Development and Comparative Analysis of Hard and Soft Wheat Flour Films Enriched with Yellow and White Chlorella vulgaris Algae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing of Soft Wheat and Durum Wheat Flour Films
2.3. Characterization of the Films
2.3.1. Thickness
2.3.2. Gloss
2.3.3. Color
2.3.4. Film Opacity and Internal Transmittance
2.3.5. Moisture Content and Water Solubility
2.3.6. Water Absorption Capacity
2.3.7. Contact Angle in Water and Oil
2.3.8. Water Vapor Permeability (WVP)
2.3.9. Mechanical Properties
2.3.10. Microstructure
2.3.11. Statistical Analysis
3. Results and Discussion
3.1. Film Characterization
3.1.1. Optical and Color Properties
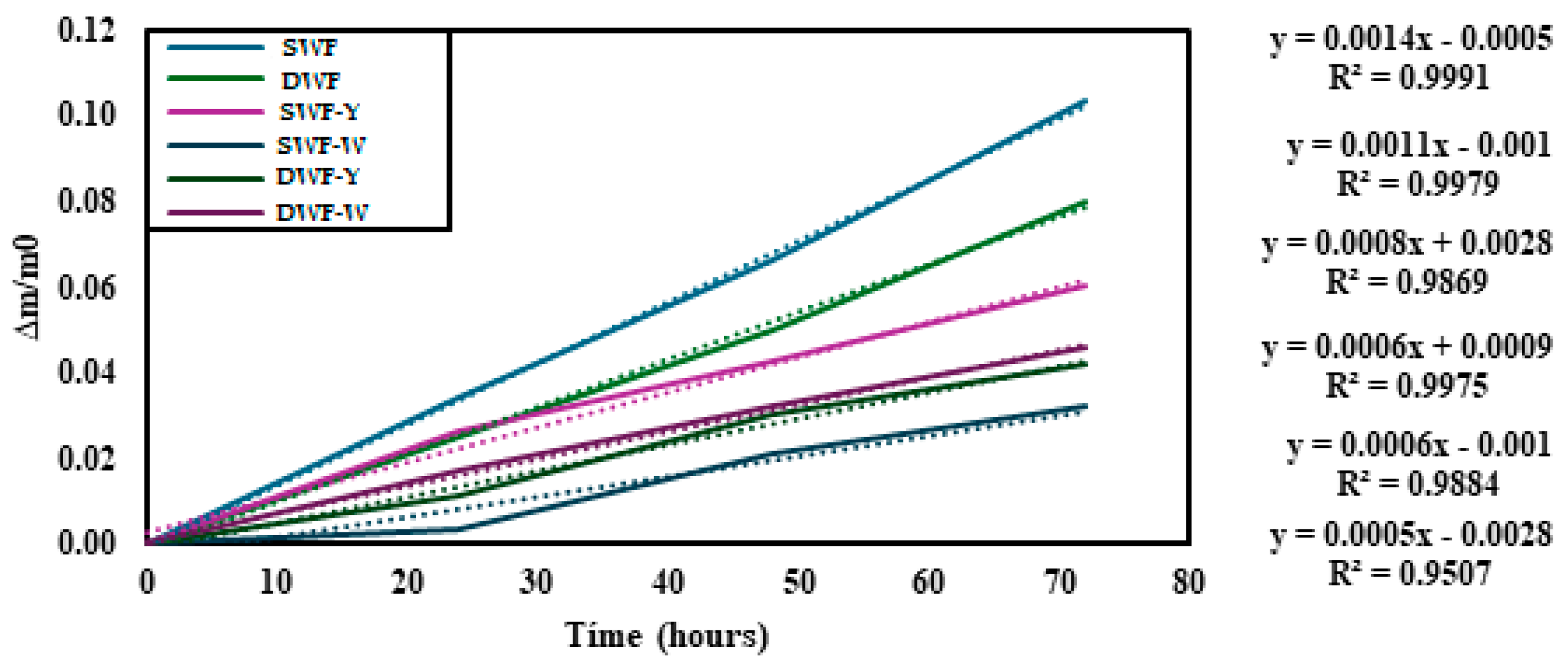
3.1.2. Physical and Water Absorption Properties
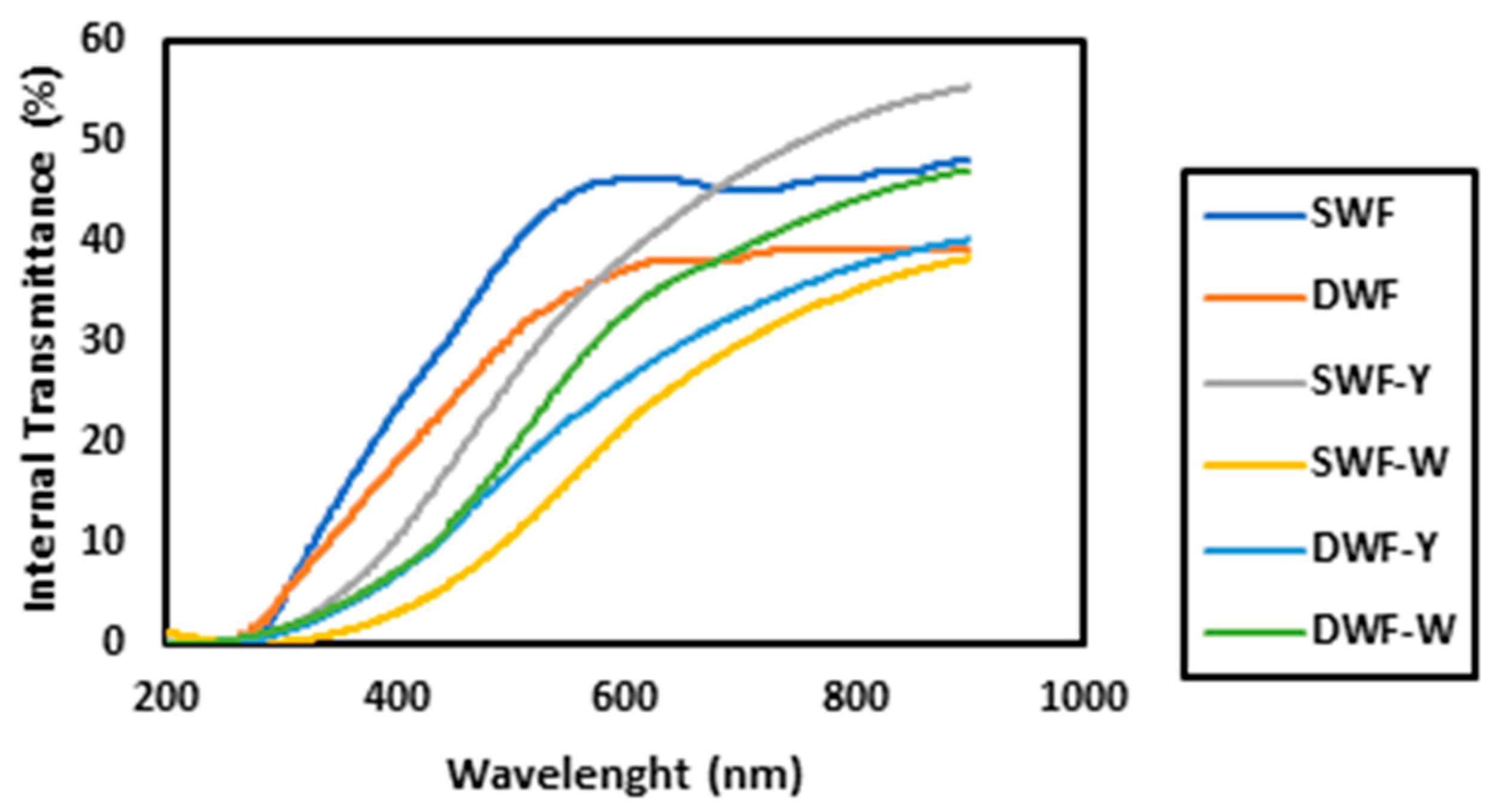
3.1.3. Internal Transmittance
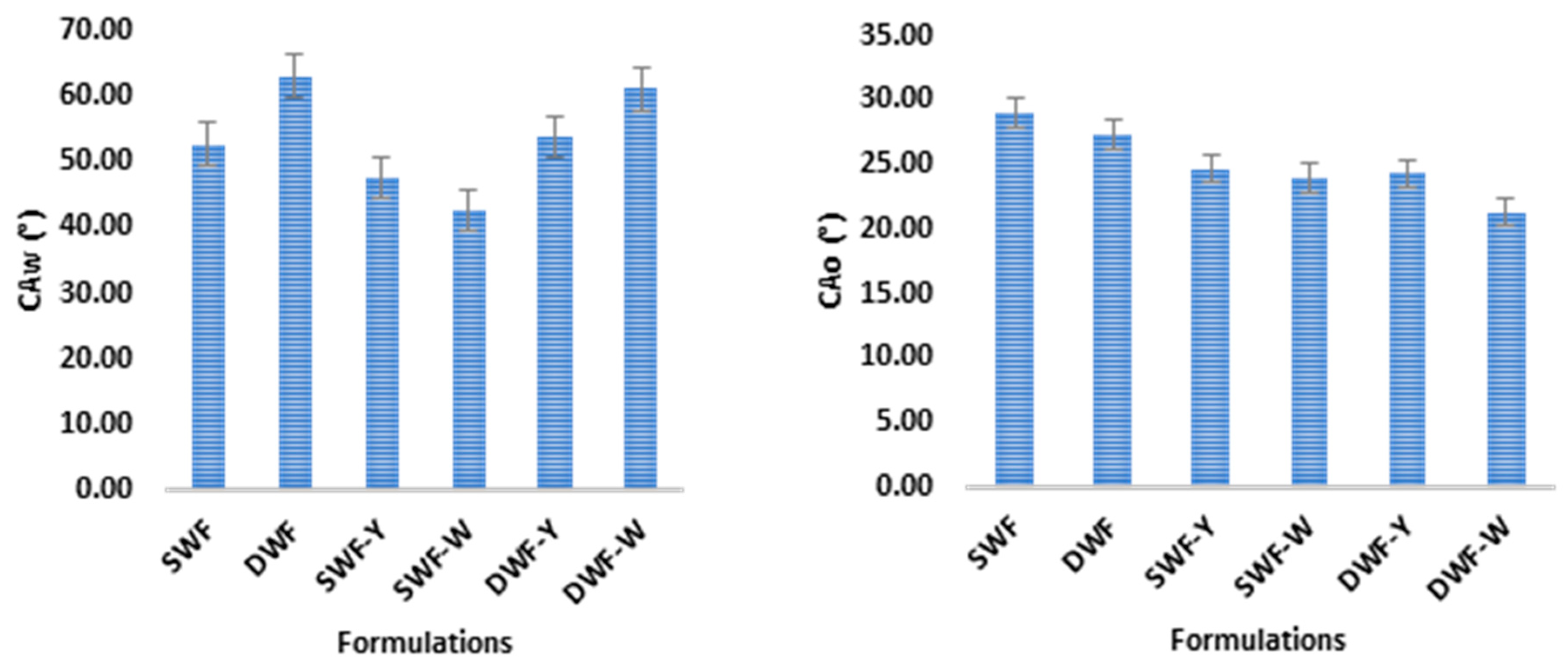
3.1.4. Contact Angle
3.1.5. Mechanical Properties
3.1.6. Microstructure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable biopolymers for active packaging: Demand, development and directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Xu, X.; Sun, Q.; Li, M.; Wang, Y.; Xie, F. Wheat flour-based edible films: Effect of gluten on the rheological properties, structure, and film characteristics. Int. J. Mol. Sci. 2022, 23, 11668. [Google Scholar] [CrossRef]
- Balan, G.C.; Paulo AF, S.; Correa, L.G.; Alvim, I.D.; Ueno, C.T.; Coelho, A.R.; Ströher, G.R.; Yamashita, F.; Sakanaka, L.S.; Shirai, M.A. Production of wheat flour/PBAT active films incorporated with oregano oil microparticles and its application in fresh pastry conservation. Food Bioprocess Technol. 2021, 14, 1587–1599. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Tan, X.; Khoo, K.S.; Ng, H.S.; Jonglertjunya, W.; Yew, G.Y.; Show, P.L. Microalgae-based bioplastics: Future solution towards mitigation of plastic wastes. Environ. Res. 2022, 206, 112620. [Google Scholar] [CrossRef]
- Moreira, A.S.; Gonçalves, J.; Sousa, F.; Maia, I.; Pereira, H.; Silva, J.; Coimbra, M.A.; Ferreira, P.; Nunes, C. Potential of Coccolithophore microalgae as fillers in starch-based films for active and sustainable food packaging. Foods 2023, 12, 513. [Google Scholar] [CrossRef]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of microalgae for biomaterial production and environmental applications at biorefineries. Sustainability 2021, 13, 3063. [Google Scholar] [CrossRef]
- Mari, A.; Fafalis, C.; Krokida, M. Evaluation of Edible Coatings from Components from Chlorella vulgaris and Comparison with Conventional Coatings. Coatings 2024, 14, 621. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—Novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef]
- Coêlho DD, F.; Tundisi, L.L.; Cerqueira, K.S.; Rodrigues JR, D.S.; Mazzola, P.G.; Tambourgi, E.B.; Souza RR, D. Microalgae: Cultivation aspects and bioactive compounds. Braz. Arch. Biol. Technol. 2019, 62, e19180343. [Google Scholar] [CrossRef]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive hydrolysates from chlorella vulgaris: Optimal process and bioactive properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef] [PubMed]
- Al-Hammadi, M.; Güngörmüşler, M. New insights into Chlorella vulgaris applications. Biotechnol. Bioeng. 2024, 121, 1486–1502. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Hyrslova, I.; Krausova, G.; Smolova, J.; Stankova, B.; Branyik, T.; Malinska, H.; Huttl, M.; Kana, A.; Curda, L.; Doskocil, I. Functional properties of chlorella vulgaris, colostrum, and bifidobacteria, and their potential for application in functional foods. Appl. Sci. 2021, 11, 5264. [Google Scholar] [CrossRef]
- Saiah, R.; Sreekumar, P.; Leblanc, N.; Saiter, J. Structure and thermal stability of thermoplastic films based on wheat flour modified by monoglyceride. Ind. Crops Prod. 2009, 29, 241–247. [Google Scholar] [CrossRef]
- ASTM D523-89; Standard Test Method for Specular Gloss. ASTM International: West Conshohocken, PA, USA, 1999; pp. 523–589.
- Arrieta, C.N.; Acevedo-Puello, V.; Ordoñez EG, F.; Fonseca-Florido, H.; Ortega-Toro, R. Biodegradable monolayer film based on the collagen extracted from Oreochromis sp. processing byproducts blended with chitosan and assembled with PCL and PLA monolayers to form bilayers films. J. Food Process Eng. 2024, 47, 7. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Hasan, Z.; Omran AA, B.; Elfaghi, A.M.; Ali, Y.H.; Akeel, N.A.; Ilyas, R.; Sapuan, S. Effect of sugar palm fibers on the properties of blended wheat starch/polyvinyl alcohol (PVA) -based biocomposite films. J. Mater. Res. Technol. 2023, 24, 1043–1055. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; León, A. Antimicrobial, mechanical and barrier properties of triticale protein films incorporated with oregano essential oil. Food Biosci. 2013, 1, 2–9. [Google Scholar] [CrossRef]
- Pirsa, S. Nanocomposite base on carboxymethylcellulose hydrogel: Simultaneous absorbent of ethylene and humidity to increase the shelf life of banana fruit. Int. J. Biol. Macromol. 2021, 193, 300–310. [Google Scholar] [CrossRef]
- Acevedo-Puello, V.; Figueroa-López, K.J.; Ortega-Toro, R. Gelatin-Based Hydrogels Containing Microcrystalline and Nanocrystalline Cellulose as Moisture Absorbers for Food Packaging Applications. J. Compos. Sci. 2023, 7, 337. [Google Scholar] [CrossRef]
- Rezaei, M.; Pirsa, S.; Chavoshizadeh, S. Photocatalytic/antimicrobial active film based on wheat gluten/ZnO nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2654–2665. [Google Scholar] [CrossRef]
- Mat Yasin, N.H.; Othman, N.A.; Adam, F. Evaluation of the properties on carrageenan bio-films with Chlorella vulgaris blending. Chem. Eng. Commun. 2024, 211, 400–414. [Google Scholar] [CrossRef]
- Muñoz-Suarez, A.; Cortes-Rodriguez, M.; Ortega-Toro, R. Biodegradable films based on tilapia collagen (Oreochromis sp.): Improvement of properties with PLA and PCL bilayers with potential use in sustainable food packaging. Rev. Mex. Ing. Quím 2024, 23, Alim24326. [Google Scholar] [CrossRef]
- Madadi, R.; Maljaee, H.; Serafim, L.S.; Ventura, S.P. Microalgae as contributors to produce biopolymers. Mar. Drugs 2021, 19, 466. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liu, C. Film transparency and opacity measurements. Food Anal. Methods 2022, 15, 2840–2846. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- Lim, R.; Kiew, P.L.; Lam, M.K.; Yeoh, W.M.; Ho, M.Y. Corn starch/PVA bioplastics—The properties and biodegradability study using Chlorella vulgaris cultivation. Asia-Pac. J. Chem. 2021, 16, e2622. [Google Scholar] [CrossRef]
- Carissimi, M.; Flôres, S.H.; Rech, R. Effect of microalgae addition on active biodegradable starch film. Algal Res. 2018, 32, 201–209. [Google Scholar] [CrossRef]
- Wan, S.; Liu, Q.; Yang, D.; Guo, P.; Gao, Y.; Mo, R.; Zhang, Y. Characterization of high amylose corn starch-cinnamaldehyde inclusion films for food packaging. Food Chem. 2023, 403, 134219. [Google Scholar] [CrossRef]
- Zhou, Y.; Dhital, S.; Zhao, C.; Ye, F.; Chen, J.; Zhao, G. Dietary fiber-gluten protein interaction in wheat flour dough: Analysis, consequences and proposed mechanisms. Food Hydrocoll. 2021, 111, 106203. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Qualitative composition. Cereal Chem. 2023, 10, 23–35. [Google Scholar] [CrossRef]
- Turksoy, S.; Erturk, M.Y.; Kokini, J. Behavior of semolina, hard, soft wheat flour dough at different aging times and temperatures through LAOS properties and molecular interactions of proteins. J. Food Eng. 2021, 301, 110549. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Aloui, H.; Khomlaem, C.; Negi, A.; Yun, J.H.; Kim, H.S.; Kim, B.S. Biodegradable films based on chitosan and defatted Chlorella biomass: Functional and physical characterization. Food Chem. 2021, 337, 127777. [Google Scholar] [CrossRef] [PubMed]
- Bof, M.J.; Locaso, D.E.; García, M.A. Corn starch-chitosan proportion affects biodegradable film performance for food packaging purposes. Starch-Stärke 2021, 73, 2000104. [Google Scholar] [CrossRef]
- Ghizdareanu, A.I.; Banu, A.; Pasarin, D.; Ionita, A.; Nicolae, C.A.; Gabor, A.R.; Pătroi, D. Enhancing the mechanical properties of corn starch films for sustainable food packaging by optimizing enzymatic hydrolysis. Polymers. 2023, 15, 1899. [Google Scholar] [CrossRef]
- Wieser, H.; Seilmeier, W. The influence of nitrogen fertilisation on quantities and proportions of different protein types in wheat flour. J. Sci. Food Agric. 1998, 76, 49–55. [Google Scholar] [CrossRef]
- Fonseca-García, A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Preparation of a novel biodegradable packaging film based on corn starch-chitosan and poloxamers. Carbohydr. Polym. 2021, 251, 117009. [Google Scholar] [CrossRef]
- Peron-Schlosser, B.; Carpiné, D.; Matos Jorge, R.M.; Rigon Spier, M. Optimization of wheat flour by product films: A technological and sustainable approach for bio-based packaging material. J. Food Sci. 2021, 86, 4522–4538. [Google Scholar] [CrossRef]
- Drakos, A.; Pelava, E.; Evageliou, V. Properties of flour films as affected by the flour’s source and particle size. Food Res. Int. 2018, 107, 551–558. [Google Scholar] [CrossRef]




| Formulations | Soft Wheat Flour | Durum Wheat Flour | Yellow Chlorella vulgaris | White Chlorella vulgaris |
|---|---|---|---|---|
| SWF | 1.0 | 0.0 | 0.0 | 0.0 |
| DWF | 0.0 | 1.0 | 0.0 | 0.0 |
| SWF-Y | 1.0 | 0.0 | 0.25 | 0.0 |
| SWF-W | 1.0 | 0.0 | 0.0 | 0.25 |
| DWF-Y | 0.0 | 1.0 | 0.25 | 0.0 |
| DWF-W | 0.0 | 1.0 | 0.0 | 0.25 |
| Formulations | Gloss at 60° | Color Parameters | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C | h | |||
| SWF | 9.3 ± 1.6 a | 31.1 ± 0.3 a | −5.6 ± 0.1 b | 3.3 ± 0.1 f | 6.2 ± 0.3 f | 149.8 ± 0.6 a | - |
| DWF | 8.6 ± 0.9 a | 35.3 ± 1.2 b | −3.6 ± 0.4 c | 8.4 ± 0.2 e | 24.1 ± 0.01 e | 78.7 ± 1.3 b | - |
| SWF-Y | 12.8 ± 0.5 ab | 54.1 ± 0.5 bc | −4.0 ± 0.2 b | 16.7 ± 0.5 d | 17.1 ± 0.4 d | 95.0 ± 0.7 c | 9.9 ± 0.5 d |
| SWF-W | 11.6 ± 1.7 b | 53.0 a ± 0.9 cd | −3.7 ± 0.2 b | 40.3 ± 0.5 b | 40.5 ± 0.5 b | 95.3 ± 0.3 c | 33.3 ± 1.1 b |
| DWF-Y | 7.4 ± 0.9 c | 50.5 a ± 0.4 e | −2.5 ± 0.6 a | 22.7 ± 0.8 c | 22.9 ± 0.7 c | 96.3 ± 1.6 c | 16.5 ± 0.6 c |
| DWF-W | 7.3 ± 0.9 c | 52.6 ± 0.6 d | −3.5 ± 0.2 c | 42.8 ± 1.0 a | 42.9 ± 1.0 a | 94.7 ± 0.1 c | 35.6 ± 1.7 d |
| Formulations | Thickness (μm) | WVP | Sw | Wca | Xw |
|---|---|---|---|---|---|
| SWF | 177.8 ± 0.02 c | 4.14 ± 0.02 a | 0.034 ± 0.08 b | 0.37 ± 0.03 a | 0.147 ± 0.22 a |
| DWF | 183.5 ± 0.02 b | 4.20 ± 0.06 a | 0.015 ± 0.15 c | 0.40 ± 0.03 a | 0.130 ± 0.42 a |
| SWF-Y | 199.4 ± 0.04 e | 0.04 ± 0.01 c | 0.028 ± 0.17 b | 0.41 ± 0.08 a | 0.223 ± 1.11 b |
| SWF-W | 297.7 ± 0.05 cd | 0.05 ± 0.02 bc | 0.027 ± 0.25 b | 0.40 ± 0.02 a | 0.215 ± 0.46 b |
| DWF-Y | 404.7 ± 0.02 a | 0.04 ± 0.01 bc | 0.081 ± 0.31 a | 0.36 ± 0.07 a | 0.272 ± 1.15 c |
| DWF-W | 273.0 ± 0.02 d | 0.11 ± 0.07 b | 0.029 ± 0.17 b | 0.39 ± 0.02 a | 0.220 ± 0.84 b |
| Formulations | TS (Mpa) | E(%) | EM (Mpa) |
|---|---|---|---|
| SWF | 7.6 ± 0.2 d | 140 ± 2 a | 27.2 ± 0.6 e |
| DWF | 8.8 ± 0.3 c | 121 ± 2 b | 31.7 ± 0.5 |
| SWF-Y | 9.1 ± 0.4 bc | 115 ± 3 c | 40.2 ± 0.5 |
| SWF-W | 9.5 ± 0.2 b | 100 ± 3 d | 45.0 ± 0.4 |
| DWF-Y | 10.7 ± 0.2 a | 105 ± 2 d | 45.5 ± 0.4 |
| DWF-W | 11.2 ± 0.3 a | 90 ± 2 e | 50.2 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Padilla, A.; Cortés-Rodríguez, M.; Ortega-Toro, R. Development and Comparative Analysis of Hard and Soft Wheat Flour Films Enriched with Yellow and White Chlorella vulgaris Algae. Polymers 2025, 17, 785. https://doi.org/10.3390/polym17060785
López-Padilla A, Cortés-Rodríguez M, Ortega-Toro R. Development and Comparative Analysis of Hard and Soft Wheat Flour Films Enriched with Yellow and White Chlorella vulgaris Algae. Polymers. 2025; 17(6):785. https://doi.org/10.3390/polym17060785
Chicago/Turabian StyleLópez-Padilla, Alexis, Misael Cortés-Rodríguez, and Rodrigo Ortega-Toro. 2025. "Development and Comparative Analysis of Hard and Soft Wheat Flour Films Enriched with Yellow and White Chlorella vulgaris Algae" Polymers 17, no. 6: 785. https://doi.org/10.3390/polym17060785
APA StyleLópez-Padilla, A., Cortés-Rodríguez, M., & Ortega-Toro, R. (2025). Development and Comparative Analysis of Hard and Soft Wheat Flour Films Enriched with Yellow and White Chlorella vulgaris Algae. Polymers, 17(6), 785. https://doi.org/10.3390/polym17060785






