62 |
| HMW-Cs/CNTs | 54 |
| Films | %DS | %SW | IEC (meq g−1) |
|---|---|---|---|
| LMW-Cs * | 334.5 | 18.2 | 0.04 |
| LMW-Cs/CNTs | 450.8 | 23.0 | 0.057 |
| MMW-Cs * | 798.9 | 18.3 | 0.041 |
| MMW-Cs/CNTs | 517.3 | 28.4 | 0.047 |
| HMW-Cs * | 747.0 | 18.3 | 0.041 |
| HMW-Cs/CNTs | 665.0 | 28.4 | 0.047 |
| Chitosan Molecular Weight | CrI (nm) |
|---|---|
| LMW-Cs | 0.58 |
| MMW-Cs | 0.60 |
| HMW-Cs | 0.62 |
| Composite Films | |||
|---|---|---|---|
| LCS | MCS | HCS | |
| Crystallite Size (nm) | 17.73 | 39.07 | 15.12 |
| Microstrain (1 × 10−3) | 9.27 | 18.16 | 7.05 |
| Dislocation density (1 × 10−2) | 0.43 | 1.36 | 0.47 |
| Film | BE (eV) | |
|---|---|---|
| C 1s | N 1s | |
Pure chitosan film | 284.41 | 398.77 |
| 285.47 | 399.96 | |
| 288.39 | ||
Chitosan–CNTs film | 284.44 | 399.32 |
| 284.95 | 400.38 | |
| 285.8 | ||
| 286.23 | ||
| 288.23 | ||
| Films | Tensile Strength (MPa) | Elongation-at-Break (%) | Young Modulus (MPa) | Toughness (MJ m−3) |
|---|---|---|---|---|
| LMW-Cs | 72.13 ± 20.03 | 4.75 ± 1.47 | 3189.59 ± 524.34 | 2.06 ± 0.99 |
| LMW-Cs/CNTs | 45.62 ± 4.67 | 3.82 ± 1.19 | 2122.94 ± 664.58 | 0.86 ± 0.42 |
| MMW-Cs | 35.75 ± 2.88 | 4.75 ± 0.86 | 1620.60 ± 232.64 | 1.17 ± 0.32 |
| MMW-Cs/CNTs | 51.26 ± 7.31 | 7.26 ± 3.03 | 1895.97 ± 564.78 | 2.55 ± 1.27 |
| HMW-Cs | 49.60 ± 1.55 | 4.02 ± 0.81 | 2344.19 ± 466.55 | 1.21 ± 0.65 |
| HMW-Cs/CNTs | 56.46 ± 13.51 | 4.71 ± 1.32 | 2627.82 ± 805.32 | 1.78 ± 0.81 |
| Films | Rs (mΩ/Υ) | Resistivity (Ω cm−1) | Electrical Conductivity (S cm−1) |
|---|---|---|---|
| LMW-Cs | 1 × 108 | 1.22 × 108 | 5.1 × 10−8 |
| LMW-Cs/CNTs | 202.9 | 538.15 | 0.0048 |
| MMW-Cs | 3.9 × 107 | 6.71 × 107 | 5.95 × 10−8 |
| MMW-Cs/CNTs | 116.7 | 226.91 | 0.0156 |
| HMW-Cs | 6.7 × 107 | 9.43 × 107 | 5.16 × 10−8 |
| HMW-Cs/CNTs | 174.8 | 301.30 | 0.0132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magallanes-Vallejo, A.G.; López-Oyama, A.B.; González, E.R.; Del Angel-López, D.; Pulido-Barragán, E.U.; García-Guendulain, C.; Madera-Santana, T.J.; Rodríguez-Beas, C.; Gámez-Corrales, R. Study of the Influence of Chitosan-Wrapped Carbon Nanotubes on Biopolymer Film Properties. Polymers 2025, 17, 889. https://doi.org/10.3390/polym17070889
Magallanes-Vallejo AG, López-Oyama AB, González ER, Del Angel-López D, Pulido-Barragán EU, García-Guendulain C, Madera-Santana TJ, Rodríguez-Beas C, Gámez-Corrales R. Study of the Influence of Chitosan-Wrapped Carbon Nanotubes on Biopolymer Film Properties. Polymers. 2025; 17(7):889. https://doi.org/10.3390/polym17070889
Chicago/Turabian StyleMagallanes-Vallejo, Aurora G., Ana B. López-Oyama, Eugenio Rodríguez González, Deyanira Del Angel-López, Eder U. Pulido-Barragán, Crescencio García-Guendulain, Tomás J. Madera-Santana, César Rodríguez-Beas, and Rogelio Gámez-Corrales. 2025. "Study of the Influence of Chitosan-Wrapped Carbon Nanotubes on Biopolymer Film Properties" Polymers 17, no. 7: 889. https://doi.org/10.3390/polym17070889
APA StyleMagallanes-Vallejo, A. G., López-Oyama, A. B., González, E. R., Del Angel-López, D., Pulido-Barragán, E. U., García-Guendulain, C., Madera-Santana, T. J., Rodríguez-Beas, C., & Gámez-Corrales, R. (2025). Study of the Influence of Chitosan-Wrapped Carbon Nanotubes on Biopolymer Film Properties. Polymers, 17(7), 889. https://doi.org/10.3390/polym17070889








