3.1. Mechanism and Characterization of RFP-Mediated Photocrosslinking
Riboflavin phosphate (RFP), a water-soluble derivative of vitamin B2, serves as an effective visible-light-responsive photoinitiator. RFP absorbs light at wavelengths of 380 and 450 nm and forms reactive singlet oxygen in the presence of oxygen [
16]. This photochemical property enables RFP to initiate crosslinking reactions under safer visible light conditions compared with traditional UV-based systems, making it particularly attractive for biomedical applications [
19,
20,
21,
22].
To develop an effective polyurethane film for nail polish applications, we strategically combined two different water-based polyurethane dispersions, SUD960 and Akuarane5015, in a 1:1 ratio. This combination was crucial in creating a base film with suitable properties before introducing the photocrosslinking effect of the RFP.
SUD960 and Akuarane5015, both with solid contents of 30–35%, are commercially available water-based polyurethane dispersions that exhibit significantly different viscosities (500 cps and 100 cps, respectively), indicating a difference in their molecular weights. These materials are particularly suitable for enhancing water-based polyurethane films, as they can be readily integrated into existing commercial products due to their established manufacturing processes.
The contrasting properties of these individual polymers guided our formulation strategy. SUD960 forms films with high strength and high elasticity but low elongation, resulting in a rigid nature. In contrast, Akuarane5015 produces films with lower strength and elasticity, but higher elongation, leading to a more flexible character. By combining these polymers in equal proportions, we aimed to compensate for their individual limitations. However, this combination strategy, while balancing the overall properties, resulted in a significant decrease in mechanical strength—a critical drawback for practical applications. To address this limitation, we introduced RFP-mediated photocrosslinking as an additional strengthening mechanism.
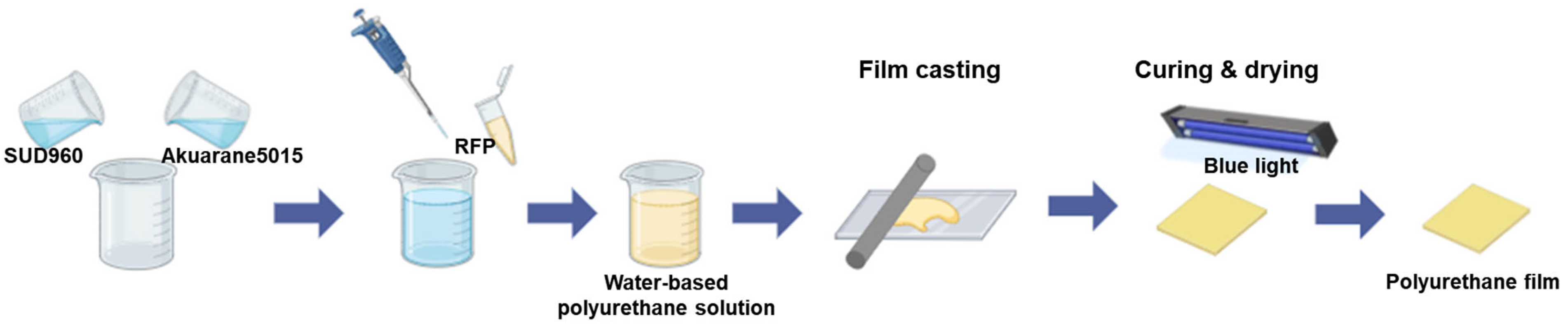
Figure 1 illustrates the fabrication process of the RFP-containing polyurethane films. The successful formation of uniform films is evident in
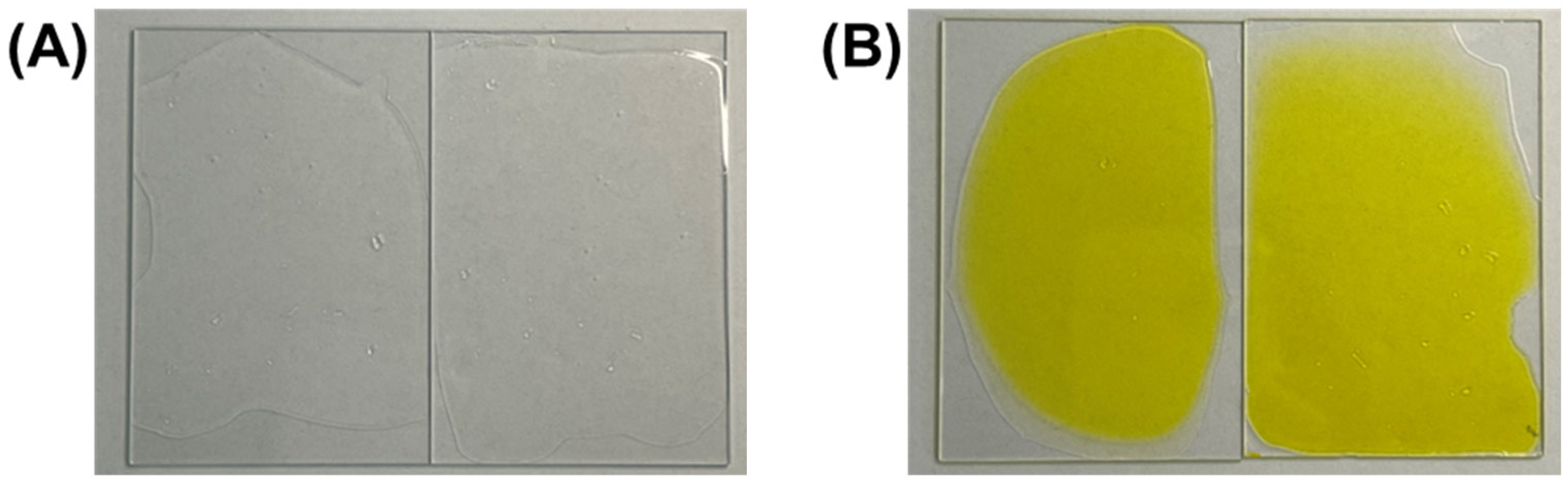
Figure 2, which compares the visual appearance of films without RFP (R0) and with 0.1% RFP (R0.1). The R0 film exhibited transparency, which is characteristic of well-formed polyurethane films. The R0.1 film displayed a slight yellow tint due to the presence of RFP, while it maintained the film’s overall morphology and appearance, indicating that the RFP integration did not disrupt the film-forming capabilities of the polyurethane blend.
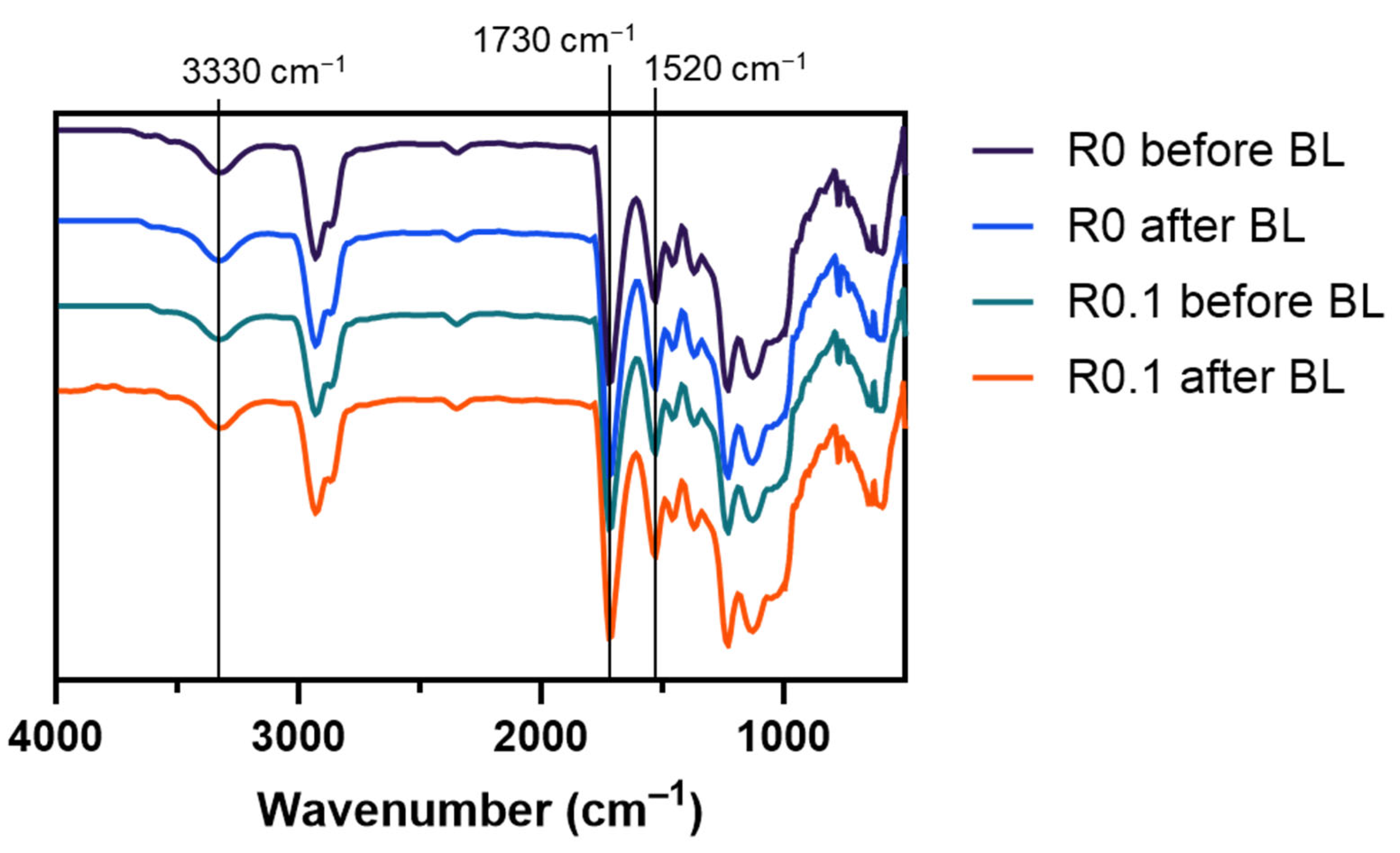
To investigate the chemical composition of the films, we conducted a Fourier-transform infrared (FT-IR) spectroscopy analysis (
Figure 3). The FT-IR spectra revealed characteristic peaks associated with polyurethane: N-H stretching (~3330 cm
−1), C=O stretching (~1730 cm
−1), and C-N stretching (~1520 cm
−1). The spectra remained consistent across all samples from the pristine film (R0) to the RFP-incorporated films after the light exposure.
This spectral consistency indicates that the incorporation of RFP and subsequent photocrosslinking process preserved the primary chemical structure of the polyurethane matrix. Any chemical modifications that resulted from the photocrosslinking reaction occurred at concentrations below the detection limit of the FT-IR spectroscopy, given that RFP was present in relatively small quantities compared with the bulk polymer matrix. The maintenance of the original chemical structure suggests favorable chemical stability of the formulation, which is essential for cosmetic applications.
The preservation of the chemical structure while achieving enhanced mechanical properties through RFP-mediated photocrosslinking demonstrated the advantages of our approach. This strategy enabled the reinforcement of polyurethane films without compromising their inherent chemical properties, which is particularly crucial for applications that require both mechanical durability and chemical stability. The combination of commercially available polyurethanes with RFP-mediated photocrosslinking provides a practical pathway for developing improved water-based nail polish formulations that can be readily implemented in current manufacturing processes.
The timing of the light exposure in relation to the drying process is an important consideration in this system. In our protocol, visible light curing was performed at the onset of drying when the polymer matrix contained sufficient moisture to facilitate the RFP mobility and reactivity. The relationship between the moisture content, crosslinking efficiency, and potential effects of ambient light exposure after the film formation represents an interesting area for future investigation to further optimize the crosslinking mechanism in these water-based systems.
3.2. Structure–Mechanical Property Relationships of Photocrosslinked Networks
The mechanical properties of photocrosslinked films were systematically evaluated through tensile testing to understand the complex relationship between the RFP concentration and network formation (
Figure 4). The incorporation of the RFP and subsequent visible light exposure significantly influenced the mechanical characteristics of the films, which revealed a non-linear concentration-dependent behavior that provided insight into the underlying network formation mechanisms.
Tensile strength exhibited an initial increase with the RFP concentration from 3.88 ± 0.15 MPa for the control (R0) to 4.93 ± 0.09 MPa at a moderate RFP level (R0.05) (
Figure 4A). This enhancement can be attributed to the formation of an optimal network structure where photoinitiated crosslinks effectively reinforced the polymer matrix without significantly disrupting the inherent polymer chain organization [
23]. While R0.075 showed a slight decrease in the tensile strength, this reduction was not statistically significant. The overall trend demonstrated a progressive increase in the tensile strength with increasing RFP concentration, ultimately reaching 5.90 ± 0.30 MPa for R0.1, representing a significant enhancement in the mechanical performance.
Young’s modulus demonstrated a similar non-linear trend with increasing RFP concentration (
Figure 4B). The control film (R0) exhibited a modulus of 78.89 ± 6.95 MPa, which initially increased to 100.09 ± 15.23 MPa at R0.05. This intermediate concentration appears to represent a critical point in the network formation, beyond which the relationship between the crosslinking density and mechanical properties became more complex. The highest RFP concentration (R0.1) ultimately achieved 138.28 ± 12.00 MPa, representing a 75% improvement over the control. Although slight decreases were observed at R0.025 and R0.075, these reductions were not statistically significant, and the overall trend showed an increase in Young’s modulus with increasing RFP concentration.
The non-linear relationship between the RFP concentration and mechanical properties provides insights into the complex interplay between the chemical crosslinking and physical interactions in network formation. At a low RFP concentration (0.025%), the introduction of sparse covalent bonds through photocrosslinking appeared to disrupt the original molecular alignment of the polyurethane chains, which partially interfered with their physical interactions without providing sufficient crosslinking density to compensate, which resulted in the observed decrease in Young’s modulus compared with R0.05. The network continued to evolve as the RFP concentration increased, where the highest concentration (0.1%) ultimately achieved the most robust mechanical properties.
This transition from a physically dominated to a covalently dominated network structure led to not only an increased crosslinking density but also improved the crosslinking homogeneity throughout the polymer network. Recent studies demonstrated that crosslinking homogeneity plays a more critical role than the crosslinking density in determining the mechanical properties of polyurethanes [
24]. At higher RFP concentrations (0.05–0.1%), the increased availability of photoinitiator molecules likely promoted a more uniform distribution of crosslinks, which resulted in a more homogeneous network structure. This enhanced network uniformity, rather than a merely increased crosslinking density, could be the primary factor that contributed to the observed improvements in the tensile strength and Young’s modulus. This interpretation aligns with recent findings that demonstrate the correlation between the crosslinking homogeneity and mechanical property enhancement in polyurethane networks [
24].
The elongation behavior further supports this network evolution mechanism (
Figure 4C). The control films (R0) showed substantial elongation at break (194 ± 109%), primarily governed by physical interactions between polymer chains. The initial decrease in elongation with RFP addition reflects the disruption of these physical interactions, while the relatively consistent elongation values among the higher RFP concentrations suggest the establishment of a stable, covalently crosslinked network structure that maintains certain molecular mobility.
These findings demonstrate that the mechanical properties were determined by the balance between covalent crosslinks and physical interactions, with the covalent bonds becoming the dominant factor at higher RFP concentrations. This understanding provides valuable guidance for optimizing the crosslinking density in photocrosslinked systems.
3.3. Dynamic Mechanical Behavior and Network Characteristics
The viscoelastic properties and network formation mechanisms of our photocrosslinked films were investigated through comprehensive rheological measurements under small deformation conditions, which provided complementary insights into the large deformation tensile testing results (
Figure 5). Small deformation behavior is governed by linear viscoelastic theory, where both physical interactions and covalent bonds contribute to the material response, while large deformation involves nonlinear behavior where the disruption of physical interactions becomes more significant [
25].
Frequency sweep measurements unveiled an intriguing trend in viscoelastic properties (
Figure 5A,B). Films with low RFP concentrations (0.025% and 0.05%) exhibited notably higher storage (G′) and loss (G″) moduli compared with the control under small deformation conditions. This behavior, which appears to contradict the tensile test results, can be explained by the different deformation regimes involved in each measurement. Under a small deformation, both physical interactions and newly formed covalent bonds collectively contribute to the material response, resulting in higher moduli. However, under the large deformation conditions of tensile testing, the disruption of physical interactions by introduced covalent bonds becomes more pronounced, leading to reduced mechanical properties at low RFP concentrations [
26].
The loss factor (tan δ), representing the ratio of viscous to elastic responses, provides crucial insights into network stability (
Figure 5C). While all samples showed tan δ values below 0.5, indicating predominantly solid-like behavior, significant variations were observed across different RFP concentrations. R0.025 exhibited the highest tan δ value despite its high storage modulus, indicating a less stable network structure, where physical and covalent crosslinks coexist without optimal organization. Conversely, R0.1 showed the lowest tan δ value, reflecting the formation of a more stable network structure dominated by uniform covalent crosslinks. Lower tan δ values indicate more elastic behavior, which is characteristic of a more stable network structure [
27].
Temperature-dependent measurements revealed particularly valuable information about network stability and the transition from physical to chemical crosslinking (
Figure 5D). R0.025 demonstrated the most pronounced decrease in the storage modulus with increasing temperature, consistent with the description of the temperature-dependent behavior in physical networks [
26]. This significant temperature sensitivity suggests a network primarily stabilized by physical interactions that were easily disrupted by thermal energy. In contrast, R0.05 and R0.075 maintained higher storage modulus retention at elevated temperatures, indicating the presence of strong covalent bonds that provided thermal stability. This thermal behavior provides strong evidence for the transition from a physically dominated to a covalently dominated network structure with increasing RFP concentration, aligning with established theories on the thermal stability differences between physical and chemical networks [
26].
This rheological behavior further explained the non-linear trends observed in tensile testing results (
Figure 4), particularly the fluctuations in Young’s modulus with increasing RFP concentration. The transitional network behavior at the 0.075% RFP concentration seen in the mechanical testing corresponded with the evolving viscoelastic properties demonstrated in these rheological measurements.
The comprehensive rheological analysis under small deformation conditions thus complemented and explained the large deformation tensile testing results, providing a more complete understanding of the network evolution with increasing RFP concentration. The apparent contradictions between the small and large deformation behaviors are resolved when considering the fundamental physics of polymer networks under different deformation regimes, highlighting the complexity of network structure development in photocrosslinked systems.
3.4. Interfacial Adhesion Mechanisms and Performance
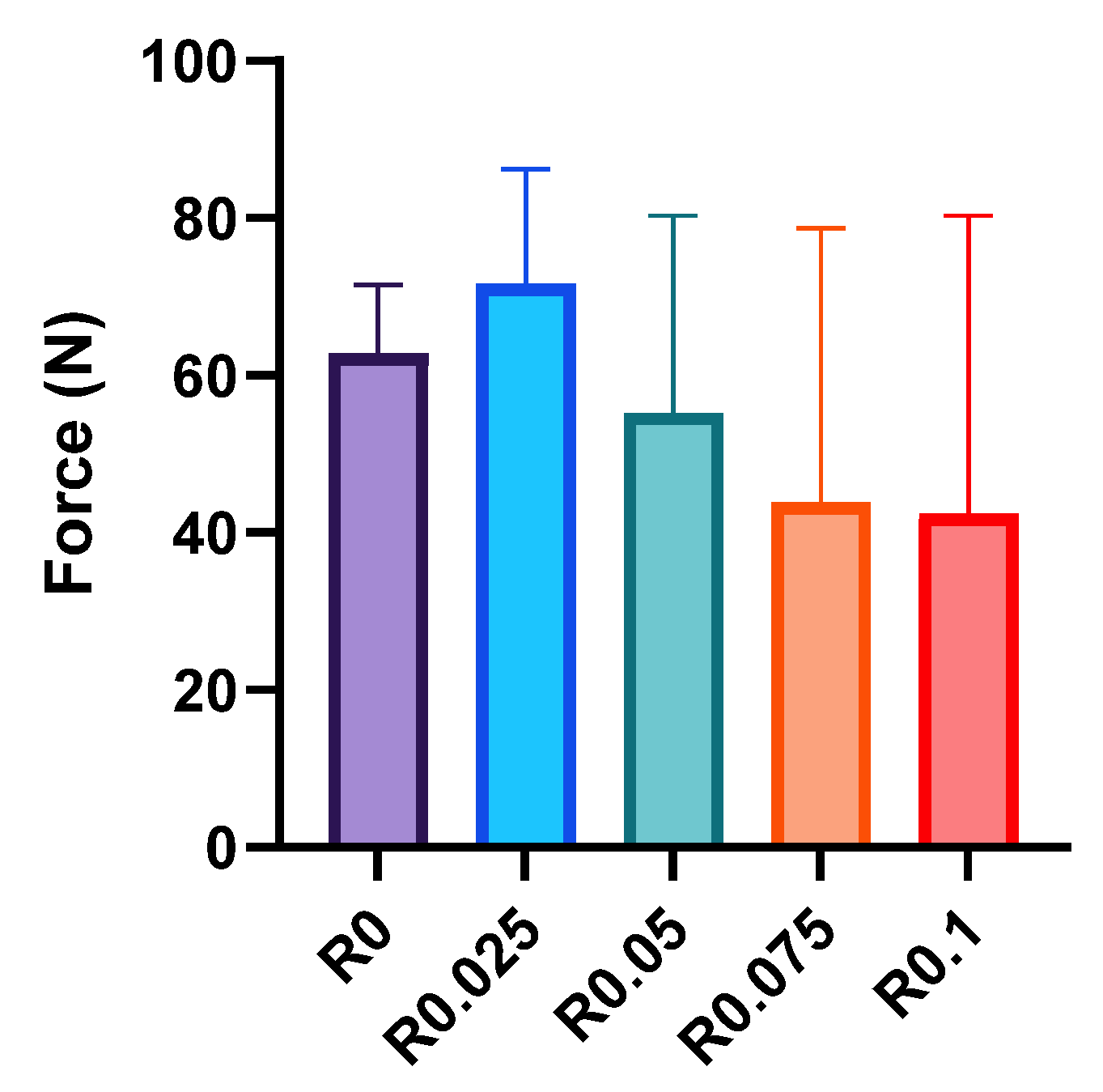
The adhesion properties of photocrosslinked films were evaluated through systematic lap shear testing using PET substrates (
Figure 6). The control film (R0) exhibited a moderate adhesion strength of 62.8 ± 7.1 N, which increased slightly to 71.6 ± 11.9 N at a low RFP concentration (R0.025). However, as the RFP concentration increased further, the adhesion strength gradually decreased to 55.2 ± 20.5 N, 43.9 ± 28.5 N, and 42.4 ± 30.9 N for R0.05, R0.075, and R0.1, respectively.
This trend in adhesion behavior can be understood through the evolution of the network structure and molecular mobility at the interface. At a low RFP concentration (R0.025), the network retained significant physical interactions while incorporating a limited number of covalent bonds. Such a network structure allows for sufficient chain mobility at the interface, enabling effective molecular contact and entanglement with the substrate surface [
27]. This molecular-level flexibility promotes stronger adhesion through multiple types of interfacial interactions.
However, as the RFP concentration increases and the network transitions from physically dominated to covalently dominated structure, the restricted chain mobility begins to limit interfacial interactions. This behavior aligned with our rheological observations: while higher RFP concentrations created more stable networks (as evidenced by lower tan δ values), the reduced molecular mobility (shown by the temperature-dependent behavior) impaired the ability of polymer chains to achieve intimate contact with the substrate surface. This trade-off between network stability and interfacial mobility explains the observed decrease in the adhesion strength at higher RFP concentrations.
The relationship between the adhesion behavior and network characteristics revealed by the mechanical and rheological analyses provides deeper insights into the structure–property relationships of our system. The higher storage modulus and loss modulus observed in the rheological measurements for films with R0.025 correlated with their enhanced adhesion performances under small deformation conditions. This suggests that the optimal balance between the physical and covalent crosslinks at low RFP concentrations created a network structure capable of both maintaining cohesive strength and facilitating interfacial interactions.
These findings demonstrate the critical role of molecular mobility and network architecture in determining the adhesion properties. While higher RFP concentrations enhance the bulk mechanical properties through the formation of stable covalent networks, the optimal adhesion performance requires sufficient chain mobility for effective interfacial interactions. This understanding suggests that future optimization strategies should focus on maintaining adequate molecular mobility at the interface while achieving the desired bulk properties through controlled network formation.
3.5. Biocompatibility Assessment and Commercial Viability
The biocompatibility of photocrosslinked films was evaluated using NIH/3T3 fibroblast cells to assess their safety for cosmetic applications (
Figure 7). Cell viability tests showed that extracts from films with various RFP concentrations (0–0.1%) maintained comparable cell viability with the control group after 24 h of exposure, although the differences were not statistically significant. The absence of cytotoxicity across all the RFP concentrations indicates the biological safety of our formulation approach.
These favorable biocompatibility results, combined with the advantages demonstrated in previous sections, position our system as a promising candidate for next-generation water-based nail polish formulations. Several key features make this technology particularly suitable for immediate commercial implementation: (1) the use of commercially available water-based polyurethanes as base materials; (2) the incorporation of RFP, an FDA-approved photoinitiator [
28,
29]; (3) a visible-light-mediated curing process that eliminates UV exposure risks; and (4) enhanced mechanical durability while maintaining easy peel-off functionality.
Furthermore, our formulation addresses growing market demands for environmentally conscious and user-friendly cosmetic products. The water-based nature of the system reduces volatile organic compound emissions, while the visible light curing process provides a safer alternative to traditional UV curing methods. The straightforward preparation process and compatibility with existing manufacturing protocols suggest that this technology could be readily integrated into current production lines, offering a practical pathway for developing improved nail polish products.