Development of Hydrogels Fabricated via Stereolithography for Bioengineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
| Material | Chemical Structure | Characteristics |
|---|---|---|
| Gelatin Methacrylate (GelMA) |  | Adhesive to cells [54,55], biodegradable [54,55] |
| Polyethylene Glycol Diacrylate (PEGDA) |  | Stable after printing [56,57], excellent printing fidelity [56,57] |
| Poly(Glycerol Sebacatemethacrylate) (PGS) |  | Native tissue-like Young’s modulus [58], highly flexible [58], stable under PBS [58], degradable by lipase [58] |
| Acrylamide (AAm) |  | High water absorption [59], stability under stress [59,60] |
| 2-Hydroxyethyl Methacrylate (HEMA) |  | Low immunogenicity [61], similar Young’s modulus to cartilage [46,61,62] |
| Acrylic Acid (AA) |  | Anionic [59], pH-sensitive [59], ion-exchange capability [59], high water absorption [59] |
| Hyaluronic Acid-Norbornene (HA-NB) | 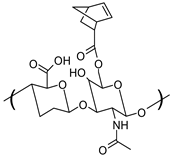 | Bioadhesive [63], promotes cell adhesion and proliferation [63], Thiol-ene reaction [63,64] |
| Pluronic F127 Diacrylate (F127DA) |  | Maintaining long-term structural stability in a PBS environment [58], high stretchability [64], cytocompatibility [64,65] |
| Photo-Initiator | Wavelength of Absorbable Light | Chemical Structure | Ref. |
|---|---|---|---|
| Lithium phenyl (2,4,6-trimethylbenzoyl) phosphinate (LAP) | 375 nm |  | [66,67] |
| 2-Hydroxy−4′-(2-hydroxyethoxy)−2-methylpropiophenone (Irgacure 2959) | 257 nm |  | [68,69] |
| 2′,4′,5′,7′-Tetrabromofluorescein disodium salt (Eosin y) | 514 nm |  | [70] |
| Tris(2,2′-bipyridyl)dichlororuthenium(II) hexahydrate/sodium persulfate(Ru/SPS) | 405 nm |  | [71,72] |
| Water-soluble 2,4,6-trimethylbenzoyl-diphenylphosphine oxide (TPO) nanoparticle | 365 nm |  | [73,74,75] |
| Phenyl bis(2,4,6-trimethylbenzoyl)phosphine oxide (Irgacure 819) | 405 nm |  | [76,77] |
| 2-Hydroxy-2-methylpropiophenone (HMPP) | 365 nm |  | [58,78] |
| Photo-Absorber | Wavelength of Absorbable Light | Chemical Structure | Ref. |
|---|---|---|---|
| Tartrazine (acid yellow 23) | 425 |  | [20,69,79,80,81] |
| Sudan 1 | 481 |  | [74,82] |
| Quinoline yellow | 415 |  | [63,83] |
| Orange G | 480 |  | [84,85] |
| Phenol red | 422,559 |  | [63] |
2.2. Methods
| SLA Type | Characteristics | Applications |
|---|---|---|
| Laser-based SLA | Very high 3D printing resolution, slow 3D printing processes, point polymerization. | soft robotics [88], neural stem cell proliferation [89] |
| DLP-based SLA | Faster 3D printing processes, 3D printing resolution can be lower than laser-based SLA, whole layer polymerization | sciatic nerve regeneration [90] osteosarcoma cell proliferation [91], cartilage tissue engineering [92] |
3. Applications
3.1. Nerve Regeneration with Hydrogels Fabricated via SLA
3.2. Bone Regeneration with Hydrogels Fabricated via SLA
3.3. Skin and Cornea Regeneration with Hydrogels Fabricated via SLA
3.4. Lab-on-a-Chip Devices Developed with Hydrogels Fabricated via SLA
3.5. Native Tissue-like Models Developed with Hydrogels Fabricated via SLA
4. Discussion and Future Application
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Singelyn, J.M.; Sundaramurthy, P.; Johnson, T.D.; Schup-Magoffin, P.J.; Hu, D.P.; Faulk, D.M.; Wang, J.; Mayle, K.M.; Bartels, K.; Salvatore, M.; et al. Catheter-Deliverable Hydrogel Derived From Decellularized Ventricular Extracellular Matrix Increases Endogenous Cardiomyocytes and Preserves Cardiac Function Post-Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld Skin Printer: In Situ Formation of Planar Biomaterials and Tissues. Lab Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Tevlek, A.; Çetin, E.A. Smart Hydrogels in Lab-on-a-Chip (LOC) Applications. React. Funct. Polym. 2024, 204, 106023. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Ahmadi, M. Degradable Hydrogels: Design Mechanisms and Versatile Applications. Mater. Today Sustain. 2023, 23, 100468. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. In Vitro Degradation, Swelling, and Bioactivity Performances of in Situ Forming Injectable Chitosan-Matrixed Hydrogels for Bone Regeneration and Drug Delivery. Biotechnol. Bioeng. 2024, 121, 2767–2779. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Rausch, M.K.; Peppas, N.A. Cross-Evaluation of Stiffness Measurement Methods for Hydrogels. Polymer 2022, 258, 125316. [Google Scholar] [CrossRef]
- Gandin, A.; Murugesan, Y.; Torresan, V.; Ulliana, L.; Citron, A.; Contessotto, P.; Battilana, G.; Panciera, T.; Ventre, M.; Netti, A.P.; et al. Simple yet Effective Methods to Probe Hydrogel Stiffness for Mechanobiology. Sci. Rep. 2021, 11, 22668. [Google Scholar] [CrossRef]
- Lee, J.; Song, B.; Subbiah, R.; Chung, J.J.; Choi, U.H.; Park, K.; Kim, S.H.; Oh, S.J. Effect of Chain Flexibility on Cell Adhesion: Semi-Flexible Model-Based Analysis of Cell Adhesion to Hydrogels. Sci. Rep. 2019, 9, 2463. [Google Scholar] [CrossRef]
- Xia, J.; Liu, Z.Y.; Han, Z.Y.; Yuan, Y.; Shao, Y.; Feng, X.Q.; Weitz, D.A. Regulation of Cell Attachment, Spreading, and Migration by Hydrogel Substrates with Independently Tunable Mesh Size. Acta Biomater. 2022, 141, 178. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Sui, B.; Hua, Y.; Zhang, Y.; Bao, B.; Lin, Q.; Liu, X.; Zhu, L.; Sun, J. A Well Defect-Suitable and High-Strength Biomimetic Squid Type II Gelatin Hydrogel Promoted in Situ Costal Cartilage Regeneration via Dynamic Immunomodulation and Direct Induction Manners. Biomaterials 2020, 240, 119841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zou, B.; Wang, X.; Zhou, X.; Yang, G.; Lai, Q.; Zhao, Y. SLA-3d Printed Building and Characteristics of GelMA/HAP Biomaterials with Gradient Porous Structure. J. Mech. Behav. Biomed. Mater. 2024, 155, 106553. [Google Scholar] [CrossRef]
- He, X.C.; Chen, X.N.; Liu, Y.H.; Zhong, X.; Qiang, L.; Wang, H.Q.; Wang, F.Z.; Wang, J.S.; Li, C.H.; Zheng, P.F. A Blue Light 3D Printable Hydrogel with Water Absorption, Antibacterial, and Hemostatic Properties for Skin Wound Healing. Chem. Eng. J. 2024, 493, 152439. [Google Scholar] [CrossRef]
- Song, K.H.; Highley, C.B.; Rouff, A.; Burdick, J.A. Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv. Funct. Mater. 2018, 28, 1801331. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Zhu, J.; Liu, F.; Xue, Y.; Huang, Y.; Zhu, B.; Wu, D.; Pan, H.; Gong, T.; et al. Hyaluronic Acid Methacrylate/Pancreatic Extracellular Matrix as a Potential 3D Printing Bioink for Constructing Islet Organoids. Acta Biomater. 2023, 165, 86–101. [Google Scholar] [CrossRef]
- Mohammad Mehdipour, N.; Rajeev, A.; Kumar, H.; Kim, K.; Shor, R.J.; Natale, G. Anisotropic Hydrogel Scaffold by Flow-Induced Stereolithography 3D Printing Technique. Biomater. Adv. 2024, 161, 213885. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Hao, J.; Gonzales, A.; Zhao, Z.; Flores, R.S.; Kuang, X.; Mu, X.; Ching, T.; Tang, G.; et al. Molecularly Cleavable Bioinks Facilitate High-Performance Digital Light Processing-Based Bioprinting of Functional Volumetric Soft Tissues. Nat. Commun. 2022, 13, 3317. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, Z.; Zeng, H.; Liu, D.; Ji, Z.; Xu, X.; Jia, X.; Jiang, P.; Fan, Z.; Wang, X.; et al. Biomechanically Compatible Hydrogel Bioprosthetic Valves. Chem. Mater. 2022, 34, 6129–6141. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, P.; Wang, Y.; Lu, Y.; Wu, J.; Xu, X.; Ji, Z.; Sun, C.; Wang, X.; Liu, W. Engineering Tridimensional Hydrogel Tissue and Organ Phantoms with Tunable Springiness. Adv. Funct. Mater. 2023, 33, 2214885. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Chapin, S.C.; Doyle, P.S. Hydrogel Microparticles from Lithographic Processes: Novel Materials for Fundamental and Applied Colloid Science. Curr. Opin. Colloid. Interface Sci. 2011, 16, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Emerson, A.E.; Mccall, A.B.; Brady, S.R.; Slaby, E.M.; Weaver, J.D. Hydrogel Injection Molding to Generate Complex Cell Encapsulation Geometries. ACS Biomater. Sci. Eng. 2022, 8, 4002–4013. [Google Scholar] [CrossRef]
- Bian, W.; Liau, B.; Badie, N.; Bursac, N. Mesoscopic Hydrogel Molding to Control the 3D Geometry of Bioartificial Muscle Tissues. Nat. Protoc. 2009, 4, 1522. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Song, K.H. Fibrous Hydrogels by Electrospinning: Novel Platforms for Biomedical Applications. J. Tissue Eng. 2023, 14, 20417314231191881. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Ortega Arevalo, M.d.P.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-Based 3D Printing of Photo-Crosslinkable Gelatin and κ-Carrageenan Hydrogel Blends for Adipose Tissue Regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef]
- Cantoni, F.; Barbe, L.; Pohlit, H.; Tenje, M. A Perfusable Multi-Hydrogel Vasculature On-Chip Engineered by 2-Photon 3D Printing and Scaffold Molding to Improve Microfabrication Fidelity in Hydrogels. Adv. Mater. Technol. 2024, 9, 2300718. [Google Scholar] [CrossRef]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D Printing of a Bladder Device for Intravesical Drug Delivery. Mater. Sci. Eng. C 2021, 120, 111773. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.C.; Ong, J.J.; Alfassam, H.; Díaz-Torres, E.; Goyanes, A.; Williams, G.R.; Basit, A.W. Fabrication of 3D Printed Mutable Drug Delivery Devices: A Comparative Study of Volumetric and Digital Light Processing Printing. Drug Deliv. Transl. Res. 2024. [Google Scholar] [CrossRef]
- Budharaju, H.; Sundaramurthi, D.; Sethuraman, S. Embedded 3D Bioprinting—An Emerging Strategy to Fabricate Biomimetic & Large Vascularized Tissue Constructs. Bioact. Mater. 2024, 32, 356–384. [Google Scholar]
- Gusmão, A.; Sanjuan-Alberte, P.; Ferreira, F.C.; Leite, M. Design, Fabrication, and Testing of a Low-Cost Extrusion Based 3D Bioprinter for Thermo-Sensitive and Light Sensitive Hydrogels. Mater. Today Proc. 2022, 70, 148–154. [Google Scholar] [CrossRef]
- Beh, C.W.; Yew, D.S.; Chai, R.J.; Chin, S.Y.; Seow, Y.; Hoon, S.S. A Fluid-Supported 3D Hydrogel Bioprinting Method. Biomaterials 2021, 276, 121034. [Google Scholar] [CrossRef]
- Zandrini, T.; Liaros, N.; Jiang, L.J.; Lu, Y.F.; Fourkas, J.T.; Osellame, R.; Baldacchini, T. Effect of the Resin Viscosity on the Writing Properties of Two-Photon Polymerization. Opt. Mater. Express 2019, 9, 2601. [Google Scholar] [CrossRef]
- Jing, X.; Fu, H.; Yu, B.; Sun, M.; Wang, L. Two-Photon Polymerization for 3D Biomedical Scaffolds: Overview and Updates. Front. Bioeng. Biotechnol. 2022, 10, 994355. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, S.; Pandit, A.; Heise, A.; Kellett, A. Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies. Adv. Sci. 2023, 10, 2204072. [Google Scholar] [CrossRef]
- Whyte, D.J.; Doeven, E.H.; Sutti, A.; Kouzani, A.Z.; Adams, S.D. Volumetric Additive Manufacturing: A New Frontier in Layer-Less 3D Printing. Addit. Manuf. 2024, 84, 104094. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Stereolithography Apparatus and Digital Light Processing-Based 3D Bioprinting for Tissue Fabrication. iScience 2023, 26, 106039. [Google Scholar] [CrossRef]
- Anandakrishnan, N.; Ye, H.; Guo, Z.; Chen, Z.; Mentkowski, K.I.; Lang, J.K.; Rajabian, N.; Andreadis, S.T.; Ma, Z.; Spernyak, J.A.; et al. Fast Stereolithography Printing of Large-Scale Biocompatible Hydrogel Models. Adv. Healthc. Mater. 2021, 10, e2002103. [Google Scholar] [CrossRef] [PubMed]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Loterie, D.; Delrot, P.; Moser, C. High-Resolution Tomographic Volumetric Additive Manufacturing. Nat. Commun. 2020, 11, 852. [Google Scholar] [CrossRef]
- Riffe, M.B.; Davidson, M.D.; Seymour, G.; Dhand, A.P.; Cooke, M.E.; Zlotnick, H.M.; McLeod, R.R.; Burdick, J.A. Multi-Material Volumetric Additive Manufacturing of Hydrogels Using Gelatin as a Sacrificial Network and 3D Suspension Bath. Adv. Mater. 2024, 36, 2309026. [Google Scholar] [CrossRef]
- Chen, Q.; Zou, B.; Lai, Q.; Zhu, K. SLA-3d Printing and Compressive Strength of PEGDA/NHAP Biomaterials. Ceram. Int. 2022, 48, 30917–30926. [Google Scholar] [CrossRef]
- Zips, S.; Hiendlmeier, L.; Weiß, L.J.K.; Url, H.; Teshima, T.F.; Schmid, R.; Eblenkamp, M.; Mela, P.; Wolfrum, B. Biocompatible, Flexible, and Oxygen-Permeable Silicone-Hydrogel Material for Stereolithographic Printing of Microfluidic Lab-On-A-Chip and Cell-Culture Devices. ACS Appl. Polym. Mater. 2021, 3, 243–258. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated Polymerization of PEG-Diacrylate with Lithium Phenyl-2,4,6-Trimethylbenzoylphosphinate: Polymerization Rate and Cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef]
- Magalhães, L.S.S.M.; Santos, F.E.P.; de Maria Vaz Elias, C.; Afewerki, S.; Sousa, G.F.; Furtado, A.S.A.; Marciano, F.R.; Lobo, A.O. Printing 3D Hydrogel Structures Employing Low-Cost Stereolithography Technology. J. Funct. Biomater. 2020, 11, 12. [Google Scholar] [CrossRef]
- Chan, V.; Jeong, J.H.; Bajaj, P.; Collens, M.; Saif, T.; Kong, H.; Bashir, R. Multi-Material Bio-Fabrication of Hydrogel Cantilevers and Actuators with Stereolithography. Lab Chip 2011, 12, 88–98. [Google Scholar] [CrossRef]
- Warner, J.; Soman, P.; Zhu, W.; Tom, M.; Chen, S. Design and 3D Printing of Hydrogel Scaffolds with Fractal Geometries. ACS Biomater. Sci. Eng. 2016, 2, 1763–1770. [Google Scholar] [CrossRef]
- Pariskar, A.; Sharma, P.K.; Murty, U.S.; Banerjee, S. Effect of Tartrazine as Photoabsorber for Improved Printing Resolution of 3D Printed “Ghost Tablets”: Non-Erodible Inert Matrices. J. Pharm. Sci. 2023, 112, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.T.; Moon, Y.W.; Park, J.; Atala, A.; Yoo, J.J.; Lee, S.J. Combinations of Photoinitiator and UV Absorber for Cell-Based Digital Light Processing (DLP) Bioprinting. Biofabrication 2021, 13, 034103. [Google Scholar] [CrossRef]
- Liz-Basteiro, P.; Reviriego, F.; Martínez-Campos, E.; Reinecke, H.; Elvira, C.; Rodríguez-Hernández, J.; Gallardo, A. Vat Photopolymerization 3D Printing of Hydrogels with Re-Adjustable Swelling. Gels 2023, 9, 600. [Google Scholar] [CrossRef]
- Ghosh, R.N.; Thomas, J.; Vaidehi, B.R.; Devi, N.G.; Janardanan, A.; Namboothiri, P.K.; Peter, M. An Insight into Synthesis, Properties and Applications of Gelatin Methacryloyl Hydrogel for 3D Bioprinting. Mater. Adv. 2023, 4, 5496–5529. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.H.; Williams, C.J.; Micallef, C.; Duarte-Martinez, F.; Afsar, A.; Zhang, R.; Wilson, S.; Dossi, E.; Impey, S.A.; Goel, S.; et al. Nanoindentation Response of 3D Printed PEGDA Hydrogels in a Hydrated Environment. ACS Appl. Polym. Mater. 2023, 5, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Valentin, T.M.; Dubois, E.M.; Machnicki, C.E.; Bhaskar, D.; Cui, F.R.; Wong, I.Y. 3D Printed Self-Adhesive PEGDA–PAA Hydrogels as Modular Components for Soft Actuators and Microfluidics. Polym. Chem. 2019, 10, 2015–2028. [Google Scholar] [CrossRef]
- Singh, D.; Harding, A.J.; Albadawi, E.; Boissonade, F.M.; Haycock, J.W.; Claeyssens, F. Additive Manufactured Biodegradable Poly(Glycerol Sebacate Methacrylate) Nerve Guidance Conduits. Acta Biomater. 2018, 78, 48–63. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Tao, J.; Zhu, S.; Liao, X.; Wang, Y.; Zhou, N.; Li, Z.; Wan, H.; Tang, Y.; Yang, S.; Du, T.; et al. DLP-Based Bioprinting of Void-Forming Hydrogels for Enhanced Stem-Cell-Mediated Bone Regeneration. Mater. Today Bio 2022, 17, 100487. [Google Scholar] [CrossRef]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. PHEMA: An Overview for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef]
- Boazak, E.M.; Greene, V.K.; Auguste, D.T. The Effect of Heterobifunctional Crosslinkers on HEMA Hydrogel Modulus and Toughness. PLoS ONE 2019, 14, e0215895. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z.; et al. Rapid Printing of Bio-Inspired 3D Tissue Constructs for Skin Regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huang, Z.; Jiang, X.; Yang, Y.; Yang, L.; Yang, S.; Niu, C.; Xu, Y.; Feng, L. Facile Synthesize of Norbornene-Hyaluronic Acid to Form Hydrogel via Thiol-Norbornene Reaction for Biomedical Application. Polymer 2022, 245, 124696. [Google Scholar] [CrossRef]
- Gao, J.; Li, M.; Cheng, J.; Liu, X.; Liu, Z.; Liu, J.; Tang, P. 3D-Printed GelMA/PEGDA/F127DA Scaffolds for Bone Regeneration. J. Funct. Biomater. 2023, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Bedell, M.L.; Torres, A.L.; Hogan, K.J.; Wang, Z.; Wang, B.; Melchiorri, A.J.; Grande-Allen, K.J.; Mikos, A.G. Human Gelatin-Based Composite Hydrogels for Osteochondral Tissue Engineering and Their Adaptation into Bioinks for Extrusion, Inkjet, and Digital Light Processing Bioprinting. Biofabrication 2022, 14, 045012. [Google Scholar] [CrossRef]
- Bhusal, A.; Dogan, E.; Nguyen, H.A.; Labutina, O.; Nieto, D.; Khademhosseini, A.; Miri, A.K. Multi-Material Digital Light Processing Bioprinting of Hydrogel-Based Microfluidic Chips. Biofabrication 2021, 14, 014103. [Google Scholar] [CrossRef]
- Paunović, N.; Marbach, J.; Bao, Y.; Berger, V.; Klein, K.; Schleich, S.; Coulter, F.B.; Kleger, N.; Studart, A.R.; Franzen, D.; et al. Digital Light 3D Printed Bioresorbable and NIR-Responsive Devices with Photothermal and Shape-Memory Functions. Adv. Sci. 2022, 9, 2200907. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, F.; Han, D.; Zhang, S.Y.; Dong, Y.; Li, X.; Ling, L.; Deng, Z.; Cao, X.; Tian, J.; et al. 3D Bioprinting of Corneal Decellularized Extracellular Matrix: GelMA Composite Hydrogel for Corneal Stroma Engineering. Int. J. Bioprint 2023, 9, 774. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Tran, K.A.; DeOre, B.J.; Ikejiani, D.; Means, K.; Paone, L.S.; De Marchi, L.; Suprewicz, Ł.; Koziol, K.; Bouyer, J.; Byfield, F.J.; et al. Matching Mechanical Heterogeneity of the Native Spinal Cord Augments Axon Infiltration in 3D-Printed Scaffolds. Biomaterials 2023, 295, 122061. [Google Scholar] [CrossRef] [PubMed]
- Viray, C.M.; Van Magill, B.; Zreiqat, H.; Ramaswamy, Y. Stereolithographic Visible-Light Printing of Poly(l-Glutamic Acid) Hydrogel Scaffolds. ACS Biomater. Sci. Eng. 2022, 8, 1115–1131. [Google Scholar] [CrossRef]
- Wang, R.; Damanik, F.; Kuhnt, T.; Jaminon, A.; Hafeez, S.; Liu, H.; Ippel, H.; Dijkstra, P.J.; Bouvy, N.; Schurgers, L.; et al. Biodegradable Poly(Ester) Urethane Acrylate Resins for Digital Light Processing: From Polymer Synthesis to 3D Printed Tissue Engineering Constructs. Adv. Healthc. Mater. 2023, 12, 2202648. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, Z.; Cheng, J.; Zhang, B.; Zhang, Y.F.; Li, H.; He, X.; Yuan, C.; Liu, J.; Magdassi, S.; et al. 3D Printing of Highly Stretchable Hydrogel with Diverse UV Curable Polymers. Sci. Adv. 2021, 7, eaba4261. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Zhang, J.; He, X.; Liu, F.; Cui, J.; Lu, Z.; Hu, G.; Yang, J.; Zhou, Z.; et al. General One-Pot Method for Preparing Highly Water-Soluble and Biocompatible Photoinitiators for Digital Light Processing-Based 3d Printing of Hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 55507–55516. [Google Scholar] [CrossRef] [PubMed]
- Mochi, F.; De Matteis, F.; Prosposito, P.; Burratti, L.; Francini, R.; Casalboni, M. One Photon 3D Polymerization via Direct Laser Writing; Trans Tech Publications, Ltd.: Bäch, Switzerland, 2018. [Google Scholar]
- Ding, G.; He, Y.; Shi, Y.; Maimaitimin, M.; Zhang, X.; Huang, H.; Huang, W.; Yu, R.; Wang, J. Sustained-Drug-Release, Strong, and Anti-Swelling Water-Lipid Biphasic Hydrogels Prepared via Digital Light Processing 3D Printing for Protection against Osteoarthritis: Demonstration in a Porcine Model. Adv. Healthc. Mater. 2023, 12, 2203236. [Google Scholar] [CrossRef] [PubMed]
- Curti, C.; Kirby, D.J.; Russell, C.A. Systematic Screening of Photopolymer Resins for Stereolithography (SLA) 3D Printing of Solid Oral Dosage Forms: Investigation of Formulation Factors on Printability Outcomes. Int. J. Pharm. 2024, 653, 123862. [Google Scholar] [CrossRef]
- Levato, R.; Lim, K.S.; Li, W.; Asua, A.U.; Peña, L.B.; Wang, M.; Falandt, M.; Bernal, P.N.; Gawlitta, D.; Zhang, Y.S.; et al. High-Resolution Lithographic Biofabrication of Hydrogels with Complex Microchannels from Low-Temperature-Soluble Gelatin Bioresins. Mater. Today Bio 2021, 12, 100162. [Google Scholar] [CrossRef]
- Vera, D.; García-Díaz, M.; Torras, N.; Castillo, Ó.; Illa, X.; Villa, R.; Alvarez, M.; Martinez, E. A 3D Bioprinted Hydrogel Gut-on-Chip with Integrated Electrodes for Transepithelial Electrical Resistance (TEER) Measurements. Biofabrication 2024, 16, 035008. [Google Scholar] [CrossRef]
- Torras, N.; Zabalo, J.; Abril, E.; Carré, A.; García-Díaz, M.; Martínez, E. A Bioprinted 3D Gut Model with Crypt-Villus Structures to Mimic the Intestinal Epithelial-Stromal Microenvironment. Biomater. Adv. 2023, 153, 213534. [Google Scholar] [CrossRef]
- Yue, L.; Macrae Montgomery, S.; Sun, X.; Yu, L.; Song, Y.; Nomura, T.; Tanaka, M.; Jerry Qi, H. Single-Vat Single-Cure Grayscale Digital Light Processing 3D Printing of Materials with Large Property Difference and High Stretchability. Nat. Commun. 2023, 14, 1251. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Han, Y.; Peng Hao, X.; Chao Yu, H.; Yin, J.; Du, M.; Zheng, Q.; Liang Wu, Z.; Dong, M.; Hao, X.P.; et al. Digital Light Processing 3D Printing of Tough Supramolecular Hydrogels with Sophisticated Architectures as Impact-Absorption Elements. Adv. Mater. 2022, 34, 2204333. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, S.; Mau, R.; Kara, S.; Seitz, H.; Kragl, U.; Meyer, J. 3D Printed and Stimulus Responsive Drug Delivery Systems Based on Synthetic Polyelectrolyte Hydrogels Manufactured via Digital Light Processing. J. Mater. Chem. B 2023, 11, 6547–6559. [Google Scholar] [CrossRef] [PubMed]
- Brossier, T.; Benkhaled, B.T.; Colpaert, M.; Volpi, G.; Guillaume, O.; Blanquer, S.; Lapinte, V. Polyoxazoline Hydrogels Fabricated by Stereolithography. Biomater. Sci. 2022, 10, 2681–2691. [Google Scholar] [CrossRef]
- Fei, G.; Parra-Cabrera, C.; Li, Y.; Kravchenko, D.E.; Dochy, R.; Van Looy, L.; Ameloot, R. Stereolithographic 3D Printing of Graded Porous Materials via an Integrated Digital Exposure and Selective Dissolution Strategy. Cell Rep. Phys. Sci. 2023, 4, 101504. [Google Scholar] [CrossRef]
- Champeau, M.; Alves Heinze, D.; Nunes Viana, T.; Rodrigues de Souza, E.; Cristine Chinellato, A.; Titotto, S.; Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; et al. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Odent, J.; Vanderstappen, S.; Toncheva, A.; Pichon, E.; Wallin, T.J.; Wang, K.; Shepherd, R.F.; Dubois, P.; Raquez, J.M. Hierarchical Chemomechanical Encoding of Multi-Responsive Hydrogel Actuators via 3D Printing. J. Mater. Chem. A Mater. 2019, 7, 15395–15403. [Google Scholar] [CrossRef]
- Lee, S.J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D Printing Nano Conductive Multi-Walled Carbon Nanotube Scaffolds for Nerve Regeneration. J. Neural Eng. 2018, 15, 016018. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Huang, Y.; Wu, W.; Deng, X.; Liu, H.; Li, R.; Tao, J.; Li, X.; Liu, X.; et al. A 3D-Printed Self-Adhesive Bandage with Drug Release for Peripheral Nerve Repair. Adv. Sci. 2020, 7, 2002601. [Google Scholar] [CrossRef]
- Chang, H.K.; Yang, D.H.; Ha, M.Y.; Kim, H.J.; Kim, C.H.; Kim, S.H.; Choi, J.W.; Chun, H.J. 3D Printing of Cell-Laden Visible Light Curable Glycol Chitosan Bioink for Bone Tissue Engineering. Carbohydr. Polym. 2022, 287, 119328. [Google Scholar] [CrossRef]
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital Light Processing 3D Printed Silk Fibroin Hydrogel for Cartilage Tissue Engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef]
- Wu, W.; Dong, Y.; Liu, H.; Jiang, X.; Yang, L.; Luo, J.; Hu, Y.; Gou, M. 3D Printed Elastic Hydrogel Conduits with 7,8-Dihydroxyflavone Release for Peripheral Nerve Repair. Mater. Today Bio 2023, 20, 100652. [Google Scholar] [CrossRef]
- Singh, A.; Asikainen, S.; Teotia, A.K.; Shiekh, P.A.; Huotilainen, E.; Qayoom, I.; Partanen, J.; Seppälä, J.; Kumar, A. Biomimetic Photocurable Three-Dimensional Printed Nerve Guidance Channels with Aligned Cryomatrix Lumen for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 43327–43342. [Google Scholar] [CrossRef] [PubMed]
- Menassol, G.; van der Sanden, B.; Gredy, L.; Arnol, C.; Divoux, T.; Martin, D.K.; Stephan, O. Gelatine–Collagen Photo-Crosslinkable 3D Matrixes for Skin Regeneration. Biomater. Sci. 2024, 12, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yang, J.; Lau, A.D.S.; Chen, F.; Bu, Y.; Cai, E.; Wang, H.; Chieng, H.E.; Sun, T.; Zhou, Z.; et al. Digital Light Processing-Bioprinted Poly-NAGA-GelMA-Based Hydrogel Lenticule for Precise Refractive Errors Correction. Biofabrication 2023, 15, 035011. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mapa, M.L.; Singh, M.; Simon, O.; Mapa, J.L.; Machida, M.; Günther, A.; Roth, B.; Heinemann, D.; Terakawa, M.; Heisterkamp, A. Fabrication of a Monolithic Lab-on-a-Chip Platform with Integrated Hydrogel Waveguides for Chemical Sensing. Sensors 2019, 19, 4333. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Enders, A.; Preuss, J.A.; Bahnemann, J.; Heisterkamp, A.; Torres-Mapa, M.L. 3D Printed Microfluidic Lab-on-a-Chip Device for Fiber-Based Dual Beam Optical Manipulation. Sci. Rep. 2021, 11, 14584. [Google Scholar] [CrossRef]
- Zhang, R.; Larsen, N.B. Stereolithographic Hydrogel Printing of 3D Culture Chips with Biofunctionalized Complex 3D Perfusion Networks. Lab Chip 2017, 17, 4273–4282. [Google Scholar] [CrossRef]
- Yang, M.; Chu, L.; Zhuang, Y.; Qi, C.; Meng, S.; Liu, Z.; Kong, T. Multi-Material Digital Light Processing (DLP) Bioprinting of Heterogeneous Hydrogel Constructs with Perfusable Networks. Adv. Funct. Mater. 2024, 34, 2316456. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Zehnder, S.M.; Rowe, K.G.; Jain, S.; Nixon, R.M.; Sawyer, W.G.; Angelini, T.E. Writing in the Granular Gel Medium. Sci. Adv. 2015, 1, e1500655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, S.; Hingorani, H.; Serjouei, A.; Larush, L.; Pawar, A.A.; Goh, W.H.; Sakhaei, A.H.; Hashimoto, M.; Kowsari, K.; et al. Highly Stretchable Hydrogels for UV Curing Based High-Resolution Multimaterial 3D Printing. J. Mater. Chem. B 2018, 6, 3246–3253. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.-i.; et al. Precisely Printable and Biocompatible Silk Fibroin Bioink for Digital Light Processing 3D Printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Puza, F.; Lienkamp, K.; Puza, F.; Lienkamp, K. 3D Printing of Polymer Hydrogels—From Basic Techniques to Programmable Actuation. Adv. Funct. Mater. 2022, 32, 2205345. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.; Chong, C.M.; Lin, D.; Chen, S.; Zhen, Y.; Ding, H.; Zhong, H.J. Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers 2024, 16, 2131. [Google Scholar] [CrossRef]
- Mei, Q.; Rao, J.; Bei, H.P.; Liu, Y.; Zhao, X. 3D Bioprinting Photo-Crosslinkable Hydrogels for Bone and Cartilage Repair. Int. J. Bioprint 2021, 7, 367. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, R.; Wu, X. Recent Progress in the Development of Conductive Hydrogels and the Application in 3D Printed Wearable Sensors. RSC Appl. Polym. 2023, 1, 132–157. [Google Scholar] [CrossRef]
- Moon, S.H.; Hwang, H.J.; Jeon, H.R.; Park, S.J.; Bae, I.S.; Yang, Y.J. Photocrosslinkable Natural Polymers in Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1127757. [Google Scholar] [CrossRef]
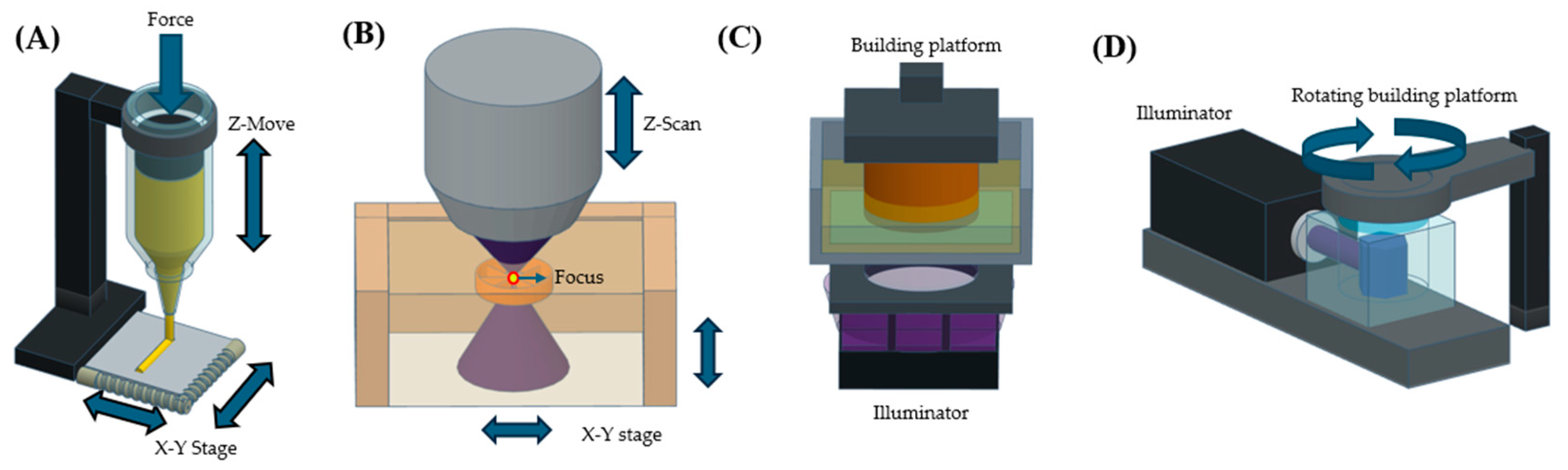







| Feature | SLA | Extrusion | Two-Photon Polymerization | Volumetric Printing |
|---|---|---|---|---|
| Resolution | High (10–100 µm) | Moderate (100–500 µm) | Very high (0.1–1 µm) | High (50–200 µm) |
| Printing speed | Moderate | Slow | Very slow | Fast |
| Precursor viscosity | Low | High | Low | Moderate |
| Cost efficiency | Moderate | Low | Very high | Moderate |
| Limitation in fabricable structure | Long overhanging structures can be limited | Support bath is required for overhanging structure printing | Thickness of constructs can be limited [37] | Structure stability can be limited |
| References | [35,40,41] | [33,34,42] | [36,37] | [43,44] |
| Hydrogel | Mechanical Properties | Degradation Rate | Cell Viability |
|---|---|---|---|
| (DBCO-GelMA [90] | Loss modulus: 19.98 Pa, tensile strength: 53.2 kPa | 63.8 % mass loss in PBS at 37 °C over 21 days; 46.2% of the hydrogel remained after four weeks in a murine model | 95.4% cell survival after 72 h |
| PGSm [58] | Young’s modulus: 3.2 MPa, elongation at break: 220 ± 14% | 68.2% mass reduction in lipase-containting media after 72 h; 48.0% mass is left after 5 weeks of in vivo condition | 94.8% cell survival after 72 h |
| GelMA/SF-MA [93] | Compressive strength: 16 N, tensile strength: 0.027–3.51 MPa depending on ratio | 40.5% mass reduction after 14 days with Protease XIV; 49.3% of hydrogel remained after 4 weeks of in vivo condition | 91.2% cell survival after 48 h |
| Hydrogel | Mechanical Properties | Degradation Rate | Cell Viability |
|---|---|---|---|
| GelMA [60] | Young’s modulus: about 0.5 KPa | 40% mass reduction over 14 days in collagenase | 90.0% of cell survival after 72 h |
| GelMA/PEGDA/F-127 [65] | Compressive modulus: 92.34 ± 6.80 kPa, sweeling ratio 3.90 ± 0.62 | 57.81 ± 3.64% mass is left after 50 days | - |
| AAm/IBOA/WPUA [77] | Tensile strength: 9.9 MPa, elongation at break: 561–216%, | - | - |
| Hydrogel | Mechanical Property | Degradation Rate | Cell Viability |
|---|---|---|---|
| GelMA/HA-NB [63] | Young’s modulus: 30.53 kPa | 83% mass is retained after 4 h in PBS | 95% of cell survival after 5 days |
| GelMA/Collagen [95] | Young’s modulus: 191 ± 35 kPa | - | 96 ± 2% of cell survival ater 7 days |
| GelMA/CECM [69] | Storage modulus (G’): 4979.64 Pa, loss modulus (G’’): 132.66 Pa, Young’s modulus: 26.68 kPa: compressive strength of the hydrogel structure: 73.665 kPa | 83% mass is retained after 4 h in PBS | 94.84% of cell survival after 14 days |
| (NAGA)/GelMA [96] | Young’s modulus: 0.145 MPa, compressive strength: 6.9879 MPa | - | over 90% of cell survival after 7 days |
| Hydrogel | Mechanical Property | Degradation Rate | Cell Viability |
|---|---|---|---|
| GelMA/PEGDA [81] | Elastic modulus: 5.94 ± 0.19 kPa, storage modulus (G’): 2.07 ± 0.41 kPa | - | 96 ± 2% of cell survival at 10 days |
| GelMA/PEGDA [80] | Fluid shear stress: 0.014–0.03 dyn/cm2 | - | 80 % on day 1, 70% on day 4 |
| PEGDA/AAm [100] | Elastic modulus: from 45 kPa (soft matrix) to 210 kPa (stiff matrix) | No significant weight loss after 7 days | Over 90% viability at day 7. |
| Hydrogel | Mechanical Property | Degradation Rate | Cell Viability |
|---|---|---|---|
| GelMA/PEGDA [41] | Elastic modulus: below 8 kPa | 28% mass reduction after 20 days | 96 ± 2% of cell survival at 10 days |
| Sil-MA [104] | Compressive strength: 910 kPa, Compressive modulus: 125.8 kPa, Tensile strength: 75 kPa, Tensile modulus: 14.5 kPa | - | 85–95% of cell survival |
| Application | Target Tissue | Structures | |
|---|---|---|---|
| Tissue regeneration | Sciatic Nerve [90,93] | Multi-layer, tube structures |  Multi-layer structure  Tube structure  Lattice structure  Dome structure |
| Fibular nerve [58] | Multi-layer, tube structures | ||
| Parietal bone [60] | Multi-layer, lattice structures | ||
| Femoral bone [65] | Multi-layer, lattice structures | ||
| Cranial dorsal labrum and lateral meniscus [77] | Multi-layer structure | ||
| Skin defects [63,95] | Multi-layer, lattice structures | ||
| Corneal stroma [69,96] | Multi-layer, dome structures | ||
| Lab-on-a-chip devices | Intestinal tissue model [80,81] | Multi-layer, lattice structures | |
| Hepatic lobule [100] | Multi-layer, channel | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.; Kim, M.; Song, K.H. Development of Hydrogels Fabricated via Stereolithography for Bioengineering Applications. Polymers 2025, 17, 765. https://doi.org/10.3390/polym17060765
Jeon Y, Kim M, Song KH. Development of Hydrogels Fabricated via Stereolithography for Bioengineering Applications. Polymers. 2025; 17(6):765. https://doi.org/10.3390/polym17060765
Chicago/Turabian StyleJeon, Youngjin, Minji Kim, and Kwang Hoon Song. 2025. "Development of Hydrogels Fabricated via Stereolithography for Bioengineering Applications" Polymers 17, no. 6: 765. https://doi.org/10.3390/polym17060765
APA StyleJeon, Y., Kim, M., & Song, K. H. (2025). Development of Hydrogels Fabricated via Stereolithography for Bioengineering Applications. Polymers, 17(6), 765. https://doi.org/10.3390/polym17060765





